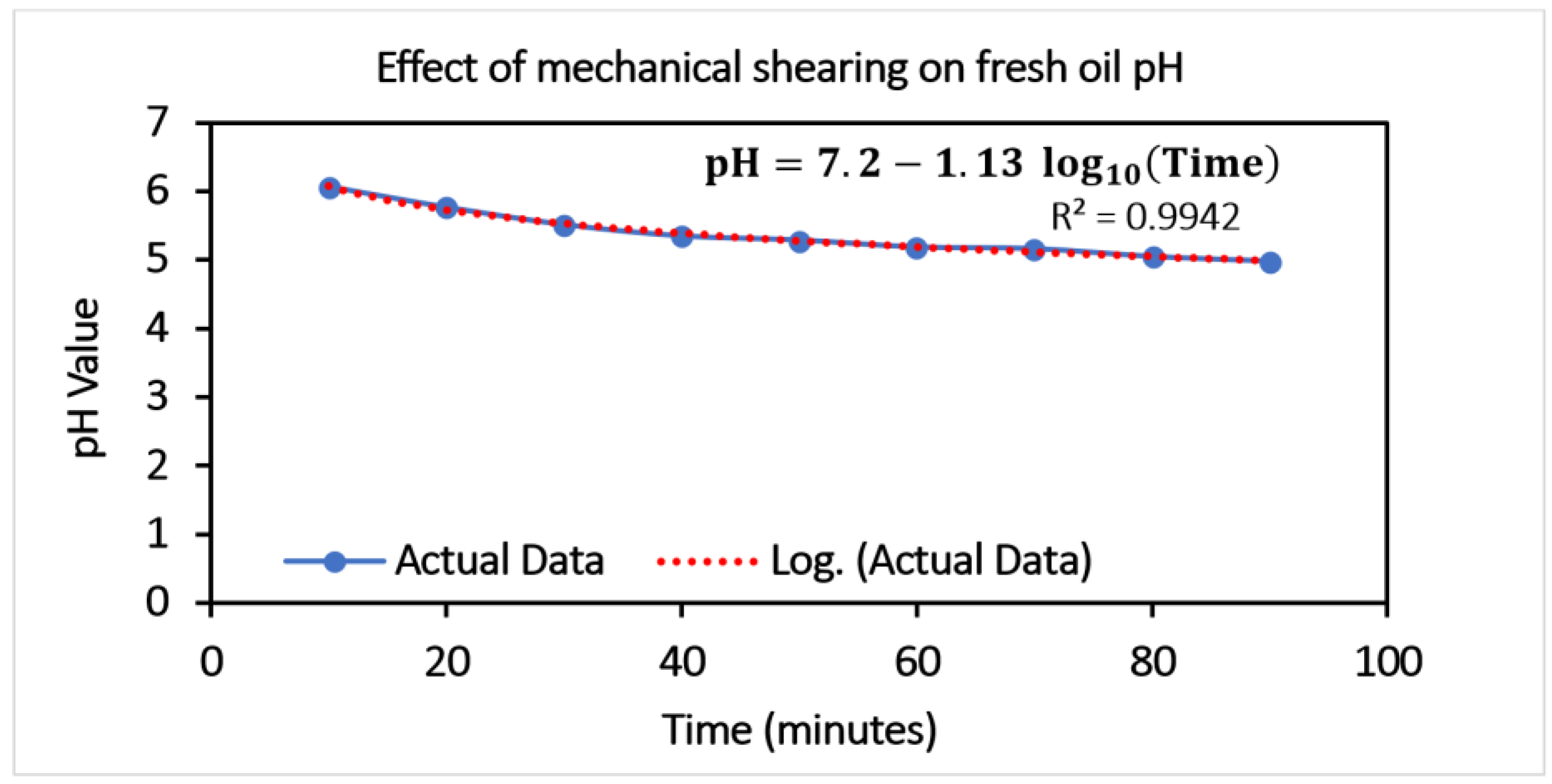
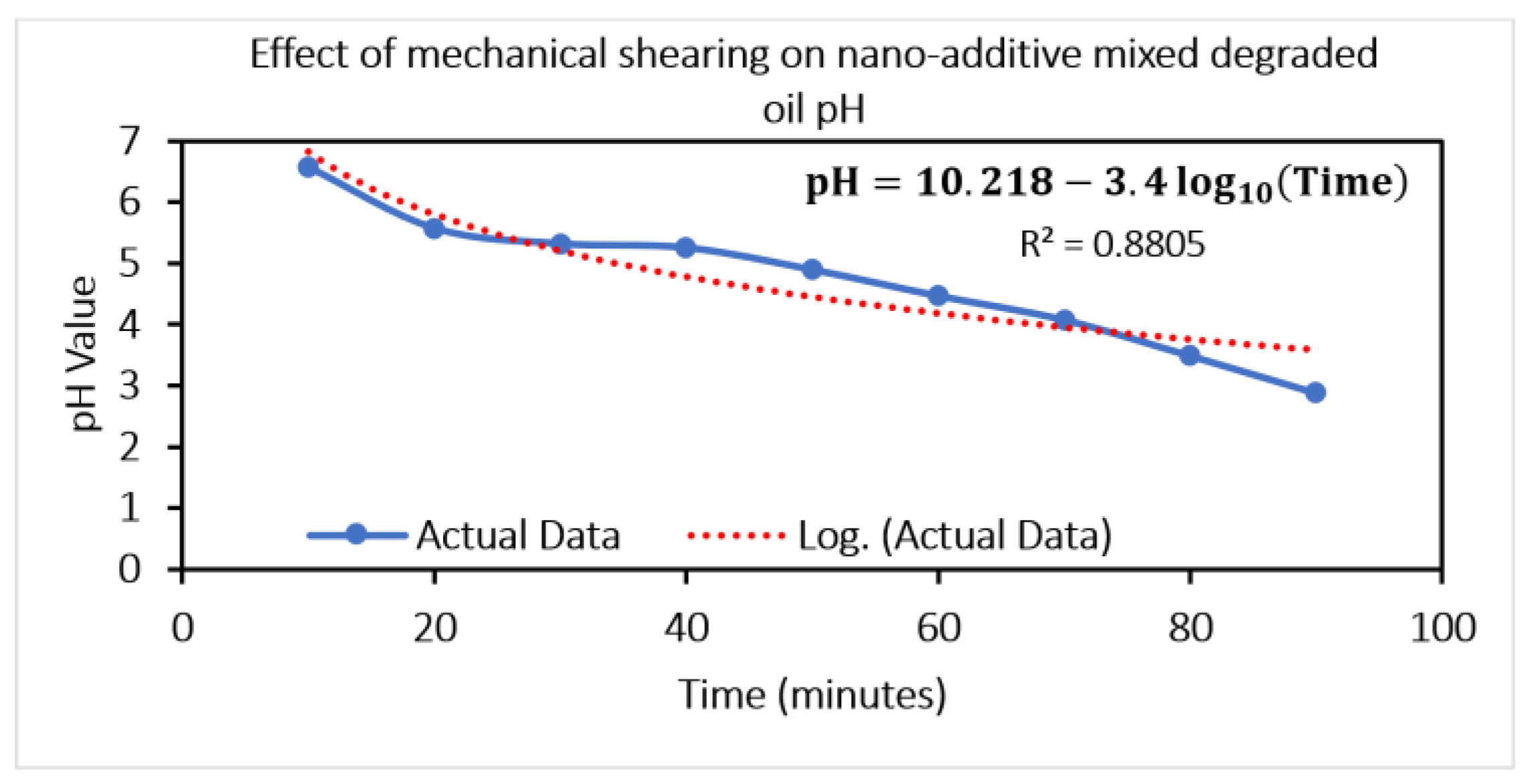
It is confirmed from Case 1 and Case 2 that oil gets oxidized over time, and the pH value of oil deteriorates over time, leading to oxide formation and water ingress, thus confirming the effect of lubricant aging. In Case 3, the main emphasis was given to selecting environment-friendly nano-additives for mixing in with the lubricant. Subsequently, the artificially aged oil was doped with nano-additives and tested in the lubricity tester to evaluate nano-additives’ performance and optimize the concentration. Finally, artificially aged oil doped with the optimum concentration (obtained from experiments on lubricity tester) of nano-additives was used in practical gear operation (on gear test rig) to see the effect on gear wear.
