Abstract
In this work, a chemical modification method was used to prepare silicone grease with high thermal conductivity. We report two preparation methods for thermal conductive fillers, which are hydroxylated boron nitride-grafted carboxylic silicone oil (h-BN-OH@CS) and amino boron nitride-grafted carboxylic silicone oil (h-BN-NH2@CS). When h-BN-OH@CS and h-BN-NH2@CS were filled with 30 wt% in the base grease, the thermal conductivity was 1.324 W m−1 K−1 and 0.982 W m−1 K−1, which is 6.04 and 4.48 times that of the base grease (0.219 W m−1 K−1), respectively. The interfacial thermal resistance is reduced from 11.699 °C W−1 to 1.889 °C W−1 and 2.514 °C W−1, respectively. Inorganic filler h-BN and organic filler carboxylic silicone oil were chemically grafted to improve the compatibility between h-BN and the base grease. The covalent bond between functionalized h-BN and carboxylic silicone oil is stronger than the van der Waals force, which can reduce the viscosity of the silicone grease.
1. Introduction
Filling thermal interface materials (TIMs) between devices and heat sinks can reduce the interface thermal resistance (IR) [1]. TIMs include silicone grease, silicone pad, thermally conductive phase change materials, and so on [2]. Lubricating grease has been widely used as a kind of TIM due to its easy processing and lightweight and excellent flexibility [3]. It is mainly composed of the base silicone grease and the thermally conductive (TC) filler [4]. The base silicone grease itself has a low thermal and high heat resistance which limits the application [5]. Therefore, the introduction of TC nano-fillers helps to reduce the IR [6,7,8]. Commonly used fillers for silicone greases include metal nanoparticles [9] and carbon black materials [10], which can also be electrically conductive. However, the applications in integrated circuits and high-power devices are hindered by the introduction of thermally and electrically conductive fillers. Therefore, it is necessary to reduce the interface resistance and form thermally conductive pathways in the base silicone grease [11].
Thermally conductive insulating fillers are mainly made of ceramic materials such as alumina oxide, silicon oxide, and so on [12]. The h-BN has high thermal conductivity, oxidation resistance, and electrical insulation [13]. Researchers have found that h-BN can effectively increase the TC, and the thermal conductivity mainly depends on the filler content, i.e., h-BN [14,15]. However, the high content of thermally conductive fillers tends to agglomerate and reduce the strength of composite materials and correspondingly increase the cost of manufacturing processes [16]. Therefore, in order to maximize the functionality of the thermally conductive filler, different methods of modification of h-BN need to be chosen [17]. It was found that h-BN modified with physical and chemical methods provides better thermal-conductivity enhancement than unmodified and agglomerated h-BN [18]. The h-BN plates were embedded in polycarbonate materials by Sun using a hot-press alignment method, and the ordered arrangement of the BN plates provided a pathway for phonon transport. When the h-BN content reaches 18.5 vol%, the composite was able to achieve a maximum TC of 3.1 W m−1 K−1 along the orientation direction [19]. Luo prepared vertically oriented, densely packed h-BN/epoxy resin composites by a vacuum filtration and slicing method. The thermal conductivity of the composites was 9.0 W m−1 K−1 when 44.0 vol% of h-BN was added [20]. Ahn modified the BN/polyvinyl butyral (PVB) composites with ellagic acid hydrate to give a higher thermal conductivity than PVB with pristine BN [21]. The BN surface is functionalized with octadecylamine and hyperbranched aromatic polyamides by the Yu group, and the TC of the epoxy resin was significantly improved [22]. Qian improved the thermal conductivity of BN nanosheet hybrids by copper phthalocyanine-grafted BN, and the TC was up to 0.63 W m−1 K−1 when the addition of the filler was 50 wt% [23]. Han polymerized PS-COOH on the surface of BN-OH. The TC of the composite material containing 12 wt% BN-OH was 1.131 W m−1 K−1, better than pure PS (0.186 W m−1 K−1) and BN/PS (0.312 W m−1 K−1) [24]. When 2–3 wt% of the hydroxyl- and amino-functionalized BN nanotubes were added to epoxy resins, Young’s modulus, strain at break, and thermal conductivity were improved [25]. Currently, h-BN is widely used in polymers, but its application in greases needs to be further developed.
In this work, hydroxyl- and amino-functionalized h-BN were bonded with carboxyl silicone oil (CS) by a chemical modification method. The thermal stability of silicone grease with modified h-BN was analyzed using thermogravimetric curves. The effects of modified h-BN-OH and h-BN-NH2 on the viscosity and thermal conductivity of silicone greases were compared. The results showed that the TC was improved by the addition of the functionalized h-BN. The mechanism was investigated, and it is suggested that the formed ester and amide bonds can bridge adjacent thermally conductive materials to form thermally conductive pathways.
2. Experimental Section
2.1. Materials
SiO2 (ca. 15 ± 5 nm) was purchased from Rohn Reagent Co., Ltd. (Shanghai, China). Dimethyl silicone oil, CS (molecular weight ca. 2000) was purchased from Anhui Aiyota Silicone Oil Co., Ltd. (Bengbu, China). h-BN (purity > 99.9%) with a particle size of 1–2 μm was purchased from Aladdin Co., Ltd. (Shanghai, China). The chemical reagents were purchased from commercial sources and, prior to usage, were not purified.
2.2. The Preparation of Functionalized BN
2.2.1. The Preparation of h-BN-OH and h-BN-NH2
We dispersed h-BN at 5 mg mL−1 in a NaOH solution, then heated the h-BN dispersion to 120 °C and stirred it magnetically at a speed of 350 r·min−1 for 48 h. After cooling down, the product was filtered, and the sample was washed with deionized water (DIW) until neutral. It was then vacuum dried at 60 °C for 18 h to obtain h-BN-OH.
The h-BN-NH2 was prepared by a one-step method. The h-BN powder (1.0 g) and urea (4.0 g) were added into the isopropanol solution (25 mL), and the solution was premixed and ball milled for 48 h at a rate of 300 r·min−1. After the ball mill operation, the mixture was removed into a beaker and left to stand, and the upper suspension was injected into an extraction flask. The washed sample was vacuum dried at 60 °C for 24 h to obtain h-BN-NH2.
