A Computational Study on the Role of Lubricants under Boundary Lubrication
Abstract
:1. Introduction
2. Materials and Methods
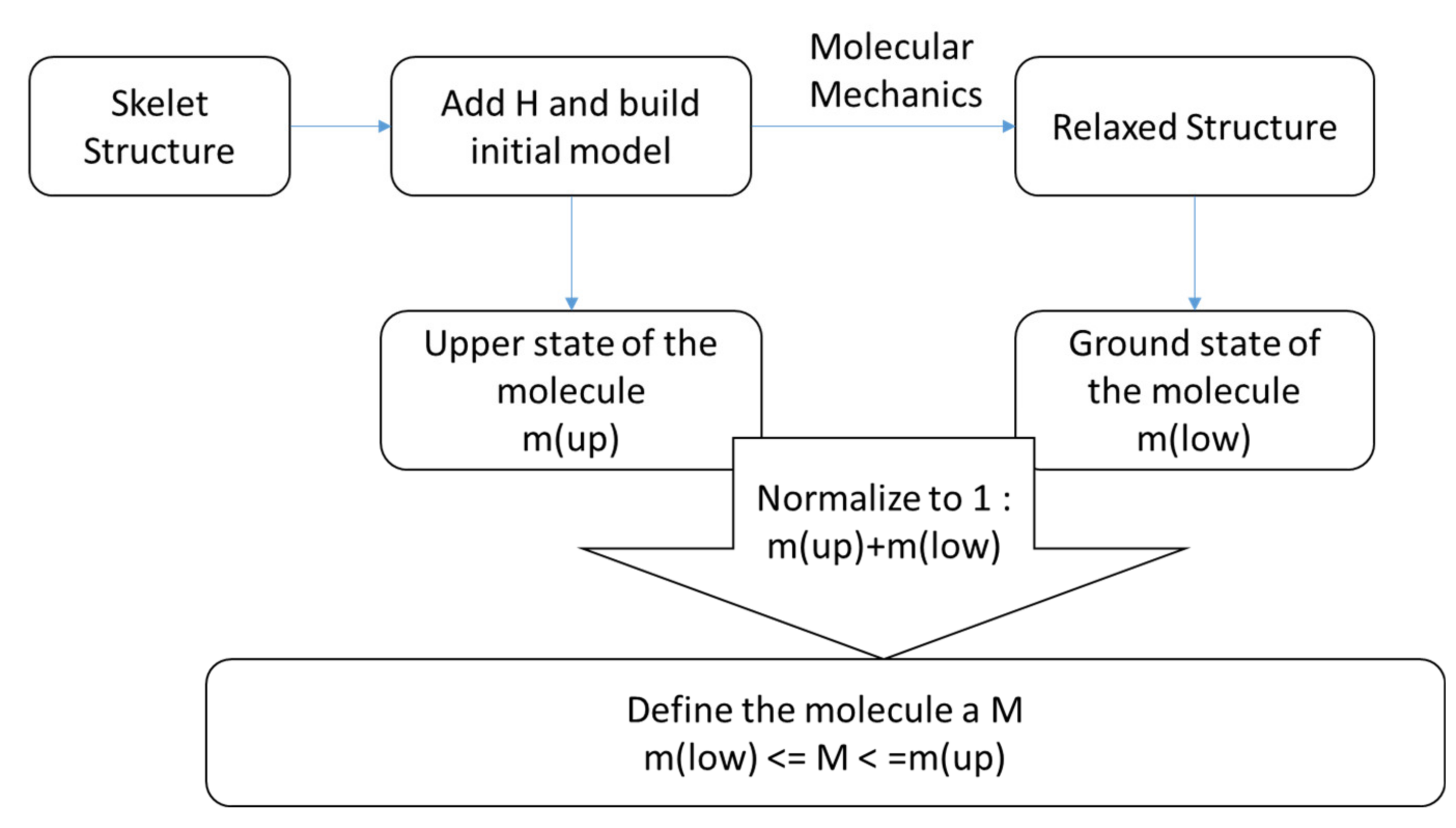
2.1. Theoretical Approach
- Solvate BxAy, with x >> y, meaning that plenty of base oil molecules cover the additive;
- Clustering Bx [Ay]g, with x ~ y, meaning that B dissolves additives clustering together. In extreme cases, micelles are formed with a shell of base oil encapsulating self-aggregates of the additives, expressed as degenerated by a degree of ;
- Clusters of the base oil [B] temporarily created random with a degree of ;
- Clusters of the additive [A] temporarily created random with a degree of ;
- Adsorption at surface sites S:
- 5.1.
