Influence of Deposition Temperature on the Structure and Current-Carrying Friction Performance of Cu Films by DC Magnetron Sputtering Technology
Abstract
1. Introduction
2. Experimental Details
2.1. Film Deposition
2.2. Film Characterization
2.3. Tribological Properties Test
3. Results and Discussion
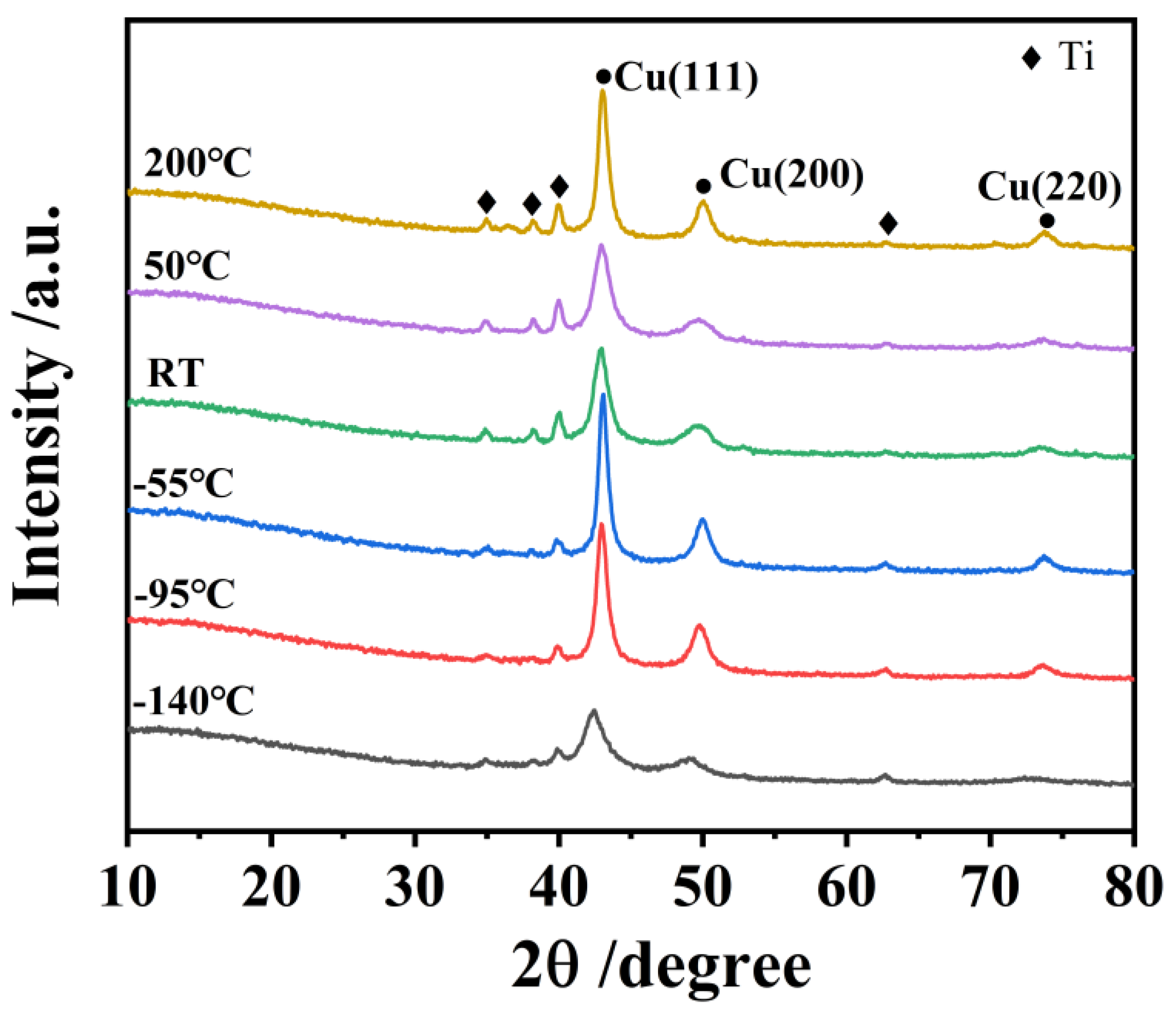
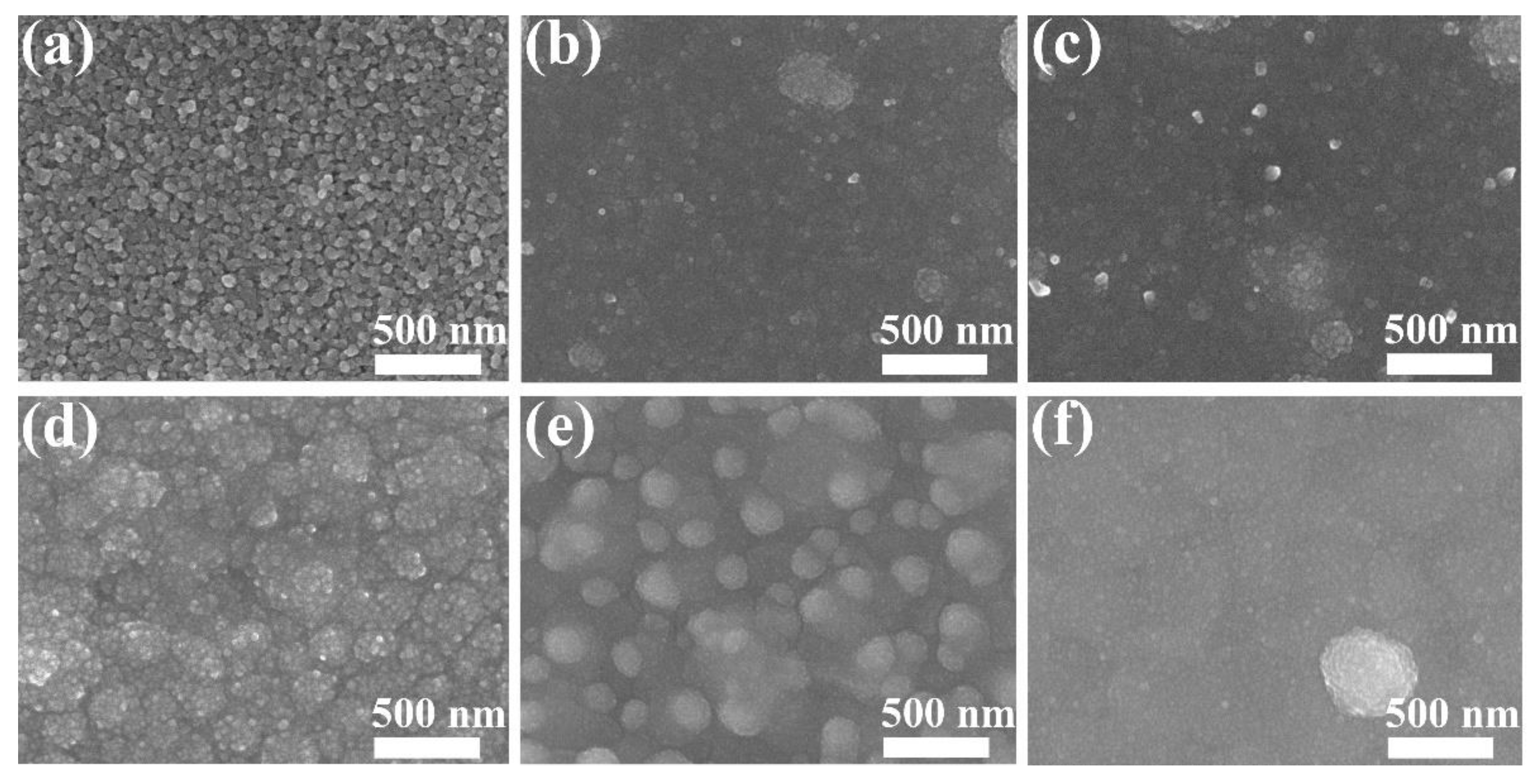
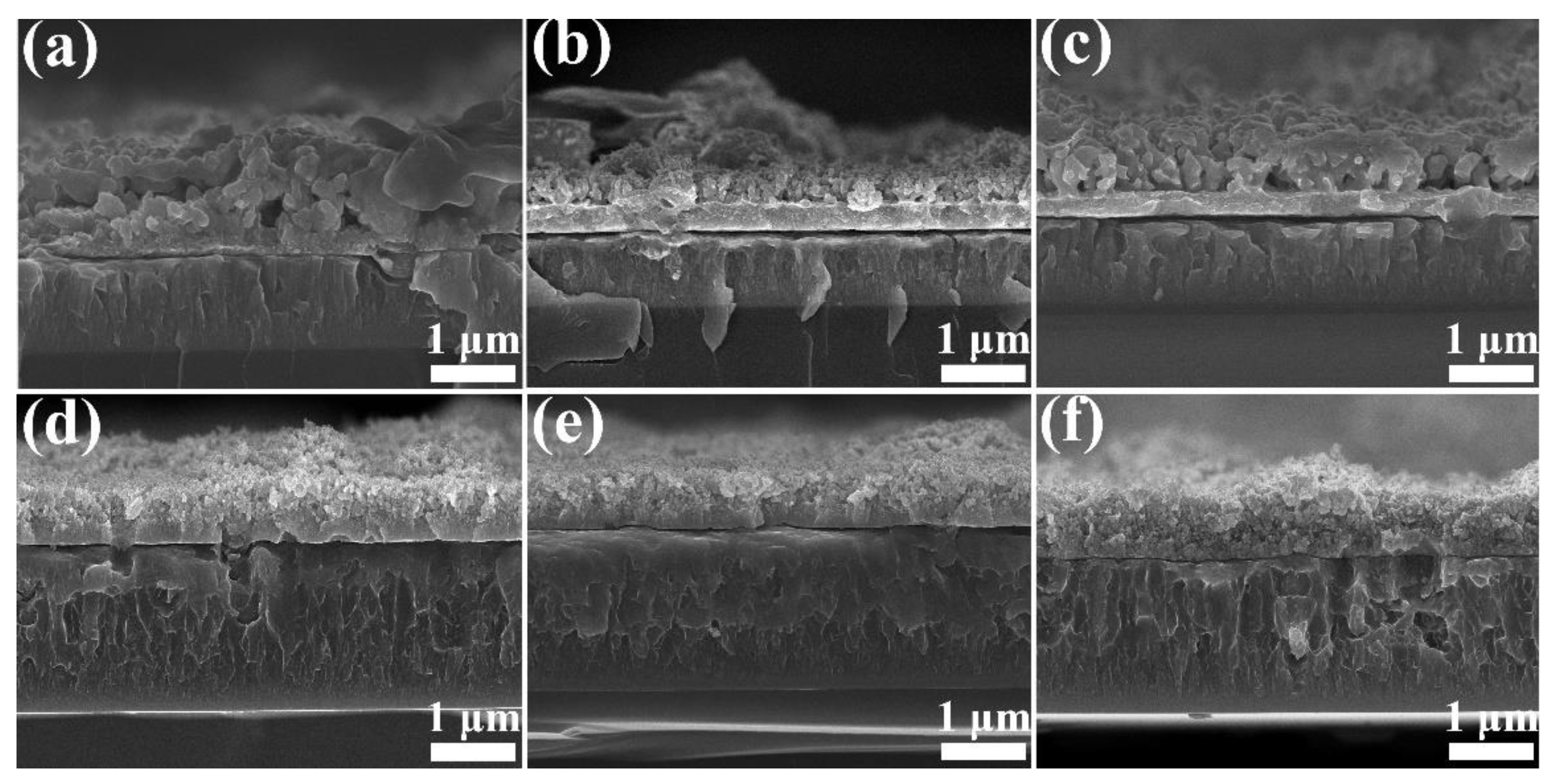
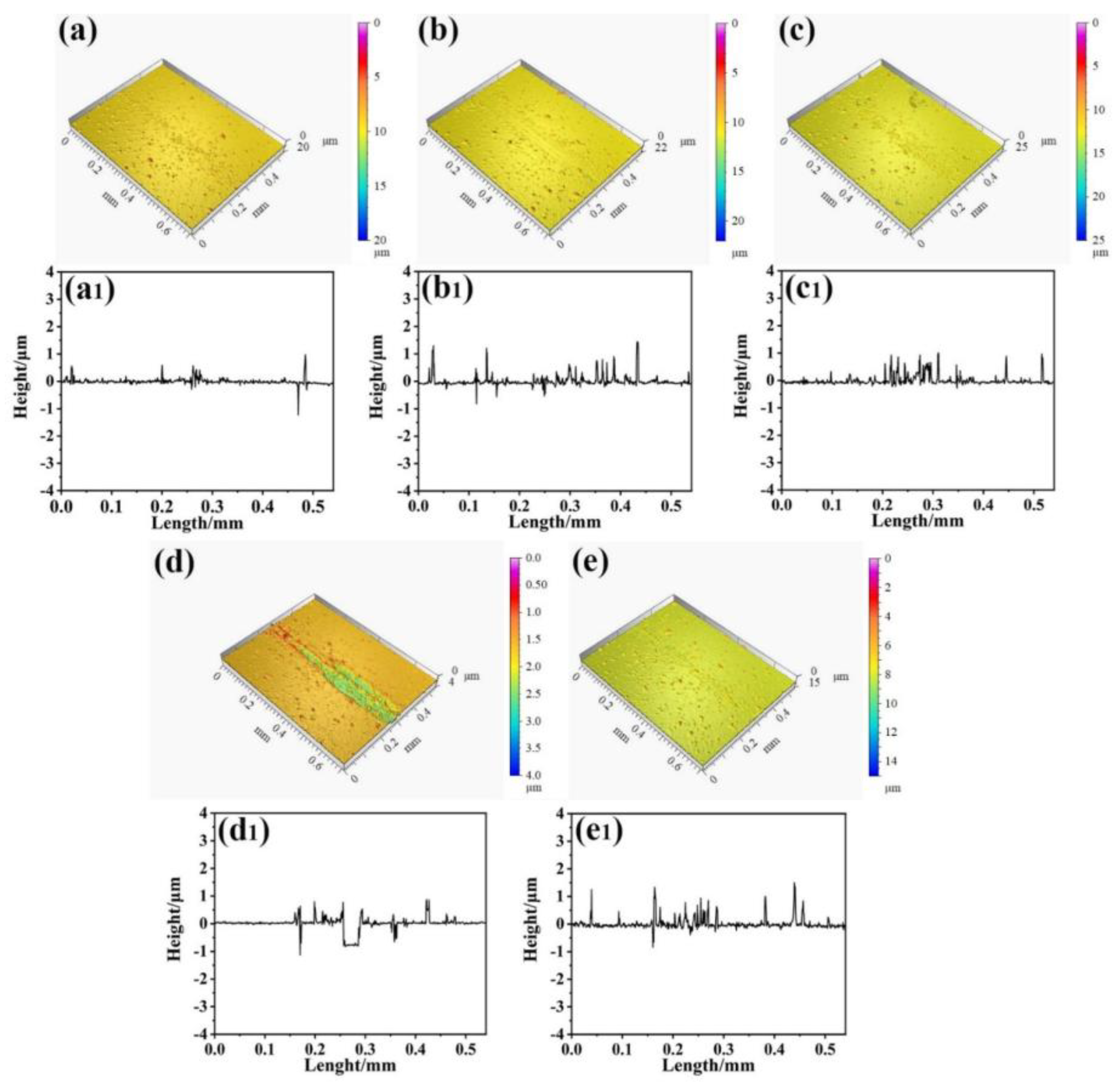
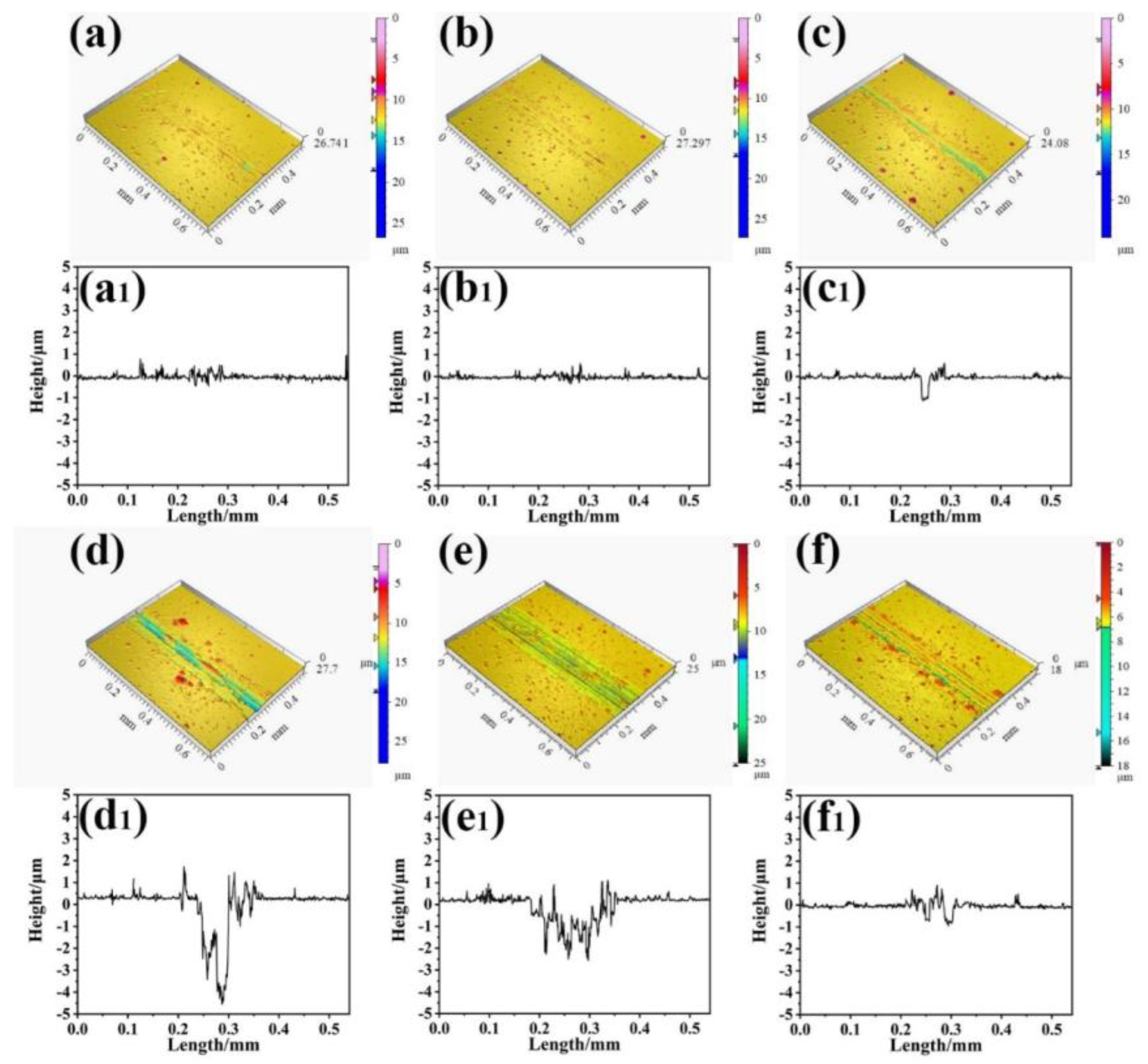
3.1. Structure of Film
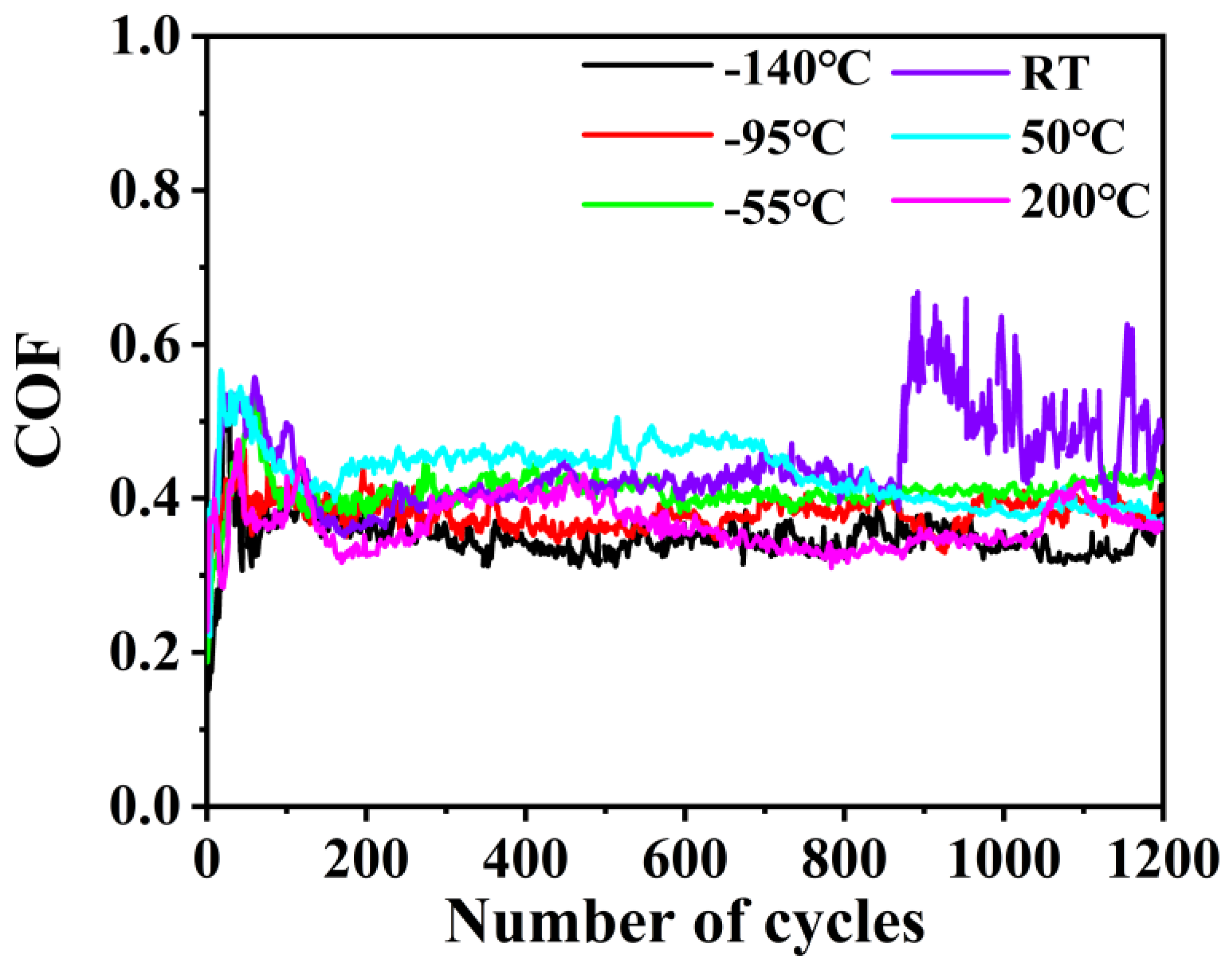
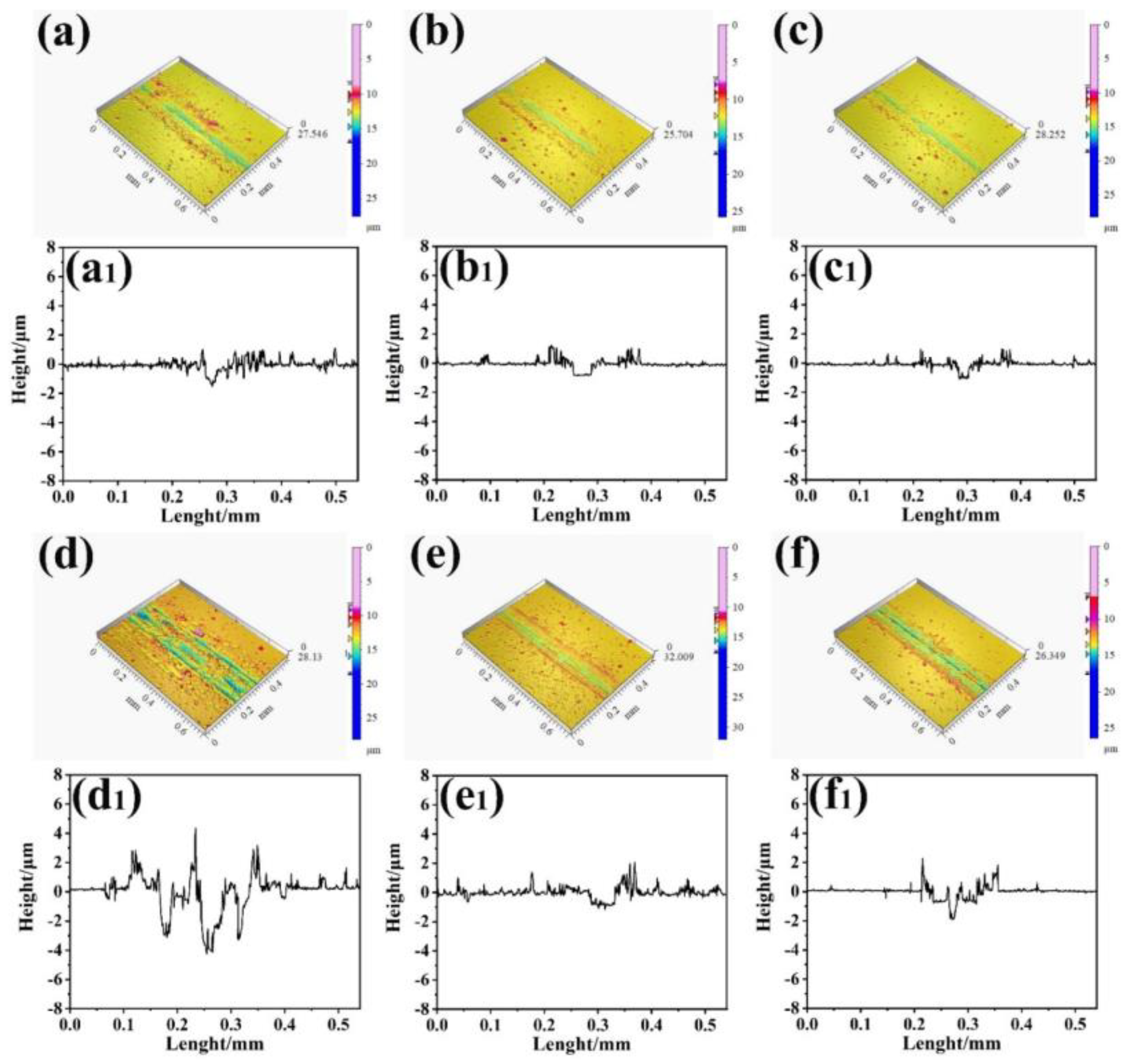
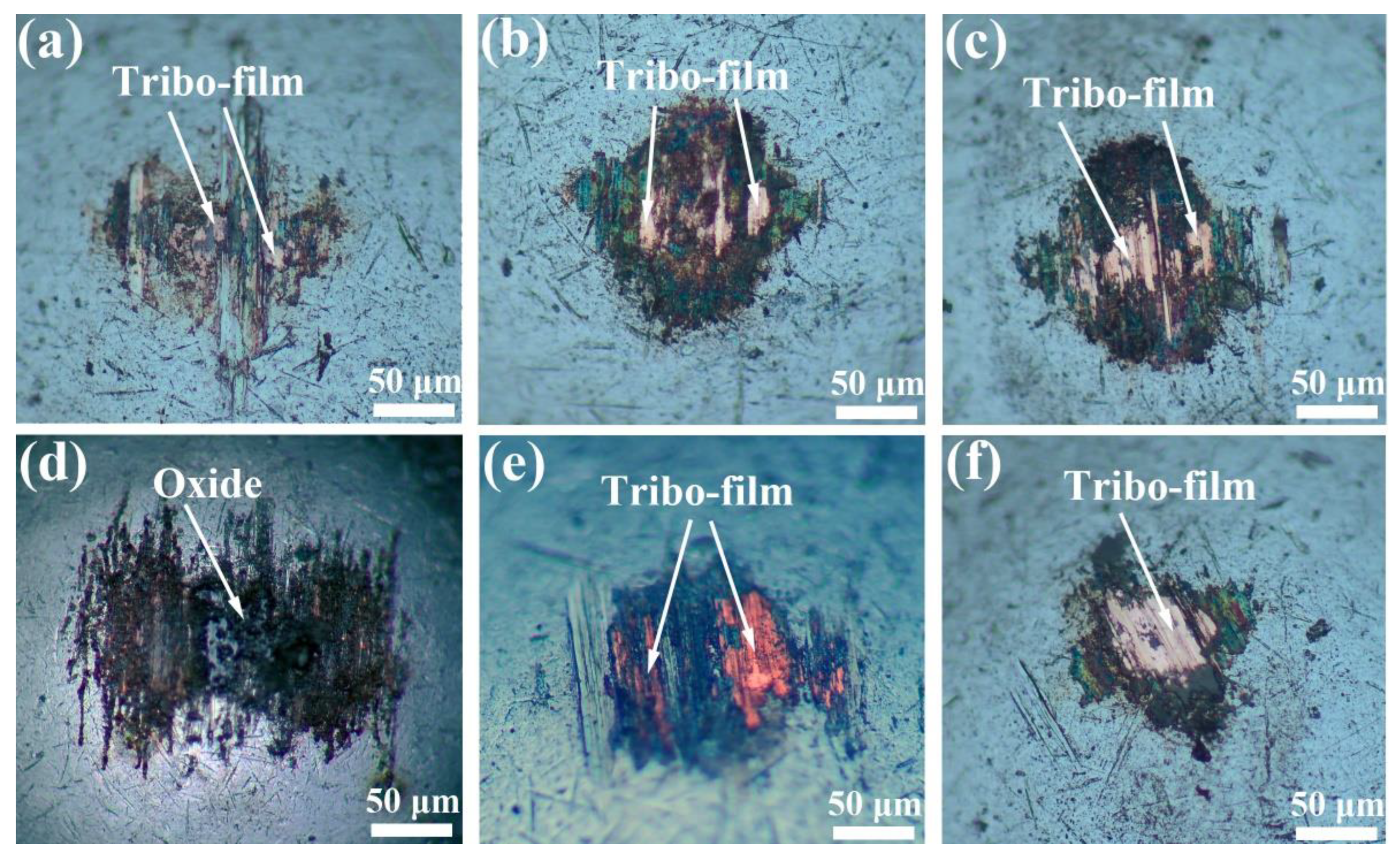
3.2. Tribological Properties
3.2.1. Tribological Performance without Electric Current
3.2.2. Tribological Performance with Electric Current
4. Conclusions
- (1)
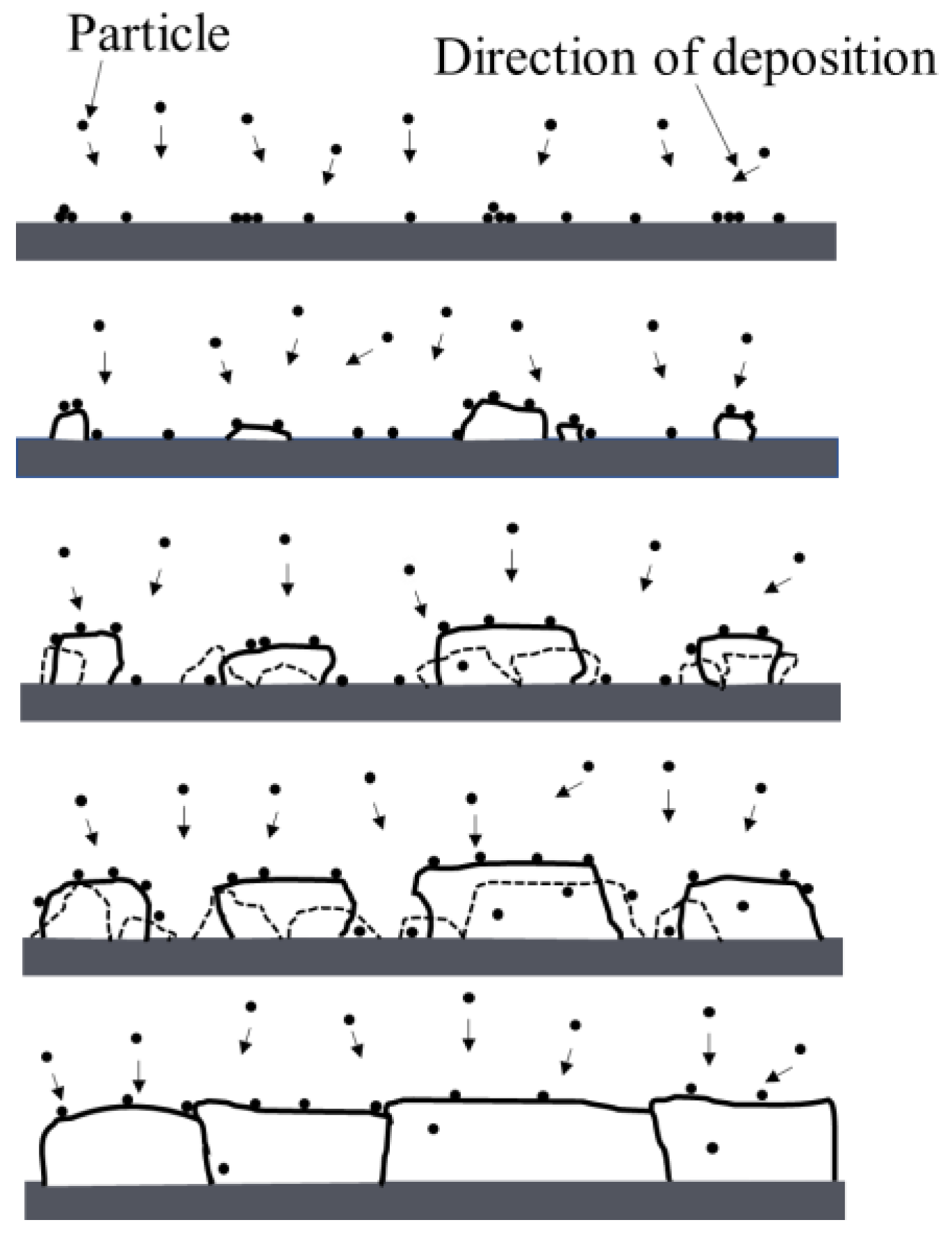
- All the Cu films exhibited a strong orientation of the (111) and (200) planes, which is attributed to the lowest surface energy of the (111) plane and lowest strain energy of the (200) plane of the Cu (fcc) crystal. The Cu films deposited at low temperatures below −55 °C exhibited a typical bilayer structure. It can be attributed to the mobility and surface diffusion of the adatoms during the growth of the Cu films. When the deposition temperature increases above the RT, the bilayer structure of Cu films disappears and is replaced by the dense structure.
- (2)
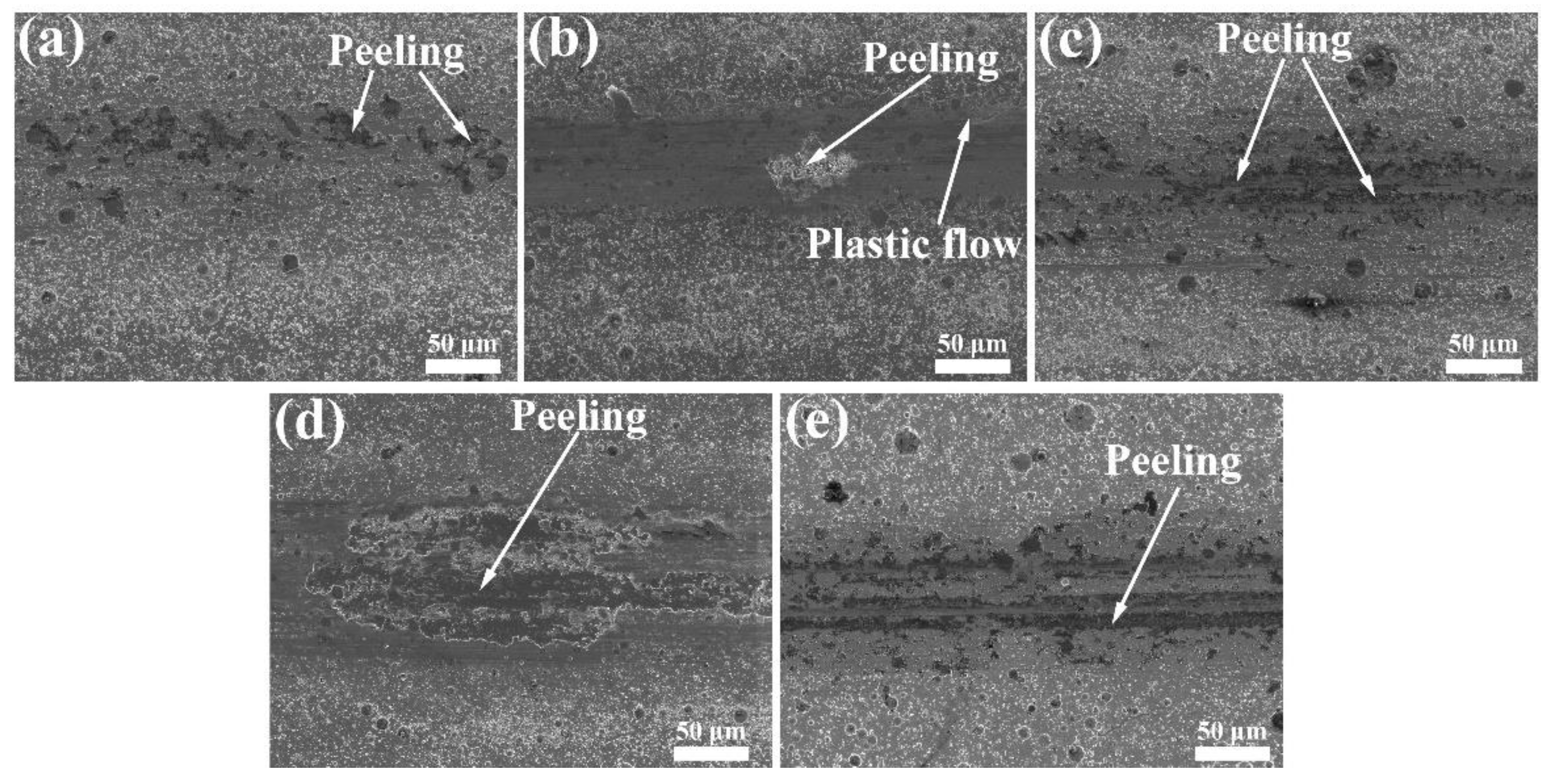
- The loose Cu film deposited below −55 °C had relatively stable low friction and anti-wear performances as compared with the Cu film with a dense structure. The loose-structure of Cu film could rearrange on the wear tracks during the current-carrying friction process because the plastic deformation caused by the contact pressure and kinetic frictional interaction easily occurred, which could effectively provide synchronized deformation of the film with the ductile