2.2.2. The Preparation of h-BN-OH@CS
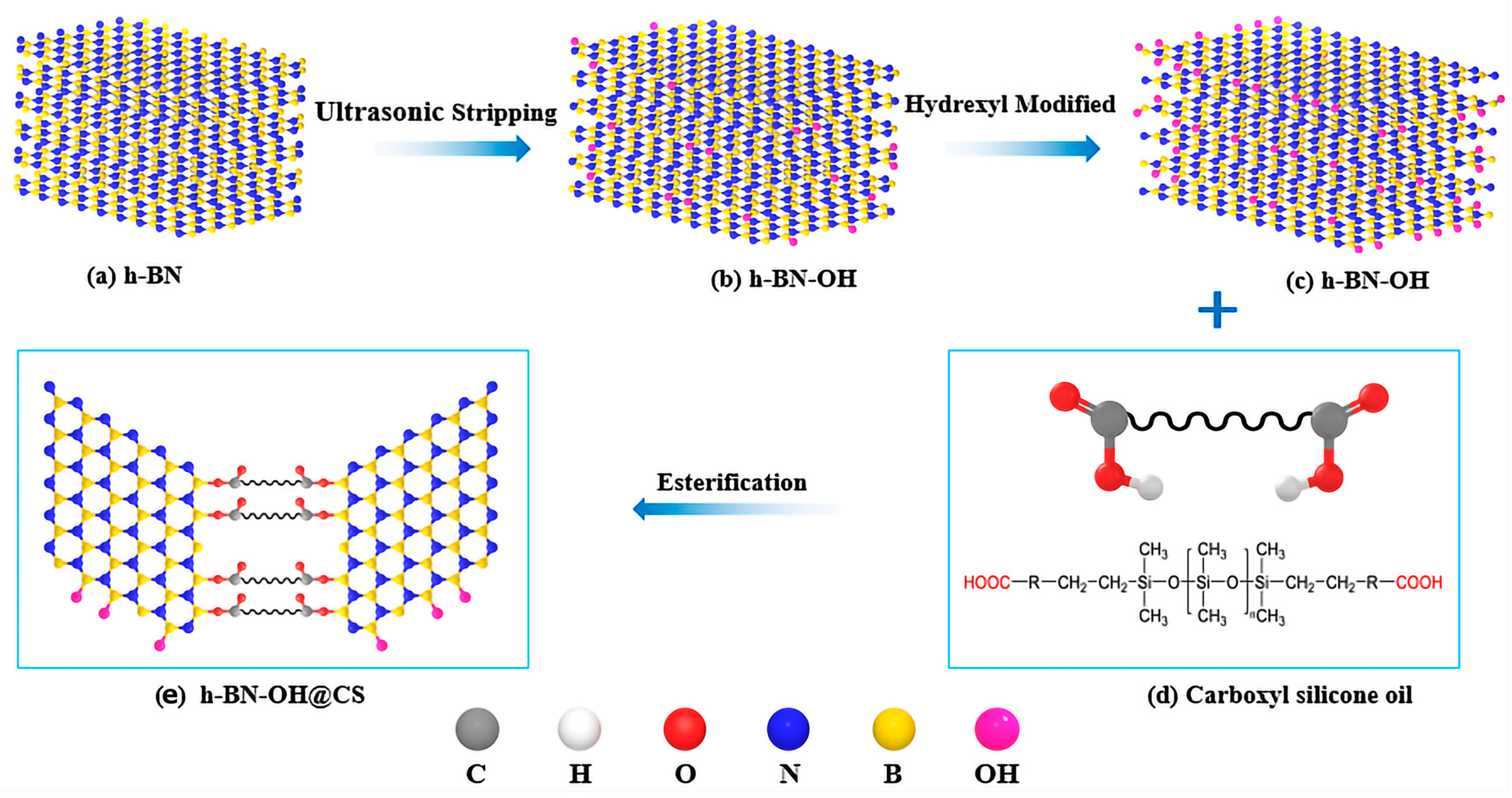
The preparation of h-BN-OH@CS is shown in Figure 1. Toluene (40 mL) was used as the solvent, h-BN-OH (2 g) and CS (18 g) were added to a container, and we adjusted the pH of the reaction system to 3 with concentrated sulphuric acid. The oil bath was heated to 90 °C, and the pre-reaction was carried out for 1–1.5 h. The p-toluenesulfonic acid was added to the reaction flask as a catalyst and heated to 120 °C for 72 h. The crude product after the reaction is washed with a 5 wt% NaOH solution. The upper solution was washed with DIW to neutral and rotary evaporated to remove the solvent and dried for 24 h to obtain h-BN-OH@CS (10 wt%).
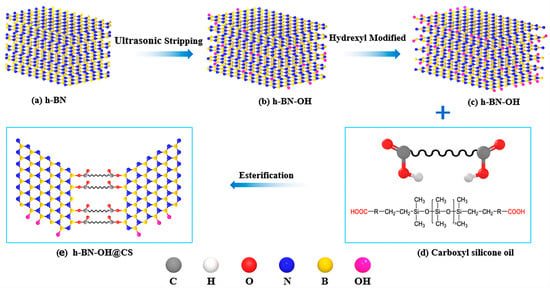
Figure 1.
The preparation of h-BN-OH@CS.
2.2.3. The Preparation of h-BN-NH2@CS
The preparation of h-BN-NH2@CS was similar to that of h-BN-OH@CS. N, N-dimethylformamide (40 mL) was used as a solvent, CS (9 g) and h-BN-NH2 (1 g) were added to the beaker, and dicyclohexylcarbodiimide (0.5 g) was used as a catalyst. After the amidation process, h-BN-NH2 (10 wt%) was grafted with CS to give h-BN-NH2@CS.