2.3.1. Selection of Nano-Additives
ATR-FTIR results shown in
Figure 2 indicate degradation (formation of the oxide products (1720–1732 cm
−1)) of the lubricant and moisture ingress (increase in the O–H stretching at 3410 cm
−1) in the used oil after experimentation on spur gears lubricated with fresh gear oil (API GL-4 EP90) and oil sample artificially aged with aqueous HCl. Considering this kind of lubricant degradation, some allotropes of carbon (i.e., Graphene, Graphite) can be used as lubricant additives (as green additives or as additives that are environment friendly) to reduce the wear of gear-pair and to enhance the service life of gear oil. Considering these aspects, three nano-additives—graphite, graphene, and GO@SiO
2—were selected. The effect of the selected nano-additive is given in
Figure A2 in the
Appendix A. The use of nano-additives decreases the moisture peak, which is confirmed by the ATR-FTIR as the peak at 3410 cm
−1 is reduced. The SEM morphology of the nano-additives used in this study is shown in
Figure 5. The graphene particles (
Figure 5a) are in the form of flacks, platelets, and graphite (
Figure 5b) particles are in the shape of flacks and spheres, and GO@SiO
2 (
Figure 5c) are in true nanoscale and spherical.
The approximate thickness of graphene, graphite, and GO@SiO2 nanoparticles 400 nm, 200 nm, and 95 nm, respectively, have been observed.
These particles were then mixed with a lubricant and homogenized using an ultrasonic homogenizer, which disintegrated the nanoparticles, and it is plausible that the particles may be even thinner/smaller [
73] in the lubricant suspension.
Raman spectroscopy (as shown in
Figure 5d) is a widely used technique for characterizing the structural properties of materials, including graphite, graphene, and graphene oxide (GO) compounds. This method is based on the inelastic scattering of light, known as Raman scattering, which can be used to identify specific vibrational modes of the material in question. In the case of graphene and GO, the most commonly observed Raman bands are the G band, which corresponds to in-plane vibrations of the carbon atoms in the sp
2-bonded network, and the D band, which is associated with out-of-plane vibrations of the sp
3-bonded carbon atoms that result from defects in the hexagonal structure of the graphene lattice. The presence of the 2D band in the Raman spectrum of graphene oxide is also a useful indicator of the presence of oxygen-containing functional groups (like silicon oxide), which are known to introduce structural defects into the graphene lattice. This band typically appears in the range of 2720–2740 cm
−1 and is attributed to phonon scattering from the oxygen-modified graphene structure. The intensity ratio of the G band to the D band, also known as the I(D)/I(G) ratio, is a useful indicator of the degree of structural disorder in the graphene lattice. A lower ratio indicates a higher degree of structural disorder, which is commonly associated with the presence of oxygen-containing functional groups and defects in the graphene lattice. In
Figure 5d, the presence of carbon strains in the form of graphite and graphene through the observation of the D band (1350 cm
−1) and G band (1580 cm
−1) in the spectra is confirmed. The presence of SiO
2 in the GO compound leads to a higher degree of defects in the GO molecules, as evident by the strong intensity of the D and G bands. The peaks at 520 cm
−1 and 976 cm
−1 ensure the presence of low-intensity SiO
2 in the spectra of GO@SiO
2. This attributes low concentration of SiO
2.
The nano-additives are mixed in the lubricant and first stirred manually for 15 min, then homogenized using the ultrasonic homogenizer for 30 min. The surfactant is not used along with the nano-additives, so it becomes crucial to check their sedimentation rate. The sedimentation effect was checked by observing for 24 h (the 24 h scale is chosen according to AGMA9005-F16 guidance).
Figure 6 confirms the dispersion stability of the lower percentage of the graphene.
2.3.2. Experimental Design for the Tribological Performance of Nano-Additives Doped in Lubricant
Lubricity Tester: The lubricity tester test setup is used to evaluate the tribological performance of the nano-additives. The schematic diagram of the lubricity tester, non-conformal block on disk, and photographs of the lubricity tester are shown in
Figure 7.