substrate. Furthermore, the transferred softened Cu phase on the tribo-pair surface is conducive to the tribological performance under the current-carrying conditions. Nevertheless, the electric arc can be responsible for the oxidation of Cu phase. This work shows a novel low-temperature deposition strategy of Cu films to improve the friction and wear performances under the electric current condition, and further study would focus on how to enhance its anti-oxidation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ECR | Electrical contact resistance |
| Ra | Surface roughness |
| v | The surface diffusion rate |
| v0 | The frequency of atom vibration |
| D | The average grain diameter |
| D0 | The initial grain diameter |
| n | The grain growth exponent |
| K(T) | The grain growth constant |
| h | The Plank constant |
| ΔE | The activation energy |
| KB | The Boltzmann constant |
| T | Temperature |
| t | Time |
References
- Holmberg, K.; Erdemir, A. The impact of tribology on energy use and CO2 emission globally and in combustion engine and electric cars. Tribol. Int. 2019, 135, 389–396. [Google Scholar] [CrossRef]
- Gong, H.J.; Yu, C.C.; Zhang, L.; Xie, G.X.; Guo, D.; Luo, J.B. Intelligent lubricating materials: A review. Compos. Part B-Eng. 2020, 202, 108450. [Google Scholar] [CrossRef]
- Yang, M.S.; Liu, X.B.; Fan, J.W.; He, X.M.; Shi, S.H.; Fu, G.Y.; Wang, M.D.; Chen, S.F. Microstructure and wear behaviors of laser clad NiCr/Cr3C2-WS2 high temperature self-lubricating wear-resistant composite coating. Appl. Surf. Sci. 2012, 258, 3757–3762. [Google Scholar] [CrossRef]
- Lu, X.L.; Liu, X.B.; Yu, P.C.; Fu, G.Y.; Zhu, G.X.; Wang, Y.G.; Chen, Y. Effects of annealing on laser clad Ti2SC/CrS self-lubricating anti-wear composite coatings on Ti6Al4V alloy: Microstructure and tribology. Tribol. Int. 2016, 101, 356–363. [Google Scholar] [CrossRef]