2.2.4. Preparation of Thermally Conductive Silicone Grease
The filler (30 wt%) was filled with the base silicone grease at room temperature, and the mixed components were stirred in a three-roller mill at 150 r min−1 for 20 min. Then the grease was degassed by a vacuum-drying oven at room temperature under 0.01 MPa for 2 h.
2.3. Characterization
Fourier-transform infrared spectra (FTIR) were collected by an infrared spectrometer (Spectrum ONE, PerkinElmer, Waltham, MA, USA) in the range 500–4000 cm−1. Microscopic scanning electron micrographs (SEM) of thermally conductive fillers were analyzed by a scanning electron microscope (Gemini 300, Zeiss, Tubingen, Germany). The thermally conductive fillers were observed using transmission electron microscopy (TEM) (eol2100f, JEOL, Tokyo, Japan) at 200 kV. Thermo gravimetric analysis (TGA) (HCT-4, Hengjiu, China) reached 800 °C at a heating rate of 10 °C min−1 in a nitrogen atmosphere. Thermal resistance and thermal conductivity were carried out with a heat flow method thermal conductivity tester (DRL-III, Xiangtan Xiangyi, Xiangtan, China) at an average temperature of 50 °C, pressure 50 N, sample area 615 mm2, and deviation ±5%. The Rheometer (RheoStress 6000, HAAKE, Germany) was used to test the viscosity of the sample. The apparent viscosity of the sample was obtained at a shear rate of 1.25 s−1. The modified fillers were characterized using an X-ray photoelectron spectrometer (XPS) (Thermo Scientific K-Alpha, Waltham, MA, USA) at a chamber pressure of less than 2.0 × 10−7 mbar. UV-Visible absorption spectroscopy (UV-Vis) (Evolution 350, Thermo Fisher, Waltham, MA, USA) was used to measure the absorbance of the samples. The X-ray diffraction instrument (XRD) (Ultima IV, Rigaku, Tokyo, Japan) with a scanning angle range of 5–60° and a scanning speed of 2° min−1.
3. Results and Discussions
3.1. FTIR Analysis
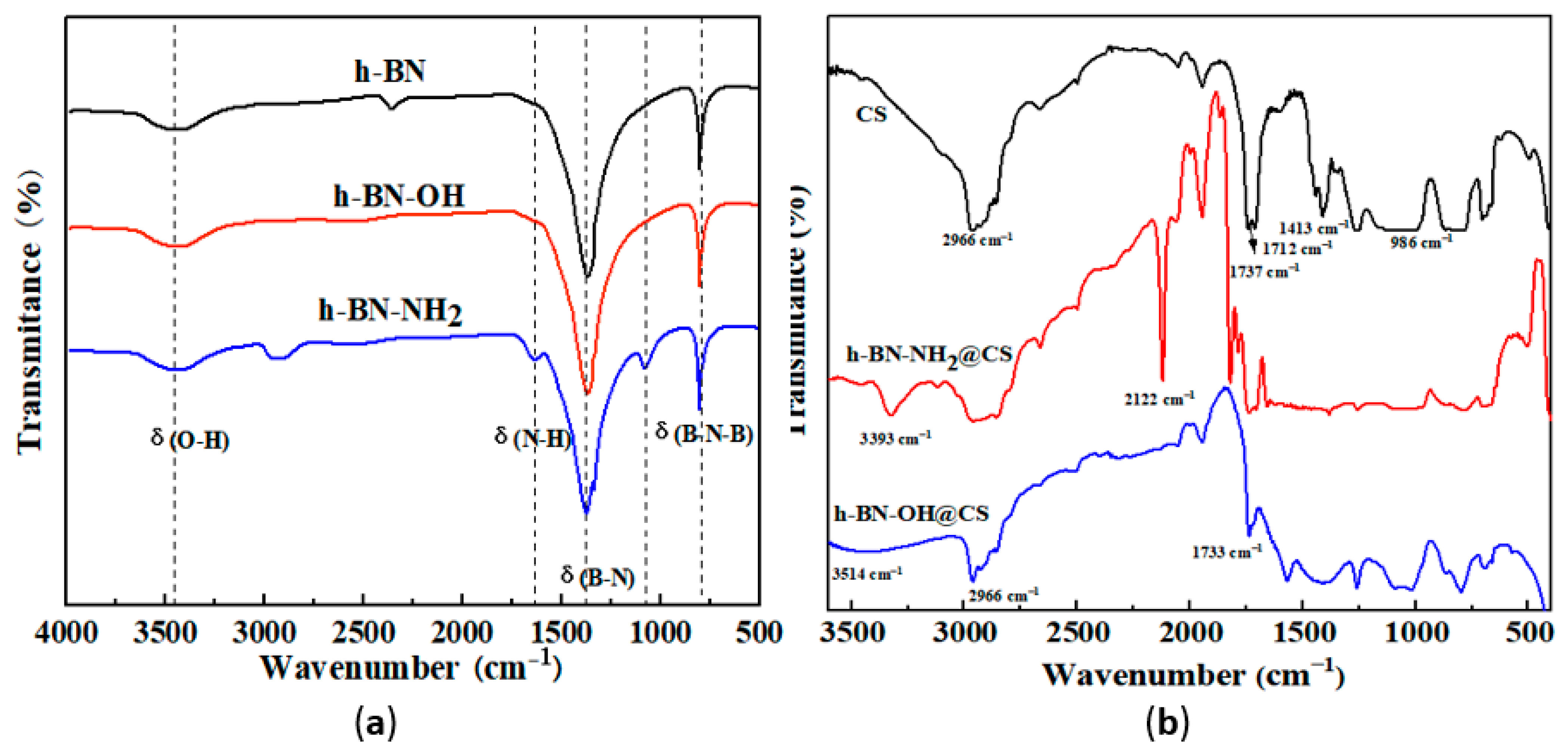
The FTIR analysis of h-BN, h-BN-OH, and h-BN-NH2 is shown in Figure 2a. Due to defects on the h-BN surface, a broad peak of hydroxyl groups at 3500 cm−1. In the spectrum of h-BN-NH2, two peaks at 1100 cm−1 and 1650 cm−1 can be attributed to the bending vibrations of the N-H bond. The peak at 3208–3433 cm−1 in h-BN-NH2 indicates that the NH2 group was grafted onto the h-BN surface after the amination process [26]. The FTIR analysis of h-BN-OH@CS and h-BN-NH2@CS is shown in Figure 2b. The peaks at 1737 cm−1 and 1712 cm−1 are attributed to the C=O double bond stretching vibrations in the carboxyl group, and the peak at 986 cm−1 is attributed to the C-O single bond stretching vibrations. The grafted sample shows a methyl and methylene structure at 2966 cm−1 and a broad peak representing B-OH and -OH at 3000–3600 cm−1. The stretching vibration peak at 1733 cm−1 is attributed to the ester group formed by the esterification reaction. The presence of the ester group peaks demonstrated that h-BN-NH2 and h-BN-OH were grafted onto the CS [27]. As is shown in FTIR curves of h-BN-NH2@CS, at peaks of 3500–3100 cm−1, 1680–1630 cm−1, 1655–1590 cm−1, and 1420–1400 cm−1, the vibrations correspond to N-H, C=O, N-H, and C-N stretching vibration, respectively [28].