- Solvates from (1) interact: {[BxAy]}-S either single with a degeneration ;
- 5.2.
- Clusters () from (1) {[BxAy]}-S;
- 5.3.
- Base oil directly [B]-S with ;
- 5.4.
- Additives directly [A]-S with ;
- 5.5.
- Base oil oil clusters [B]-S with ;
- 5.6.
- Additives clusters [A]-S with .
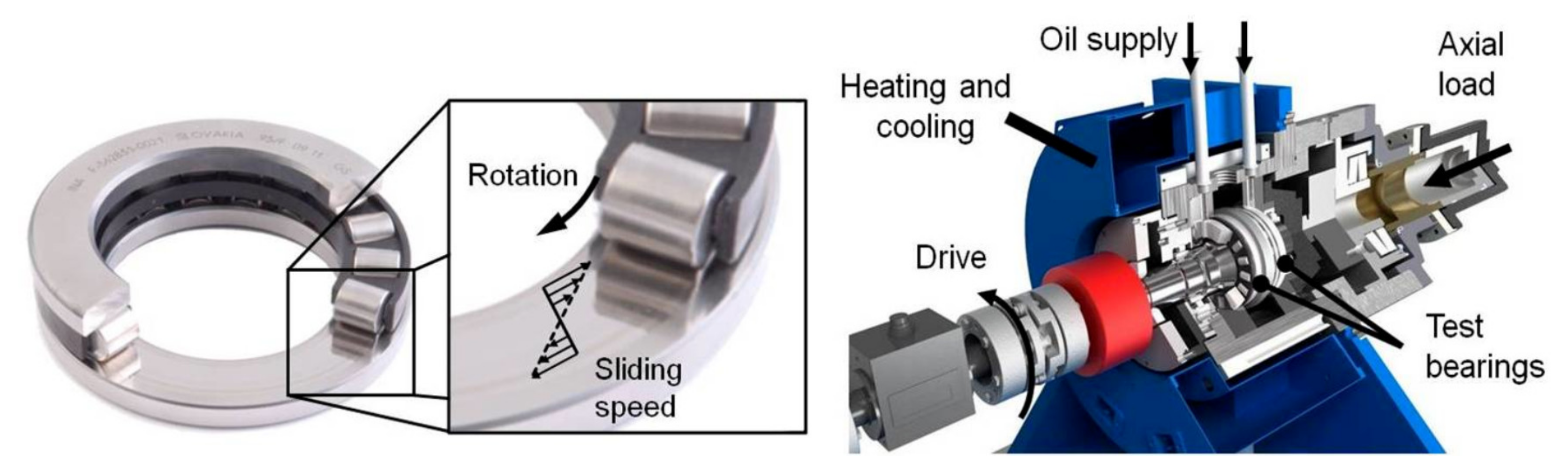
2.2. Experimental Approach—FE8 Test Rig
- 81212 Cylindrical roller bearing (CRB)
- Axial Load: 80 kN
- Temperature (held constant): 80 °C
- 7.5 rpm
- Contact pressure: 1890 MPa, 15 Rollers
- Brass
- Housing washer
- Outer diameter: 95 mm
- Bore diameter: 62 mm
- Outer diameter: 95 mm
- Bore diameter
- Mean diameter of the bearing: 78 mm
- SAE: 52100
- Martensitic heat treatment
- Hardness: 800 ± 20 HV
- Residual stress: +10 MPa (tension)
- Retained austenite: 10–12%
- Roughness Rq (washer) 0.02–0.04
2.3. The Lubricants
3. Results
FE8 Test Runs
4. Discussion
- (A)
- An oil chemistry could be separated into predictor sets, comprising the (induced) dipolar activities apparent at the surface;
- (B)
- The (induced) dipolar activity as one predictor of the lubricant corresponds to apparent wear in a bearing test rig;
- (C)
- The “inner” structure, defined here as the (induced) dipolar activity is a measure of the expected wear in a bearing test rig;
- (D)
- This strong relation allows the prediction of the expected wear in an FE8 test device as a parametrized bearing application with respect to a given lubricant.