- Das, A.K. Effect of solid lubricant addition in coating produced by laser cladding process: A review. Mater. Today. Proc. 2022, 56, 1274–1280. [Google Scholar] [CrossRef]
- Holmberg, K.; Matthews, A.; Ronkainen, H. Coatings tribology—Contact mechanisms and surface design. New Dir. Tribol. 1998, 251, 107–120. [Google Scholar] [CrossRef]
- Holmberg, K.; Ronkainen, H.; Matthews, A. Tribology of thin coatings. Ceram. Int. 2000, 26, 787–795. [Google Scholar] [CrossRef]
- Scharf, T.W.; Prasad, S.V. Solid lubricants: A review. J. Mater. Sci. 2013, 48, 511–531. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C. Lubricious oxide coatings for extreme temperature applications: A review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Kumar, R.; Hussainova, I.; Rahmani, R.; Antonov, M. Solid Lubrication at High-Temperatures-A Review. Materials 2022, 15, 1695. [Google Scholar] [CrossRef]
- Ghotbi, M.Y.; Rahmati, Z. Nanostructured copper and copper oxide thin films fabricated by hydrothermal treatment of copper hydroxide nitrate. Mater. Des. 2015, 85, 719–723. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, J.H.; Kang, J.H. Structural and optical characterization of Cu3N films prepared by reactive RF magnetron sputtering. J. Cryst. Growth 2001, 222, 767–772. [Google Scholar] [CrossRef]
- Cale, T.S.; Merchant, T.P.; Borucki, L.J.; Labun, A.H. Topography simulation for the virtual wafer fab. Thin Solid Films 2000, 365, 152–175. [Google Scholar] [CrossRef]
- Vancea, J.; Hofmann, F.; Hoffmann, H. Grain size-dependent Hall coefficient in polycrystalline copper films. J. Phys.-Condens. Matter 1989, 1, 7419–7424. [Google Scholar] [CrossRef]
- Geetha, M.; Kumar, N.; Panda, K.; Dhara, S.; Dash, S.; Panigrahi, B.K.; Tyagi, A.K.; Jayavel, R.; Kamaraj, V. Tribological and electrical properties of nanocrystalline Cu films deposited by DC magnetron sputtering with varying temperature. Tribol. Int. 2013, 58, 79–84. [Google Scholar] [CrossRef]