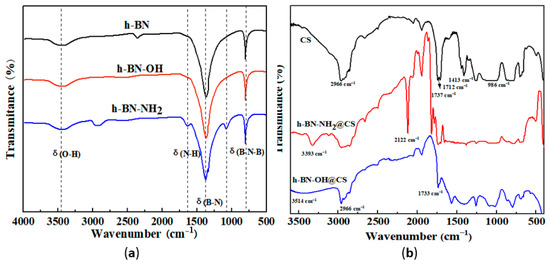
Figure 2.
FTIR analyses of (a) h-BN, h-BN-OH, and h-BN-NH2; (b) CS, h-BN-OH@CS, and h-BN-NH2@CS.
3.2. SEM Analysis
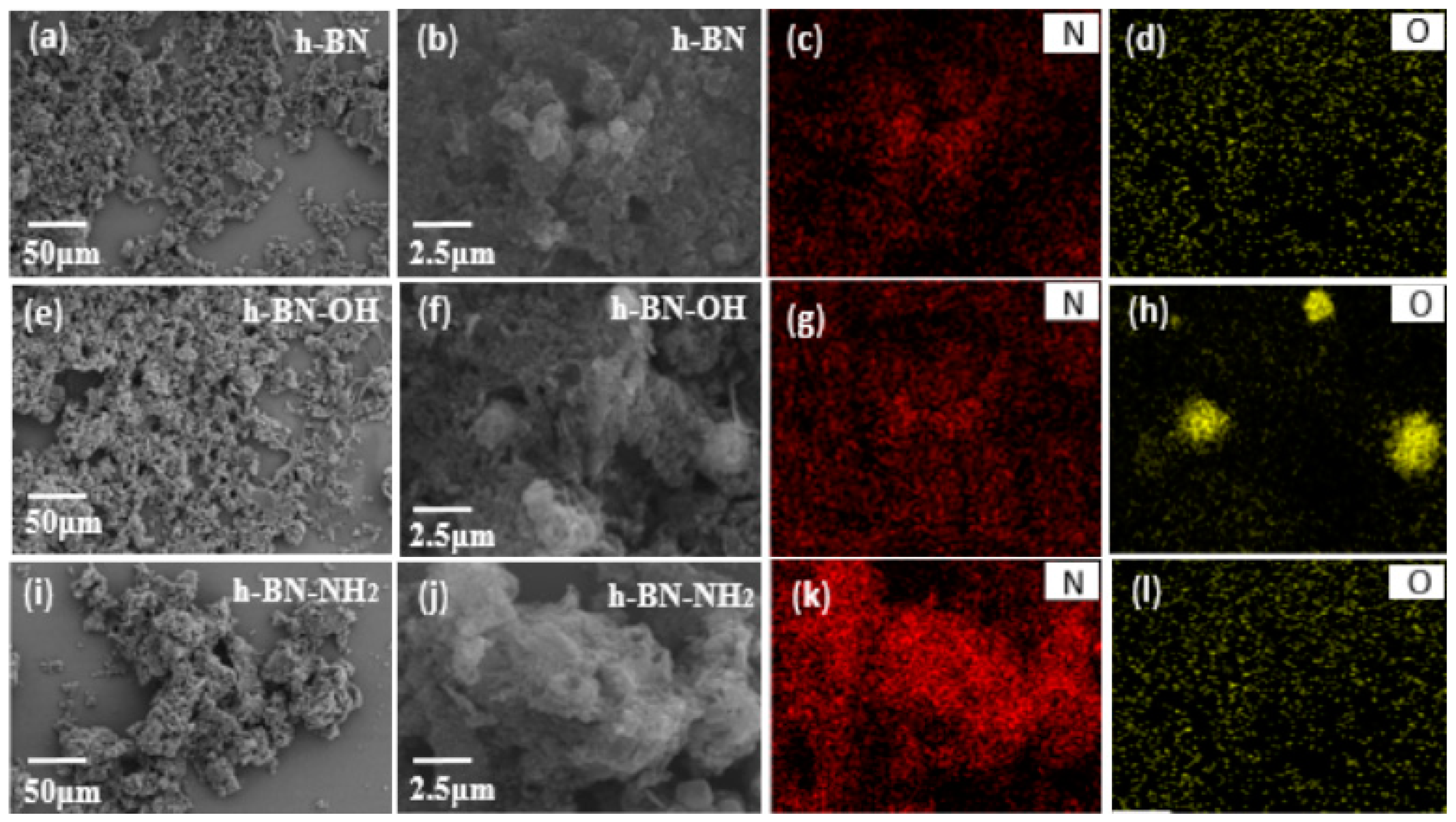
The SEM-EDS analyses of h-BN, h-BN-OH, and h-BN-NH2 are shown in Figure 3. It can be seen in Figure 3a,b that a small amount of –OH can be found on the surface of unmodified h-BN. The EDS mapping images of N and O elements in h-BN are shown in Figure 3c,d, where the percentages of B, N, and O atoms are 51.48%, 46.41%, and 2.12%, respectively. Figure 3e,f show the SEM analysis of h-BN exfoliated by NaOH. In the presence of NaOH, more -OH can be found on the surface of h-BN. The EDS mapping images of N and O elements in h-BN-OH are shown in Figure 3g,h, where the percentages of B, N, and O atoms are 50.63%, 42.66%, and 6.71%, respectively. Figure 3i,j show the SEM analysis of h-BN-NH2 treated by the ball milling method. The EDS images of N and O elements in h-BN-NH2 are shown in Figure 3k,l, where the percentages of B, N, and O atoms are 50.45%, 47.37%, and 2.18%, respectively. Therefore, the surface of the ball-ground h-BN was loaded with a certain quantity of hydroxyl or amino groups.