5. Conclusions
- (A)
- A lubricant can approach the surface by transportation across its viscosity, temperature and shearing.
- (B)
- The surface interacts with the most attractive components, defined by the dipolar and induced dipolar interactions, normalized to a “relative” dipole moment as a dimensionless parameter.
- (C)
- The presence of these species is found to be more essential in the initiation of the wear processes in mixed friction and boundary lubrication rather than their assumed chemical reactions.
- (D)
- The nature of the specie, e.g., single or clustered, is thought to be an irreversible predictor for wear.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dowson, D.; Higginson, G.R. A Numerical Solution to the Elastohydrodynamic Problem. J. Eng. Sci. 1959, 1, 6–16. [Google Scholar]
- Liran, M.A.; Luo, J. Thin film lubrication in the past 20 years. Friction 2016, 4, 280–302. [Google Scholar]
- Mathas, D.; Holweger, W.; Wolf, M.; Bohnert, C.; Bakolas, V.; Procelewska, J.; Wang, L.; Bair, S.; Skylaris, C.-K. Evaluation of Methods for Viscosity Simulations of Lubricants at Different Temperatures and Pressures: A Case Study on PAO-2. Tribol. Trans. 2021, 64, 1138–1148. [Google Scholar] [CrossRef]
- Fan, F.R.; Xie, S.; Wang, G.W.; Tian, Z.Q. Tribocatalysis: Challenges and perspectives. Sci. China Chem. 2021, 64, 1609–1613. [Google Scholar] [CrossRef]
- Somorjai, G.A. Introduction to Surface Chemistry and Catalysis; John Wiley and Sons, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Catlow, C.R.A.; Bell, R.G.; Gale, J.D.; Lewis, D.W.; Jayle, D.L.; Sinclair, P.E.; Derouna, E.G.; Haber, J.; Lemos, F.; Ribeiro, F.R.; et al. (Eds.) An Introduction to Molecular Hetergenous Catalysis, in Catalytic Activation and Functionalization of Light Alkanes; Kluwer Acad. Publ.: Dordrecht, The Netherlands, 1998; p. 189. [Google Scholar]