- Weng, L.; Sun, J.; Ming, H.; Gao, X.; Xue, Q. Structure and tribological properties of Ag films deposited at low temperature. Vacuum 2007, 81, 997–1002. [Google Scholar] [CrossRef]
- The, W.; Koh, L.; Chen, S.; Xie, J.; Lie, C.; Fou, P. Study of microstructureand resistivity evolution for electroplated copper films at near room-temperature. Microelectron. J. 2001, 32, 579–585. [Google Scholar] [CrossRef]
- Li, X.; Ji, G.; Lv, H.; Min, W.; Du, Y. Microwave absorbing properties and enhanced infrared reflectance of Fe/Cu composites prepared by chemical plating. J. Magn. Magn. Mater. 2014, 355, 65–69. [Google Scholar] [CrossRef]
- Bai, P.; Yang, G.R.; You, L.; Lu, T.M.; Knorr, D.B. Room-temperature epitaxy of Cu on Si(111) using partially ionized beam deposition. J. Mater. Res. 1990, 5, 989–997. [Google Scholar] [CrossRef]
- Tillmann, W.; Dias, N.; Stangier, D.; Matveev, S.; Debus, J. Design of Cu- and Ag-containing amorphous carbon multilayers with improved tribo-mechanical properties. Mater. Lett. 2020, 284, 128905. [Google Scholar] [CrossRef]
- Kataria, S.; Srivastava, S.K.; Kumar, P.; Srinivas, G.; Siju; Khan, J.; Rao, D.; Barshilia, H.C. Nanocrystalline TiN coatings with improved toughness deposited by pulsing the nitrogen flow rate. Surf. Coat. Tech. 2012, 206, 4279–4286. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, Y.; Duan, B. Microstructure evolution and anti-wear mechanism of Cu film fabricated by magnetron sputtering deposition. Mater. Lett. 2022, 315, 131941. [Google Scholar] [CrossRef]
- Medjani, F.; Sanjines, R.; Allidi, G.; Karimi, A. Effect of substrate temperature and bias voltage on the crystallite orientation in RF magnetron sputtered AIN thin films. Thin Solid Films 2006, 515, 260–265. [Google Scholar] [CrossRef]
- Kusaka, K.; Taniguchi, D.; Hanabusa, T.; Tominaga, K. Effect of sputtering gas pressure and nitrogen concentration on crystal orientation and residual stress in sputtered AlN films. Vacuum 2002, 66, 441–446. [Google Scholar] [CrossRef]