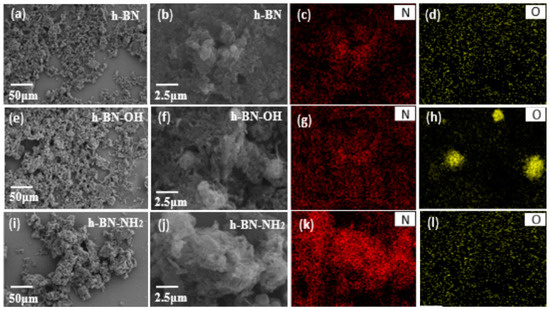
Figure 3.
SEM images of (a,b) h-BN (e,f) h-BN-OH; (i,j) h-BN-NH2 and EDS mapping images of the corresponding distribution of (c,g,k) N and (d,h,l) O element.
3.3. XRD Analysis
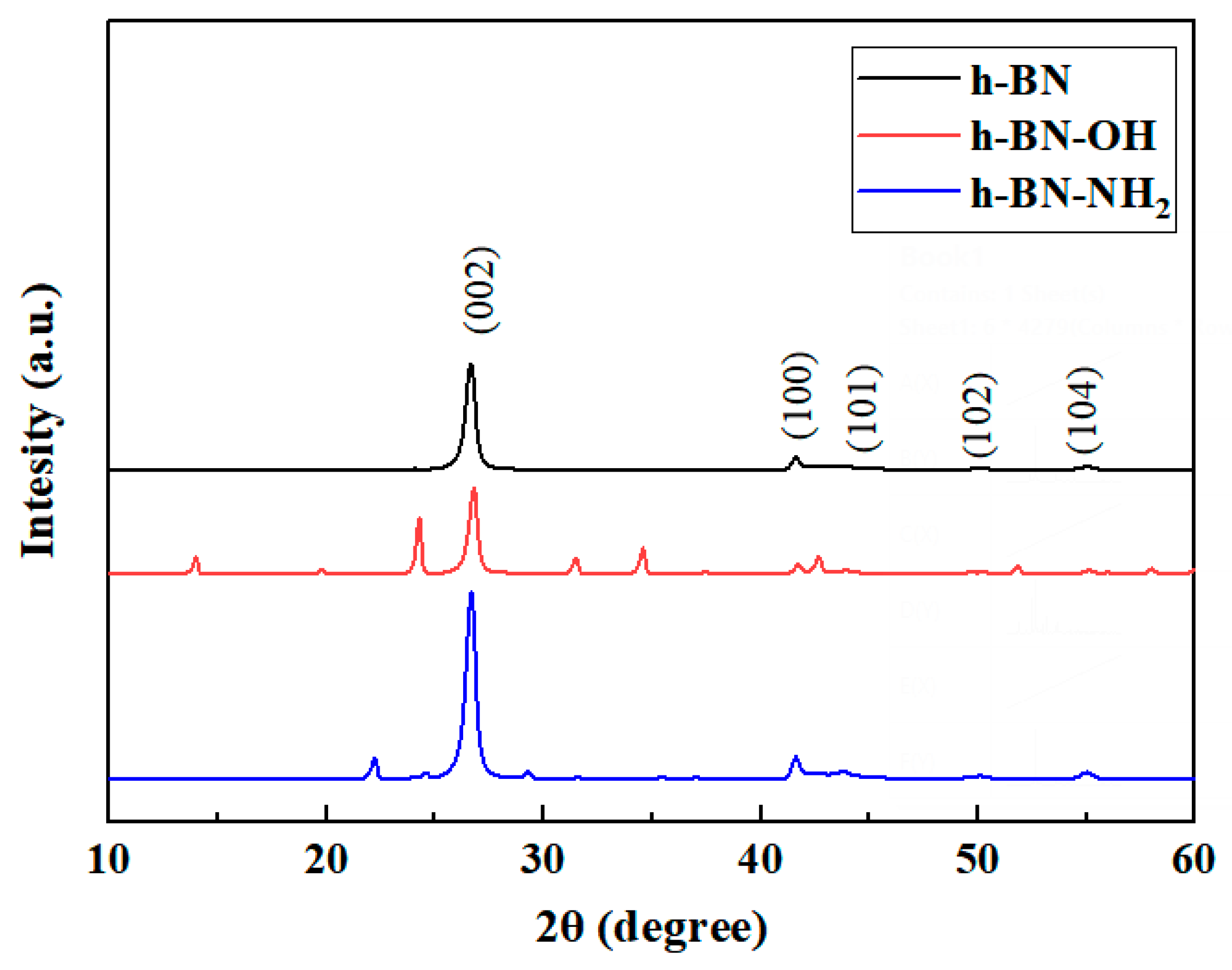
The XRD patterns of h-BN, h-BN-OH, and h-BN-NH2 samples are shown in Figure 4. According to the PDF card, functionalized h-BN exhibits a decrease in the (002) peak intensity compared to pristine h-BN [29]. Additionally, the (002) peak of functionalized h-BN shifts towards lower angles, with an increase in interlayer spacing for h-BN-OH and h-BN-NH2. Chemical modification and ball milling disrupt the van der Waals forces between h-BN layers, while the functional groups loaded onto h-BN increase the interlayer distance, achieving the goal of exfoliation.