- Weiss, W.; Schlögl, R. An Integrated Surface Science Approach Towards Metal Oxide Catalysis. Topics Catal. 2000, 13, 75. [Google Scholar] [CrossRef]
- Carley, A.F.; Davies, P.R.; Hutchings, G.J.; Spencer, M.S. (Eds.) Surface Chemistry and Catalysis; Kluwer Acad. Publ.: New York, NY, USA, 2002; p. 285. [Google Scholar]
- Spencer, N. Tailoring Surfaces; IISc Centenary Lecture Series; Mr. Spencer: London, UK, 2011. [Google Scholar]
- Holweger, W. Novel Predictors for Friction and Wear in Drivetrain Applications. In Friction, Lubrication and Wear; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Schwedt, A.; Rumpf, V.; Mayer, J.; Bohnert, C.; Wranik, J.; Spille, J.; Wang, L.; Holweger, W. A Study on Early Stages of White Etching Crack Formation under Full Lubrication Conditions. Lubricants 2022, 10, 24. [Google Scholar] [CrossRef]
- Wranik, J.; Holweger, W.; Lutz, T.; Albrecht, P.; Reichel, B.; Wang, L. A Study on Decisive Early Stages in White Etching Crack Formation Induced by Lubrication. Lubricants 2022, 10, 96. [Google Scholar] [CrossRef]
- Gachot, C.; Hsu, C.; Suárez, S.; Grützmacher, P.; Rosenkranz, A.; Stratmann, A.; Jacobs, G. Microstructural and Chemical Characterization of the Tribolayer Formation in Highly Loaded Cylindrical Roller Thrust Bearings. Lubricants 2016, 4, 19. [Google Scholar] [CrossRef]
- Azzam, B.; Harzendorf, F.; Schelenz, R.; Holweger, W.; Jacobs, G. Pattern Discovery in White Etching Crack Experimental Data Using Machine Learning Techniques. Appl. Sci. 2019, 9, 5502. [Google Scholar] [CrossRef]
- Brandt, J.M.; Benedek, M.; Guerin, J.S.; Fliege, J. Reliability-as-a-Service for bearings risk assessment investigated with Advanced Mathematical Models. Internet Things 2020, 11, 100178. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ‘t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Würdemann, R.; Walter, M. Charge transfer excitations with range separated functionals using improved virtual orbitals. J. Chem. Theory Comput. 2018, 14, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Thiyam, P.; Kurumbail, A.; Burger, F.A.; Walter, M.; Persson, C.; Brevik, I.; Parson, D.F.; Boström, M.; Buhmann, S.Y. Effective polarizability models. J. Phys. Chem. A 2017, 121/51, 9742–9751. [Google Scholar] [CrossRef] [PubMed]
- Steward, J. Optimization of parameters for semiempirical methods. I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Steward, J. Optimization of parameters for semiempirical methods. II. Applications. J. Comput. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Steward, J. Optimization of parameters for semiempirical methods. III Extension of PM3 to Be, Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb, and Bi. J. Comput. Chem. 1991, 12, 320–341. [Google Scholar] [CrossRef]
- Steward, J. Optimization of parameters for semiempirical methods. IV: Extension of MNDO, AM1, and PM3 to more main group elements. J. Mol. Model. 2004, 10, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Steward, J. PM3. In Encyclopedia of Computational Chemistry; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Schaeffler Technologies AG & Co. KG. Manual Test rig FE8 acc. to DIN 51819; Schweinfurt: Herzogenaurach, Germany, 2017. [Google Scholar]

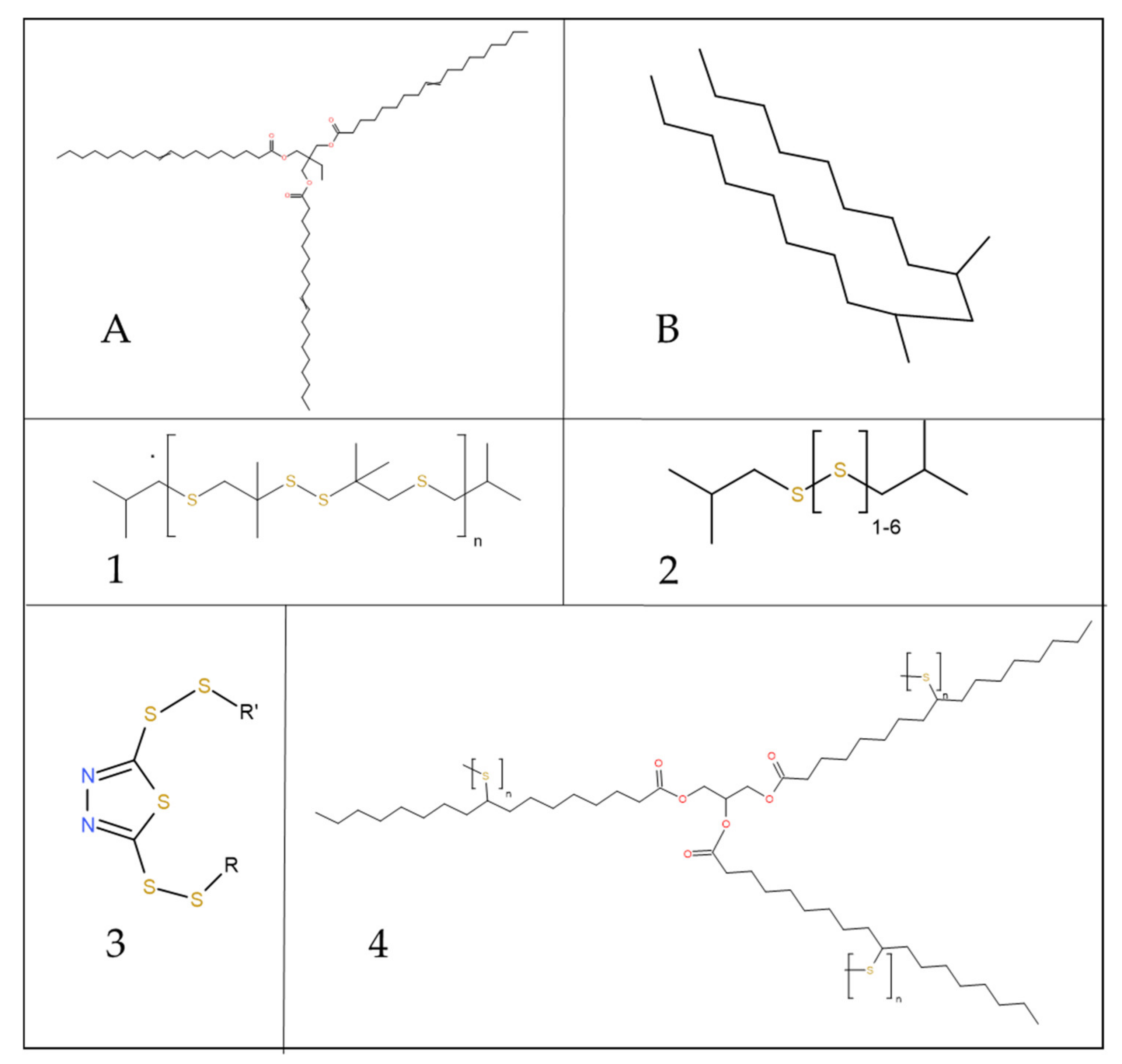
| Lubricant Tag | PAO [%]w | TMP [%]w | Additive 1 [%]w | Additive 2 [%]w | Additive 3 [%]w | Additive 4 [%]w |
|---|---|---|---|---|---|---|
| L1 | 97 | 3 | ||||
| L2 | 87.3 | 9.7 | 3 | |||
| L3 | 97 | 3 | ||||
| L4 | 87.3 | 9.7 | 3 | |||
| L5 | 97 | 3 | ||||
| L6 | 87.3 | 9.7 | 3 | |||
| L7 | 97 | 3 |
| Oil Code | Wear (mg) Housing Washer | |
|---|---|---|
| Training Set | L1 | 111.5 |
| L2 | 212.5 | |
| L3 | 170.75 | |