- Higo, M.; Fujita, K.; Tanaka, Y.; Mitsushio, M.; Yoshidome, T. Surface morphology of metal films deposited on mica at various temperatures observed by atomic force microscopy. Appl. Surf. Sci. 2006, 252, 5083–5099. [Google Scholar] [CrossRef]
- Boakye, F. Temperature dependence of the resistivity of amorphous Mn thin films. J. Non-Cryst. Solids 1999, 249, 189–193. [Google Scholar] [CrossRef]
- Kale, A.; Seal, S.; Sobczak, N.; Morgiel, J.; Sundaram, K.B. Effect of deposition temperature on the morphology, structure, surface chemistry and mechanical properties of magnetron sputtered Ti70–Al30 thin films on steel substrate. Surf. Coat. Tech. 2001, 141, 252–261. [Google Scholar] [CrossRef]
- Seiko, W.; Hoshi, Y.; Shimizu, H. Fe and Fe–N films sputter deposited at liquid nitrogen temperature. J. Magn. Magn. Mater. 2001, 235, 196–200. [Google Scholar] [CrossRef]
- Cv, T.; Carel, R. Stress and grain growth in thin films. J. Mech. Phys. Solids 1996, 44, 657–673. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, D.; Kumar, R.; Kaur, D. Effect of crystallographic orientation of nanocrystalline TiN on structural, electrical and mechanical properties of TiN/NiTi thin films. J. Alloy. Compd. 2009, 479, 166. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, J.M.; Xu, K.W. Surface-energy-driven abnormal grain growth in Cu and Ag films. Appl. Surf. Sci. 2005, 242, 55–61. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, X.; Wu, S.; Liu, J.; He, D. Kinetic Monte Carlo simulation of Cu thin film growth. Vacuum 2004, 72, 405–410. [Google Scholar] [CrossRef]
- Su, D.; Yu, M.; Zhang, G.; Jiang, S.; Li, M.Y. High thermal stable Au-Al bimetallic conductive thin films with a broadband transmittance between the UV and NIR regions. J. Mater. Chem. C 2020, 8, 2852–2860. [Google Scholar] [CrossRef]