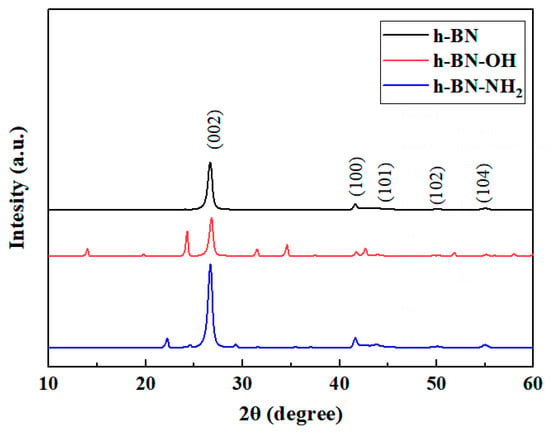
Figure 4.
XRD patterns of h-BN, h-BN-OH, and h-BN-NH2.
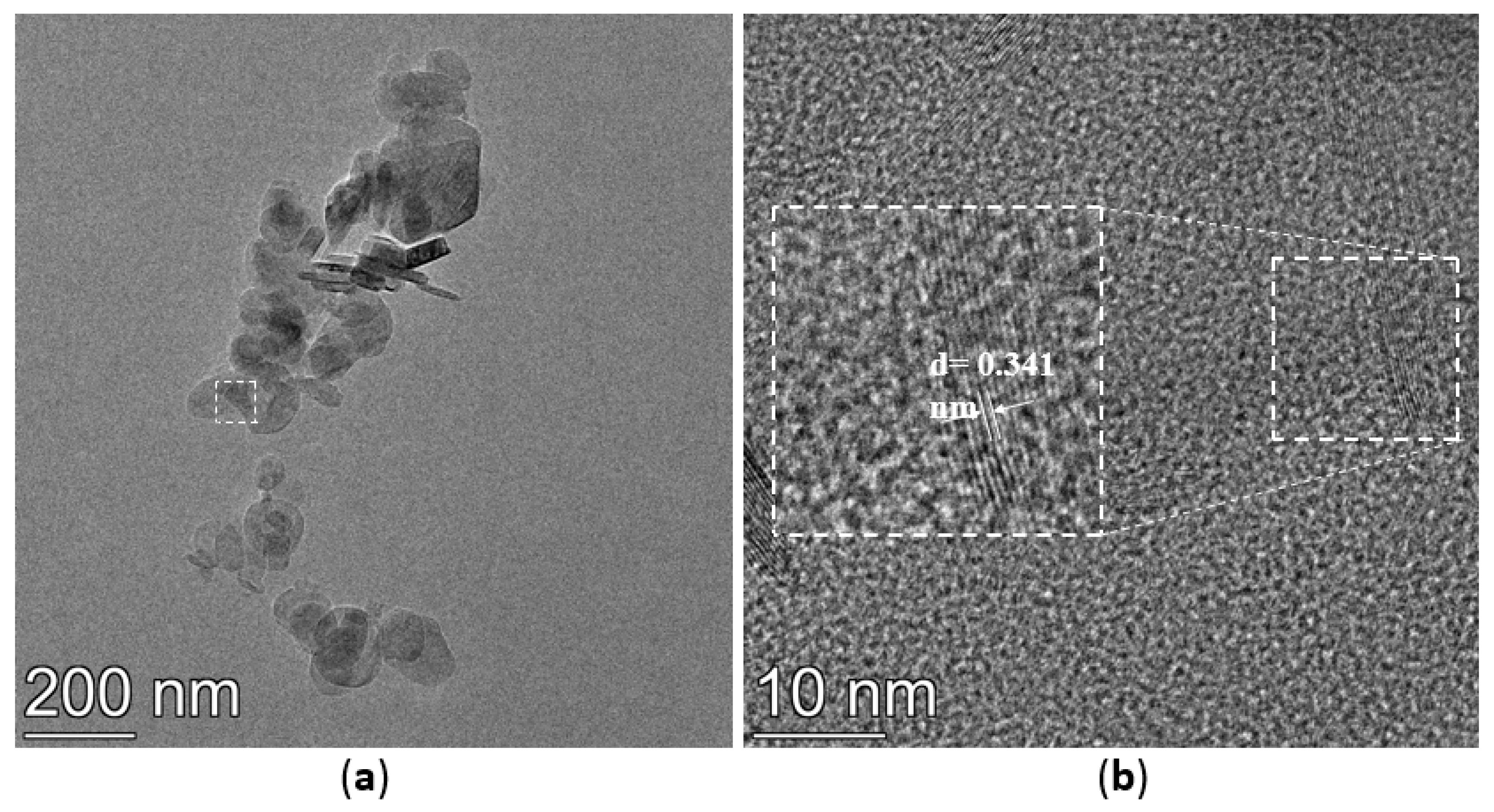
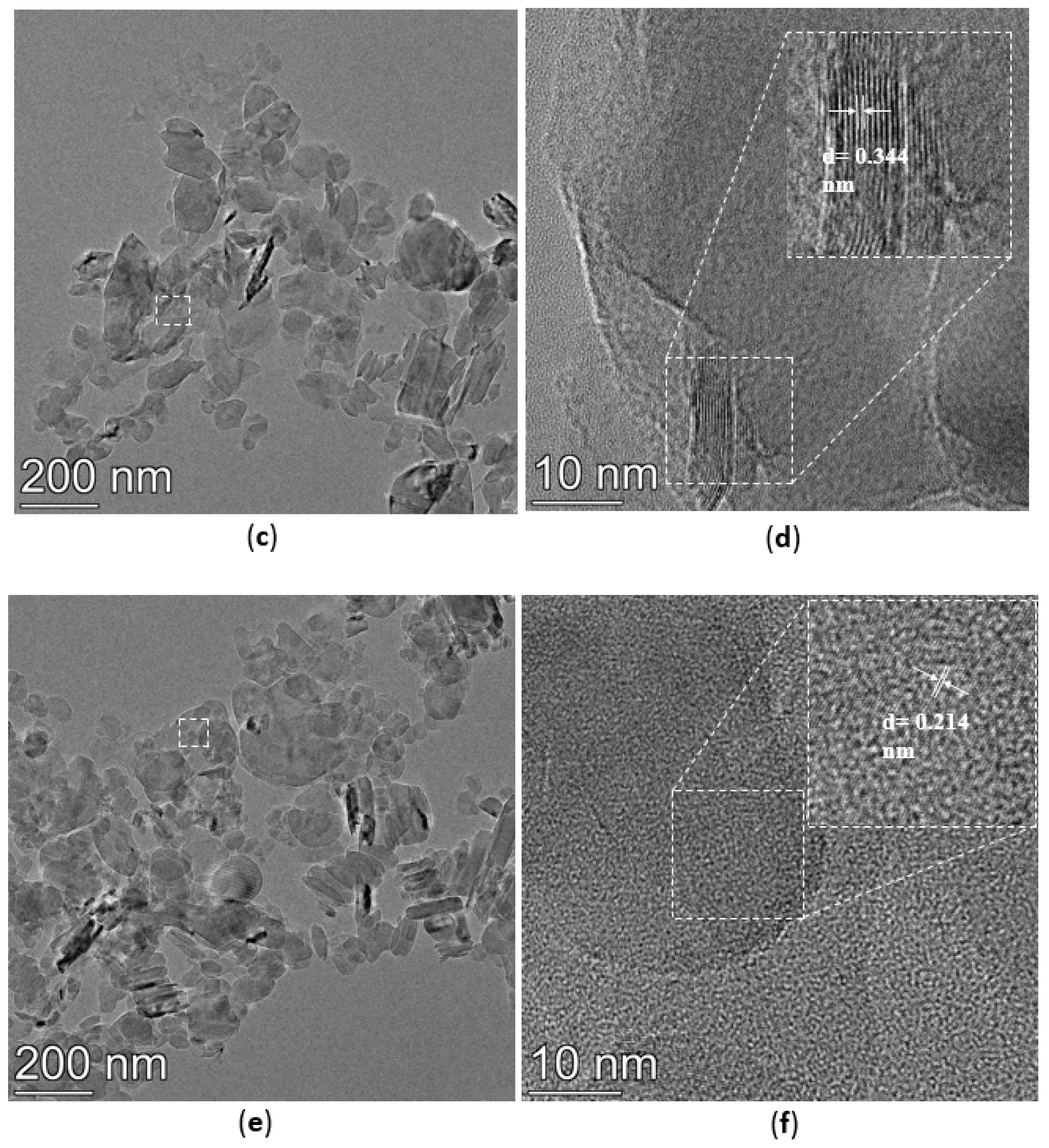
3.4. TEM Analysis
The TEM images of h-BN, h-BN-OH, and h-BN-NH2 are shown in Figure 5. The crystal plane spacing of unmodified h-BN is 0.341 nm (Figure 5a,b). After immersion in NaOH solution, the h-BN surface is loaded with hydroxyl groups, the crystal plane spacing of h-BN-OH is 0.344 nm (Figure 5c,d), and the crystal plane spacing of h-BN-OH is slightly greater than that of h-BN [30]. The TEM images of h-BN-NH2 are shown in Figure 5e,f; h-BN