| L4 | 5.75 | |
| L5 | 174.5 | |
| L6 | 153.75 | |
| L7 | 0 | |
| Commercial Oils | L8 | 0 |
| L9 | 0 | |
| L10 | 3.1 | |
| L11 | 3.1 | |
| L12 | 3.1 | |
| L13 | 3.1 | |
| L14 | 2 | |
| L15 | 2 | |
| L16 | 5 | |
| L18 | 5 | |
| L19 | 31.75 |
| Predictor ID | Description |
|---|---|
| P1 | Dipole components of the base oil at the surface |
| P2 | Polarisability (inducible dipole) of the base oil at the surface |
| P3 | Dipole components Base oil and additives at the surface |
| P4 | Polarisability (inducible dipole) of components at the surface base oil and additives |
| P5 | Dipole additives at the surface |
| P6 | Polarisability (inducible dipole) of additives at the surface |
| P7 | Cluster dipole of the base oil at the surface |
| P8 | Cluster polarisability (inducible dipole) of the base oil at the surface |
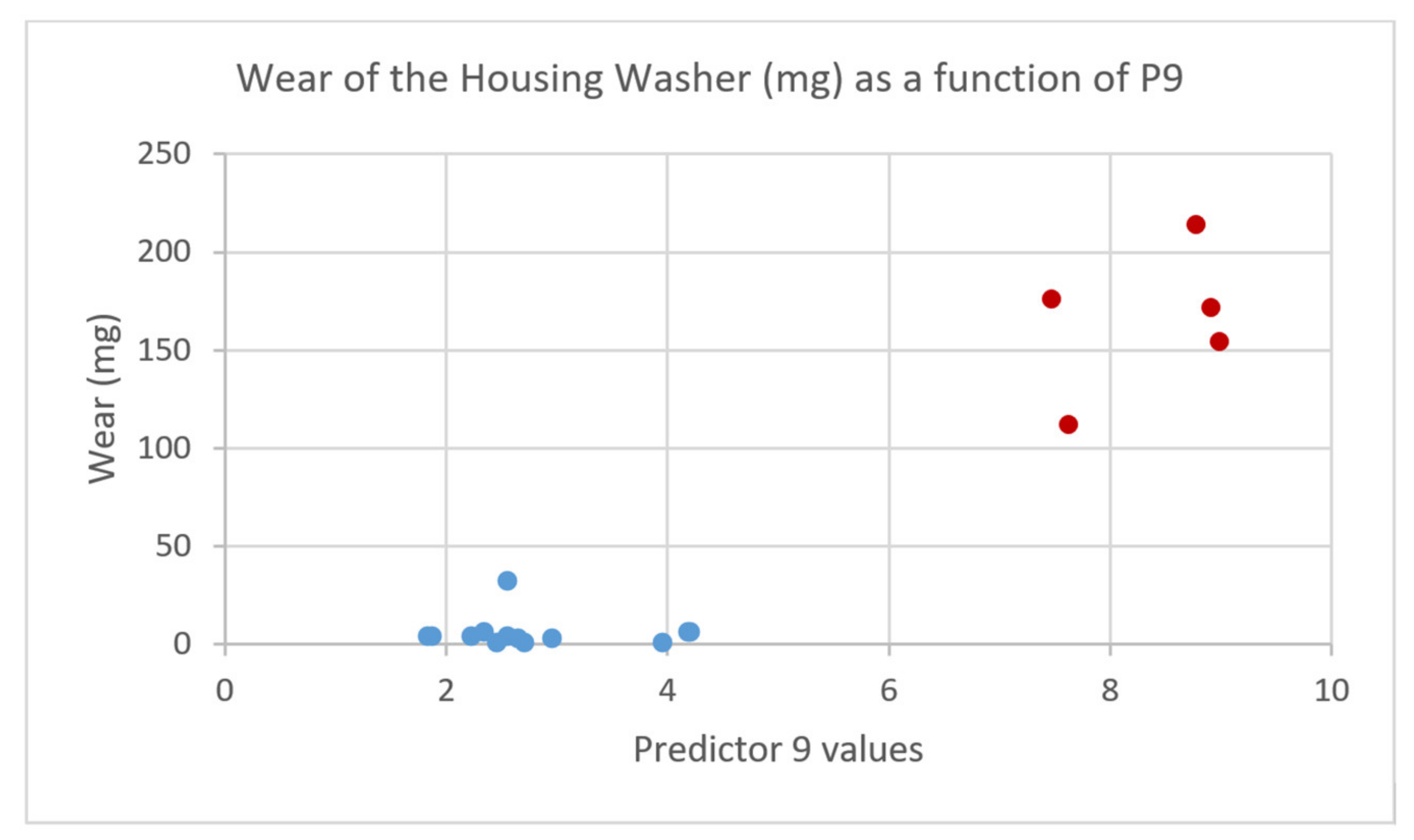
| P9 | Cluster dipole components of base oil and additives at the surface |
| P10 | Cluster polarisability (inducible dipole) of from base oil and additives at the surface |
| P11 | Cluster dipole additives at the surface |
| P12 | Cluster polarisability (inducible dipole) of additives at the surface |
| Oil | Wear (mg) | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 111.5 | 6.16 | 6.08 | 0 | 0 | 0 | 0 | 0 | 0 | 7.65 | 7.61 | 8.81 | 8.86 |
| L2 | 212.5 | 6.29 | 6.17 | 0 | 0 | 0 | 0 | 0 | 0 | 8.8 | 8.74 | 3.97 | 4.01 |
| L3 | 170.75 | 6.64 | 6.5 | 0 | 0 | 0 | 0 | 0 | 0 | 8.92 | 8.85 | 3.81 | 3.85 |
| L4 | 5.75 | 0 | 4.86 | 0 | 0 | 0 | 0 | 7.8 | 0 | 4.22 | 7.29 | 7.92 | 3.66 |
| L5 | 174.5 | 0 | 4.67 | 0 | 0 | 0 | 0 | 3.18 | 0 | 7.5 | 8.52 | 8.73 | 3.28 |
| L6 | 153.75 | 6.98 | 7.1 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 9.05 | 3.63 | 3.61 |
| L7 | 0 | 0 | 6.39 | 0 | 0 | 0 | 0 | 3.65 | 0 | 2.73 | 8.62 | 8.15 | 3.45 |
| L8 | 0 | 0 | 5.93 | 0 | 0 | 0 | 0 | 3.39 | 0 | 2.48 | 7.67 | 8.83 | 3.63 |