- Su, D.; Jiang, S.L.; Yu, M.N.; Zhang, G.Z.; Liu, H.; Li, M.Y. Facile fabrication of configuration controllable self-assembled Al nanostructures as UV SERS substrates. Nanoscale 2018, 10, 22737–22744. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.; Leinen, D.; del Campo, A.; Ares, J.R.; Sanchez, C.; Flege, J.I.; Gutierrez, A.; Prieto, P.; Soriano, L. Growth and characterization of ZnO thin films at low temperatures: From room temperature to-120 degrees C. J. Alloys Compd. 2021, 884, 161056. [Google Scholar] [CrossRef]
- Sivakumar, R.; Jayachandran, M.; Sanjeeviraja, C. Studies on the effect of substrate temperature on (VI-VI) textured tungsten oxide (WO3) thin films on glass, SnO2: F substrates by PVD: EBE technique for electrochromic devices. Mater. Chem. Phys. 2004, 87, 439–445. [Google Scholar] [CrossRef]
- Zhang, D.M.; Guan, L.; Li, Z.H.; Pan, G.J.; Sun, H.Z.; Tan, X.Y.; Li, L. Influence of kinetic energy and substrate temperature on thin film growth in pulsed laser deposition. Surf. Coat. Technol. 2006, 200, 4027–4031. [Google Scholar] [CrossRef]
- Zhao, J.L.; Li, X.M.; Bian, J.M.; Yu, W.D.; Zhang, C.Y. Growth of nitrogen-doped p-type ZnO films by spray pyrolysis and their electrical and optical properties. J. Cryst. Growth 2005, 280, 495–501. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhang, Q.Z.; Pandey, P.; Sui, M.; Kim, E.S.; Lee, J. From the Au nano-clusters to the nanoparticles on 4H-SiC (0001). Sci. Rep. 2015, 5, 13954. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.-Y.; Su, D.; Yu, M.; Kan, H.; Liu, H.; Wang, X.; Jiang, S. Broadband High Sensitivity ZnO Colloidal Quantum Dots/Self-assembled Au Nano-antennas Heterostructures Photodetectors. ACS Appl. Mater. Interfaces 2018, 10, 32516–32525. [Google Scholar] [CrossRef]
- Park, J.Y.; Dong, J.L.; Yun, Y.S.; Moon, J.H.; Lee, B.T.; Sang, S.K. Temperature-induced morphological changes of ZnO grown by metalorganic chemical vapor deposition. J. Cryst. Growth 2005, 276, 158–164. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, J.M.; Je, J.H.; Kim, S.S. Early stage growth behavior of ZnO nanoneedle arrays on Al2O3 (0001) by metalorganic chemical vapor deposition. J. Cryst. Growth 2005, 281, 446–451. [Google Scholar] [CrossRef]