The test setup, shown in
Figure 7, engages a flat EN24 steel block of 12 mm × 12 mm × 12 mm on a 15 mm thick hardened steel disk of diameter 40 mm. This disk is driven by the induction motor and remains partially immersed in the lubricant tank. The lubricant inside the tank was maintained at 40 °C with the help of an inbuilt heater and thermal cut-off switch. The static load is applied on the platform on which the block is fixed. The test specification is given in
Table 2.
To simulate the torque (50 Nm) and speed (483 mm/s) conditions of the actual gearbox test, the equivalent load against the mean contact pressure and speed equivalent to the peripheral velocity of both gears at the pitch point are considered. These values are utilized to find the pressure variation along the gear tooth profile and peripheral velocities by solving the analytical relations for the peripheral velocities and contact pressure using MatLab2022b (institute license). The theoretical values of peripheral velocities and contact pressure are shown in
Figure 8.
The equivalent speed and force are calculated following the process described by Kleemola et al. [
74]. The equivalent speed can be calculated using the peripheral velocity at pitch point (483.52 mm/s), so the equivalent relative rotational speed of the block is determined as below:
where ‘
N’ is the speed in rpm, ‘
V’ is the peripheral velocity (mm/s), and ‘
r’ is the radius of the disk (counter surface) (mm). As the block is difficult to rotate, the disc was rotated at 231 rpm to create a relative rotational speed between the disc and the block.
The load is calculated using the mean contact pressure of “436.36 MPa” considering the contact of the cylinder on a flat plat and equation of the pressure equation given by Hirani [
75]:
where ‘
Pmean’ is mean pressure on the gear tooth, ‘
F’ is acted force in N, ‘
L’ is the length of the block (mm), ‘
b’ is semi-Hertzian contact width (mm), ‘
E*’ is equivalent Young’s modulus (in this steel case is 2.07 × 10
5 MPa), ‘
R*’ is an equivalent radius.
2.3.3. Orthogonal Array Design and Optimisation of the Concentration of Nano-Additives
Initially, three nano-additives—graphite, graphene, and “graphene oxide functionalized with silicon oxide (GO@SiO
2)”—have been selected to enhance the service life of the lubricants and reduce wear for the gear applications. A preliminary study (two levels of each nano-additives) was conducted using L8 orthogonal array to estimate the performance and interaction among nano-additives.
Table 3 shows the selected variables and their levels. In the literature, a considerable variation is found for the concentration of the nano-additive mixed in the lubricant (graphite 0.1 %
w/
w to 6%
w/
w [
44,
66]; graphene 0.1%
w/
w to 4%
w/
w [
45] and GO@SiO
2 0.03% to 0.5% [
46]), so based on the literature, the values of these additives stayed in between those values.
The L8 (2
7) orthogonal array used for the wear analysis is shown in
Table A1 (see
Appendix A), along with interactions. The second, third, and fourth columns are assigned to variables, such as nano-additives (graphite, graphene, and GO@SiO
2, respectively) mixed in the lubricant and remain to their interactions.
The parameters selected for the wear analysis are shown in
Table 2. The wear analysis is performed on the lubricity tester (see
Figure 7b), in which the block (test specimen) is loaded against the disk (counter surface). Before starting the experiment, the test specimen was installed in the holder and pressed under the vertical load. Before and after the experiment, the weight of each specimen was checked using a weighing balance with the least count of 0.00001 g.
The experimental variables, their levels, and the results of conducted experiments on the wear mass are presented in
Table 4. The
F-value evaluated the model terms with a 95% confidence level.
The analysis of variance (ANOVA) was used to test the suitability of the selected nano-additives. The results of ANOVA and the percentage contribution of the process variables to wear loss are listed in
Table 5. The ‘F’ values and percentage contribution indicate the importance of the variables to wear loss. The steps followed in ANOVA are [
76]:
Step 1: Total of all responses (see
Table 4 for responses)
where ‘
R’ is the number of responses corresponding to the trial experiment
Step 2: Correction factor:
where
n = total number of trials or experiments, ‘8′
Step 3: Total sum of squares (
ST)
Step 4: Factor sum of squares (
S)
j = A, B, C, A × B, A × C, B × C
= total number of experiments where a particular factor is present (see
Table 4)
Step 5: Total and factor degree of freedom (DOF)
DOF for factor = No. of Levels − 1
DOF for array = No. of trials − 1
DOF of error = DOF for array − sum (DOF of Factors and Interactions)
Step 6: mean square variance (V)
V = (Sum of square of factor/interaction)/(DOF of factor/interaction)
Step 7: Percentage contribution:
% Contribution = (Sum of square of factor/ interaction)/ST
Step 8: F ratios
F = V of factor/V error
Based on the ANOVA analysis, it can be seen from
Table 5 that graphene is the most minor contributor in reducing wear, and GO@SiO
2 is the largest contributor. However, before concluding that GO@SiO
2 is the best nano-additive, it was decided to experiment with the graphite and GO@SiO
2 using L16 (four levels of the selected nano-additives) orthogonal array (see
Appendix A,
Table A2 for the factors and their interactions).