| L9 | 0 | 0 | 5.15 | 0 | 0 | 0 | 0 | 7.41 | 0 | 3.97 | 7.09 | 8.58 | 3.65 |
| L10 | 3.1 | 0 | 6.14 | 0 | 0 | 0 | 0 | 2.07 | 0 | 2.24 | 11.62 | 4.88 | 6.06 |
| L11 | 3.1 | 0 | 7.85 | 0 | 0 | 0 | 0 | 2.4 | 0 | 2.58 | 4.33 | 5.02 | 5.87 |
| L12 | 3.1 | 0 | 6.18 | 0 | 0 | 0 | 0 | 2.42 | 0 | 1.85 | 11.96 | 5.9 | 3.34 |
| L13 | 3.1 | 0 | 7.85 | 0 | 0 | 0 | 0 | 2.47 | 0 | 1.89 | 4.34 | 5.28 | 6.13 |
| L14 | 2 | 0 | 6.61 | 0 | 0 | 0 | 0 | 3.52 | 0 | 2.67 | 8.79 | 3.34 | 3.92 |
| L15 | 2 | 0 | 5.95 | 0 | 0 | 0 | 0 | 3.98 | 0 | 2.98 | 8.93 | 7.63 | 9.67 |
| L16 | 5 | 0 | 7.07 | 0 | 0 | 0 | 0 | 9.63 | 0 | 4.21 | 7.15 | 4.98 | 3.02 |
| L18 | 5 | 0 | 7.14 | 0 | 0 | 0 | 0 | 3.79 | 0 | 2.36 | 6.91 | 3.66 | 4.34 |
| L19 | 31.75 | 0 | 6.42 | 0 | 0 | 0 | 0 | 3.4 | 0 | 2.58 | 8.53 | 8.64 | 3.39 |
| Correlation | 0.82 | −0.17 | −0.60 | 0.94 | 0.17 | −0.18 | −0.10 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holweger, W.; Bobbio, L.; Mo, Z.; Fliege, J.; Goerlach, B.; Simon, B. A Computational Study on the Role of Lubricants under Boundary Lubrication. Lubricants 2023, 11, 80. https://doi.org/10.3390/lubricants11020080
Holweger W, Bobbio L, Mo Z, Fliege J, Goerlach B, Simon B. A Computational Study on the Role of Lubricants under Boundary Lubrication. Lubricants. 2023; 11(2):80. https://doi.org/10.3390/lubricants11020080
Chicago/Turabian StyleHolweger, Walter, Luigi Bobbio, Zhuoqiong Mo, Jörg Fliege, Bernd Goerlach, and Barbara Simon. 2023. "A Computational Study on the Role of Lubricants under Boundary Lubrication" Lubricants 11, no. 2: 80. https://doi.org/10.3390/lubricants11020080
APA StyleHolweger, W., Bobbio, L., Mo, Z., Fliege, J., Goerlach, B., & Simon, B. (2023). A Computational Study on the Role of Lubricants under Boundary Lubrication. Lubricants, 11(2), 80. https://doi.org/10.3390/lubricants11020080