- Shin, S.W.; Agawane, G.L.; Kim, I.Y.; Kwon, Y.B.; Jung, I.O.; Gang, M.G.; Moholkar, A.V.; Moon, J.H.; Kim, J.H.; Lee, J.Y. Low temperature epitaxial growth and characterization of Ga-doped ZnO thin films on Al2O3 (0001) substrates prepared with different buffer layers. Appl. Surf. Sci. 2012, 258, 5073–5079. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Chan, K.Y.; Tou, T.Y.; Teo, B.S. Effects of substrate temperature on electrical and structural properties of copper thin films. Microelectron. J. 2006, 37, 930–937. [Google Scholar] [CrossRef]
- Xue, J.; Li, Y.; Hao, L.; Gao, L.; Chen, J. Investigation on the interfacial stability of multilayered Cu–W films at elevated deposition temperatures during co-sputtering. Vacuum 2019, 166, 162–169. [Google Scholar] [CrossRef]
- Zugelj, B.B.; Kalin, M. Submicron-scale experimental analyses of the multi-asperity contact behaviour of various steels, an aluminium alloy and a polymer. Tribol. Int. 2019, 141, 105955. [Google Scholar] [CrossRef]
- Jacobs, T.D.B.; Martini, A. Measuring and Understanding Contact Area at the Nanoscale: A Review. Appl. Mech. Rev. 2017, 69, 060802. [Google Scholar] [CrossRef]
- Pan, W.; Li, X.; Wang, X. Contact mechanics of elastic-plastic fractal surfaces and static friction analysis of asperity scale. Eng.Comput. 2020, 38, 131–150. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Hu, Y.; Hou, B. Tribological performance and conductive capacity of Ag coating under boundary lubrication. Tribol. Int. 2017, 110, 161–172. [Google Scholar] [CrossRef]
- Lin, X.Z.; Zhu, M.H.; Ji-Liang, M.O.; Chen, G.X.; Jin, X.S.; Zhou, Z.R. Tribological and electric-arc behaviors of carbon/copper pair during sliding friction process with electric current applied. Trans. Nonferrous Met. Soc. China 2011, 21, 292–299. [Google Scholar] [CrossRef]