after being ball-milled, h-BN surface loaded with -NH2 after ball-milling by urea, and the crystal plane spacing of h-BN-NH2 is 0.214 nm [31]. The relative thickness of h-BN can be visually estimated through its transparency. In Figure 5a, the h-BN shows low transparency and tends to stack with relatively thick layers. However, h-BN-OH and h-BN-NH2 on the substrate tend to be transparent (Figure 5c,e), indicating the few-layer structure of functionalized h-BN [32].
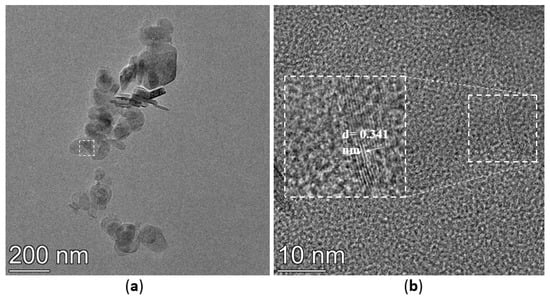
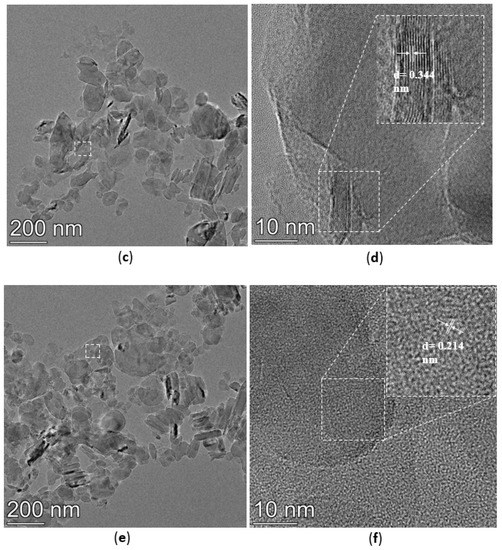
Figure 5.
TEM images of (a,b) h-BN, (c,d) h-BN-OH, and (e,f) h-BN-NH2.