Table 6 presents the experimental plan for the L16 array for four levels of the selected nano-additives.
Table 6 shows that the minimum wear mass loss (50 µg) takes place in experiment number 7 for a combination of nano-additive “graphite:0.125%
w/
w and GO@SiO
2: 0.15%
w/
w”. Furthermore, this optimum concentration was selected for the experiments on the gearbox test rig to prove the enhancement of gear lubricant oil.
The aged oil doped with the optimized concentration of the nano-additives is used for the test in the gear test rig. The test specifications are given in
Table 1. The samples collected every 10 min are processed for the pH calculation, and a decreasing trend (
Figure 9) is observed. The pH value shows a variation of 65.29 %.
Further, the comparison of the three-test ATR-FTIR spectra (
Figure 10) showed that with gear operation under lubricated conditions, the presence of moisture increases (3410 cm
−1) in Case 1 and Case 2. The moisture is not present in Case 3 as the nano-additives consume the moisture accumulated during gear operation. The oxidation products (723 cm
−1, 1720–1732 cm
−1) remain present in all tests, while glycol (879–880 cm
−1) and aromatic compound formation (1220 cm
−1) are present in Case 1 and Case 2 only. It confirms that nano-additives work effectively and slow down the aging of the lubricant.
The detailed ATR-FTIR analysis of the nano-additive-mixed lubricant is performed to observe the overall effect of the nano-additives. The gear oils are mainly composed of base oil (mineral or synthetic) and additives (anti-wear, extreme pressure, etc.). The different peaks and the baseline of the ATR-FTIR provide information about the compounds in the gear oil.
Table A3 (see
Appendix A) details the corresponding ATR-FTIR peaks and possible compounds.
The ATR-FTIR curves of the gear oil under different conditions, such as fresh oil, oil mixed with aqueous HCl, and chemically degraded oil mixed with nano-additives, are shown in
Figure 11. The spectra results indicate that the gear oil is mineral-based and has a long-chain alkane (723 cm
−1). The peaks corresponding to the OH (3410 cm
−1), C=O (900–1800 cm
−1) stretches appear, indicating the presence of secondary products like ketones, aldehydes, carboxylic acid, esters, and alcohols. The intensity at 1380 cm
−1 indicates the existence of the –CH
3 group in gear oil. The bend corresponding to 2850 cm
−1 and 2920 cm
−1 is assigned to the stretching of the –CH
2 group presence in oil. The peak corresponding to 1300 cm
−1 is attributed to the H–O–H bending; it indicates the absorption of the hydroxyl group into a carboxylic acid (confirming the acidification of the oil). The baseline shift of about 2000 cm
−1 confirms the presence of the soot in the gear oil. Adding aqueous HCl leads to the soot formation in the gear oil and acidification of the gear oil as an increase in the peak of about 1720–1732 cm
−1. The addition of the nano-additives initially decreases the soot component as the baseline shift (2000 cm
−1) reaches a value close to that of the fresh oil and decreases the oxide products by about 1720–1732 cm
−1. This initial reaction of the nano-additive in chemically degraded oil leads to a lower acidic level and improves the pH value of the gear oil. The increase in the operation time leads to the consumption of the nano-additives, which leads to the deterioration of the baseline and increases the soot level and carbon content (2000–2200 cm
−1) in the lubricant. An increase in the soot level and oxide formation with operation time further decreases the pH level and shows an increase in the acidic level. One possible reason for such behavior of nano-additives may be due to their instability as no surfactant is used, and nano-additives may start sedimenting due to agglomeration, etc.