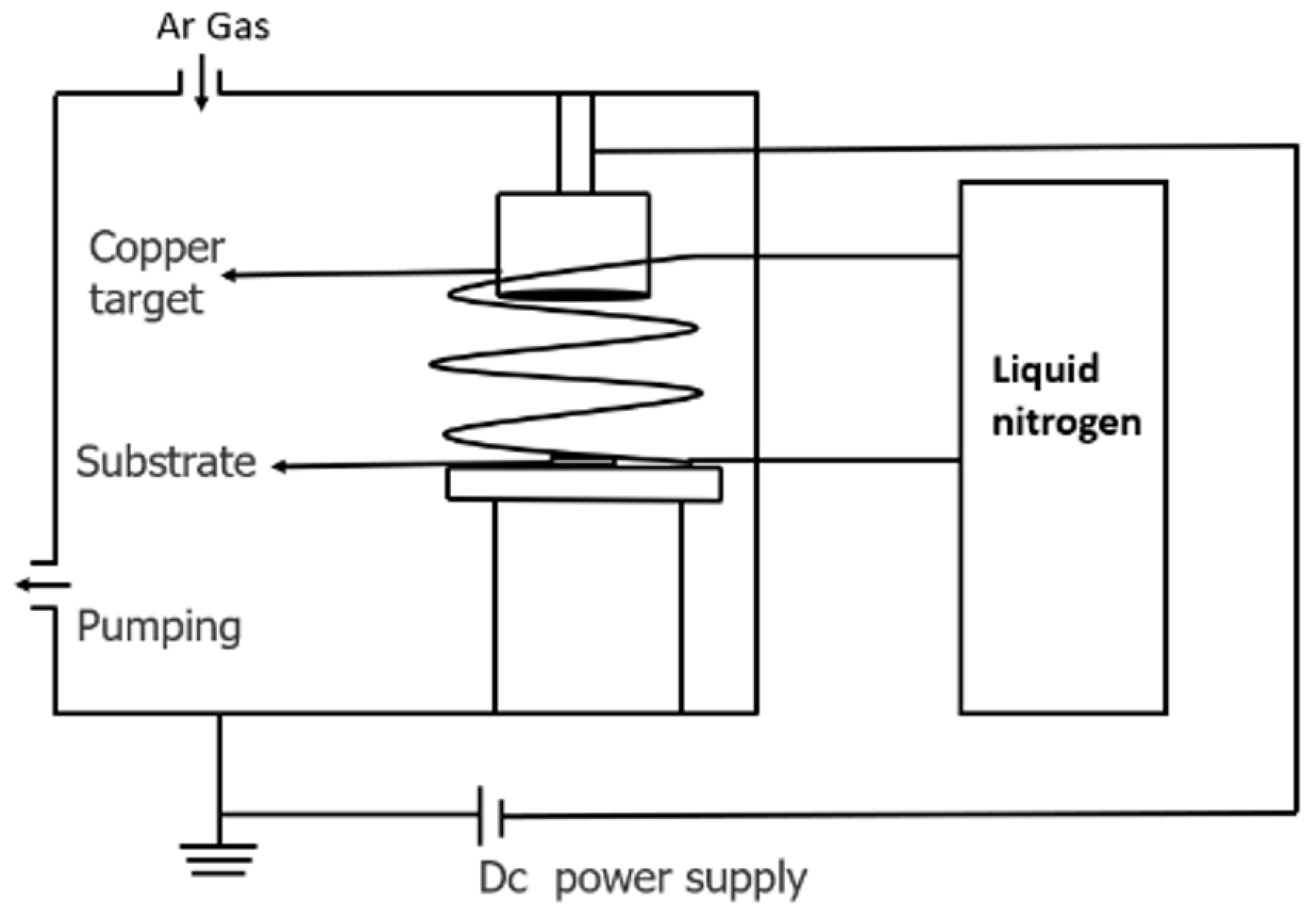
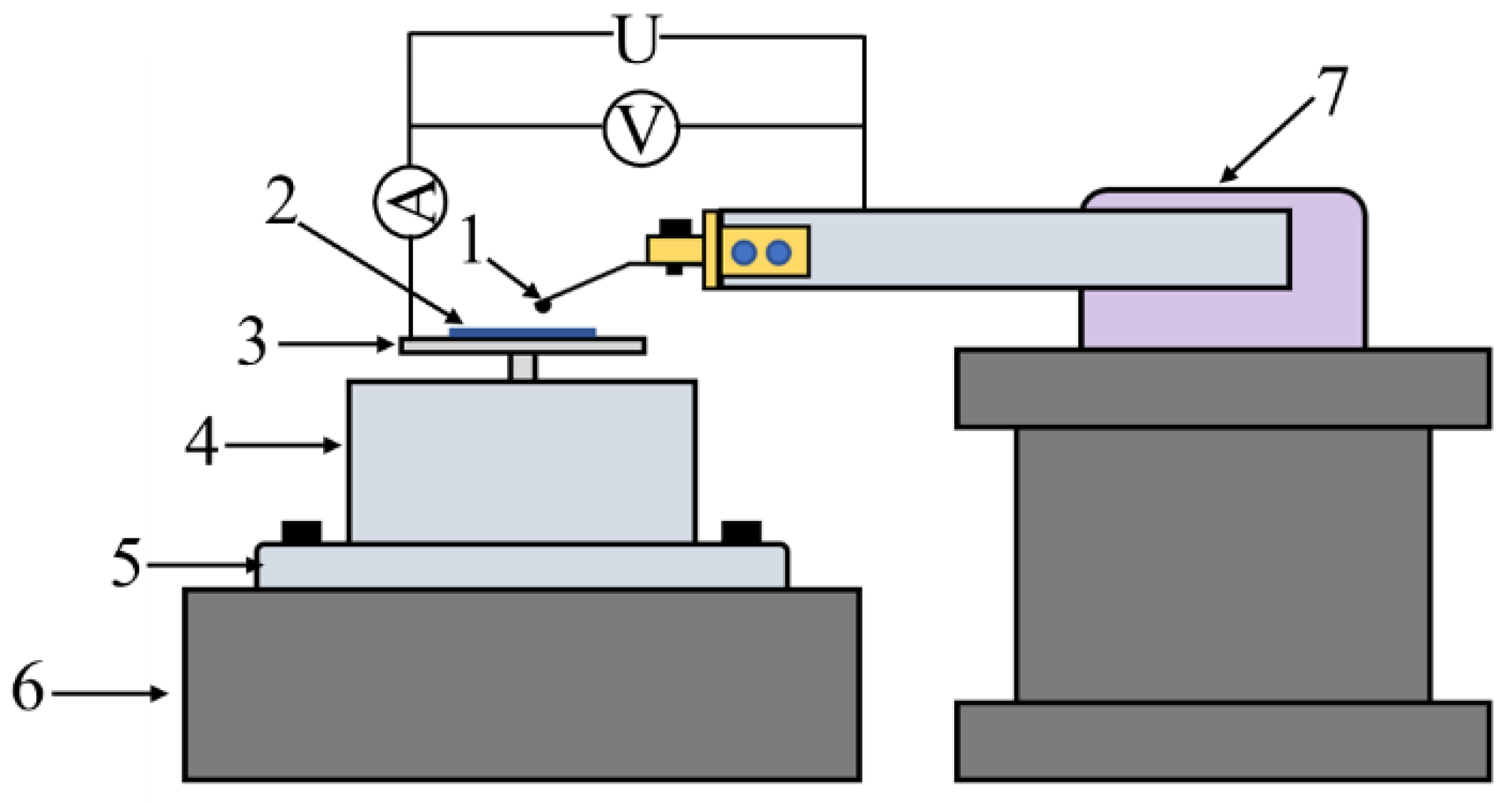




| Ar pressure | 0.5 Pa |
| Substrate bias voltage | 50 V |
| Target current | 80 A |
| Target voltage | 20 V |
| Deposition time | 20 min |
| Sputtering temperature | 200 °C |
| Standard pressure | 5 × 10−3 Pa |
| Working pressure | 0.3 Pa |
| Target current | 0.5 A |
| Target voltage | 400 V |
| Deposition time | 5 min |
| Target distance | 60 mm |
| Ar flow rate | 100 sccm |
| Substrate temperature | −140 °C, −95 °C, −55 °C, RT, 50 °C, 200 °C |
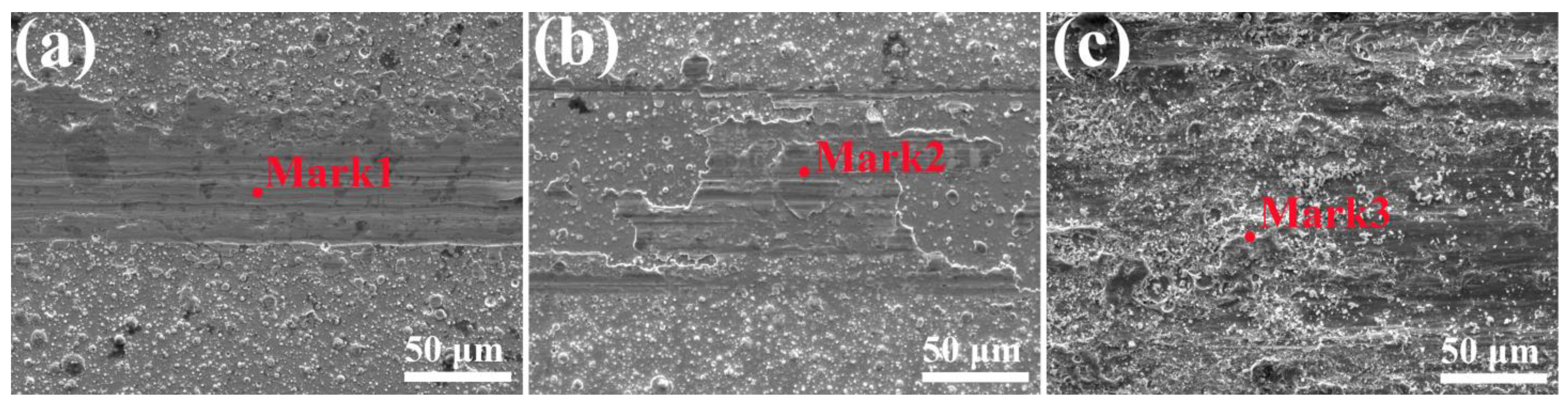
| Region | O | Ti | Fe | Cu |
|---|---|---|---|---|
| Mark 1 | 2.2% | 16.0% | 1.2% | 80.6% |
| Mark 2 | 21.8% | 71.1% | 0 | 7.1% |
| Mark 3 | 42.9% | 30.4% | 26.7% | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Le, K.; Wang, C.; Sun, J.; Xu, S.; Liu, W. Influence of Deposition Temperature on the Structure and Current-Carrying Friction Performance of Cu Films by DC Magnetron Sputtering Technology. Lubricants 2023, 11, 8. https://doi.org/10.3390/lubricants11010008
Zhang H, Le K, Wang C, Sun J, Xu S, Liu W. Influence of Deposition Temperature on the Structure and Current-Carrying Friction Performance of Cu Films by DC Magnetron Sputtering Technology. Lubricants. 2023; 11(1):8. https://doi.org/10.3390/lubricants11010008
Chicago/Turabian StyleZhang, Hao, Kai Le, Chen Wang, Jianbo Sun, Shusheng Xu, and Weimin Liu. 2023. "Influence of Deposition Temperature on the Structure and Current-Carrying Friction Performance of Cu Films by DC Magnetron Sputtering Technology" Lubricants 11, no. 1: 8. https://doi.org/10.3390/lubricants11010008
APA StyleZhang, H., Le, K., Wang, C., Sun, J., Xu, S., & Liu, W. (2023). Influence of Deposition Temperature on the Structure and Current-Carrying Friction Performance of Cu Films by DC Magnetron Sputtering Technology. Lubricants, 11(1), 8. https://doi.org/10.3390/lubricants11010008