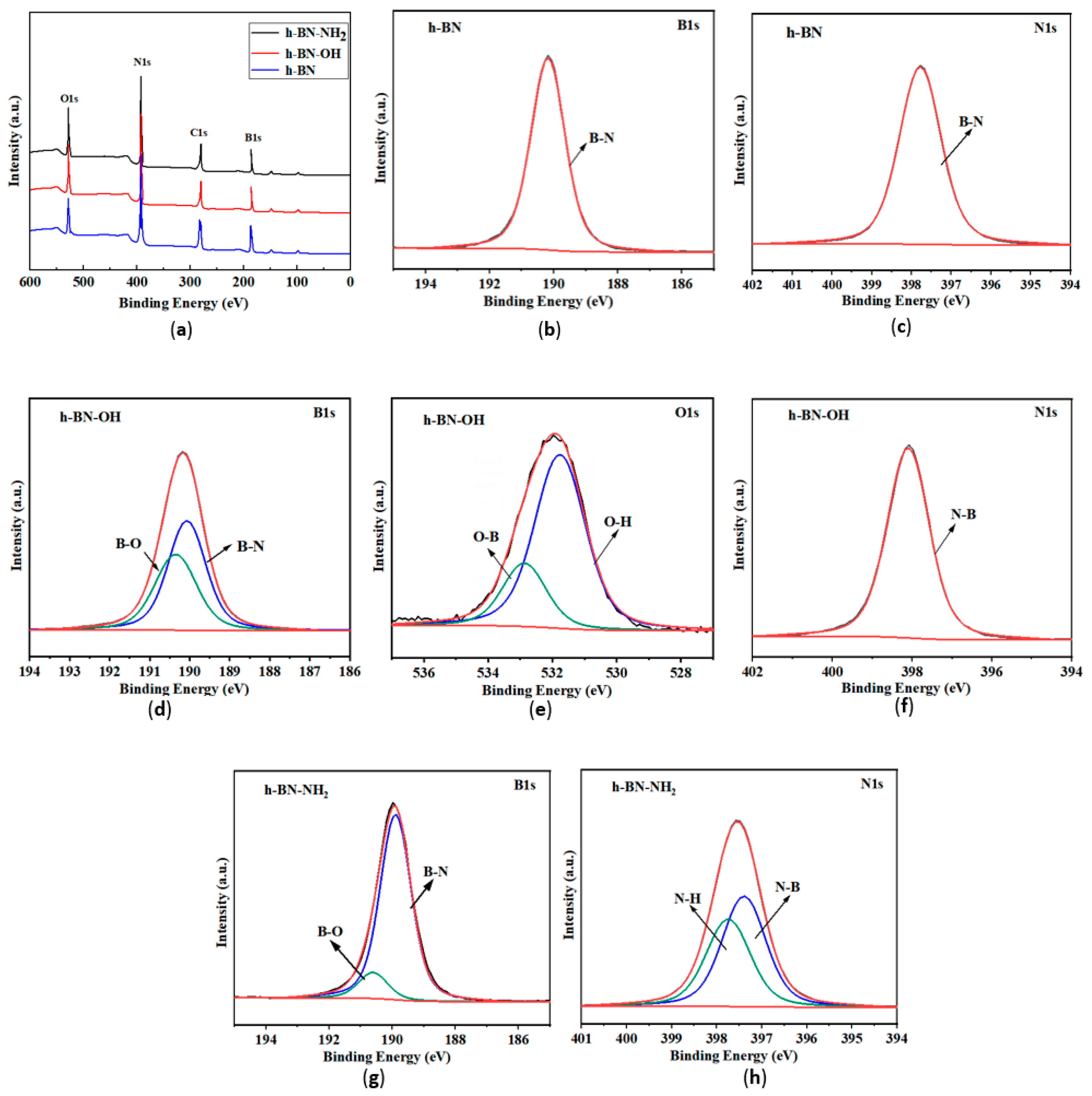
3.5. XPS Analysis
The XPS spectra of h-BN, h-BN-OH, and h-BN-NH2 are shown in Figure 6. In Figure 6a, the peaks of B 1s, N 1s, C 1s, and O 1s appear at 191 eV, 398.2 eV, 285 eV, and 532 eV, respectively. The presence of carbon elements in the XPS spectra arises from adsorbed CO2 on the h-BN surface [33]. In the B 1s and N1s spectrum (Figure 6b,c), two peaks at 190.5 and 398.2 eV correspond to the B-N and N-B bonds. As shown in Figure 6d, the peak at 192.0 eV in the B 1s spectrum is attributed to the B-O bond, indicating hydroxylation at the B site [34]. In the O 1s spectrum (Figure 6e), the main peak at 532.6 eV is caused by -OH, and the smaller peak at 533.5 eV is possibly due to the O-B bond. In N 1s spectrum (Figure 6f), the peak at 398.2 eV can be ascribed as the N-B bond in the N1s core-level spectrum, and the result demonstrates that -OH was grafted on the h-BN surface. Figure 6g,h show the B 1s and N 1s spectra of h-BN-NH2, respectively. The peak at 190.5 eV in the B 1s spectrum corresponds to the B-N bond in h-BN, and the peak at 191.5 eV is possibly due to the B-O bond formed during ball milling. In the N 1s spectrum, the two peaks at 398.9 eV and 398.2 eV are attributed to the N-H and N-B bonds, respectively, indicating the loading of -NH2 on the h-BN surface [35].
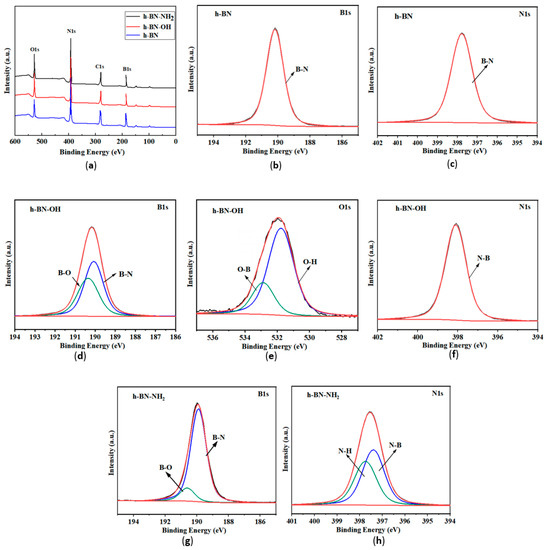
Figure 6.
(a) XPS analysis of h-BN, h-BN-OH, and h-BN-NH2; (b) B 1s and (c) N 1s spectrum of h-BN-OH; (d) B 1s (e) O 1s and (f) N 1s spectrum of h-BN-OH; (g) B 1s and (h) N 1s spectrum of h-BN-NH2.
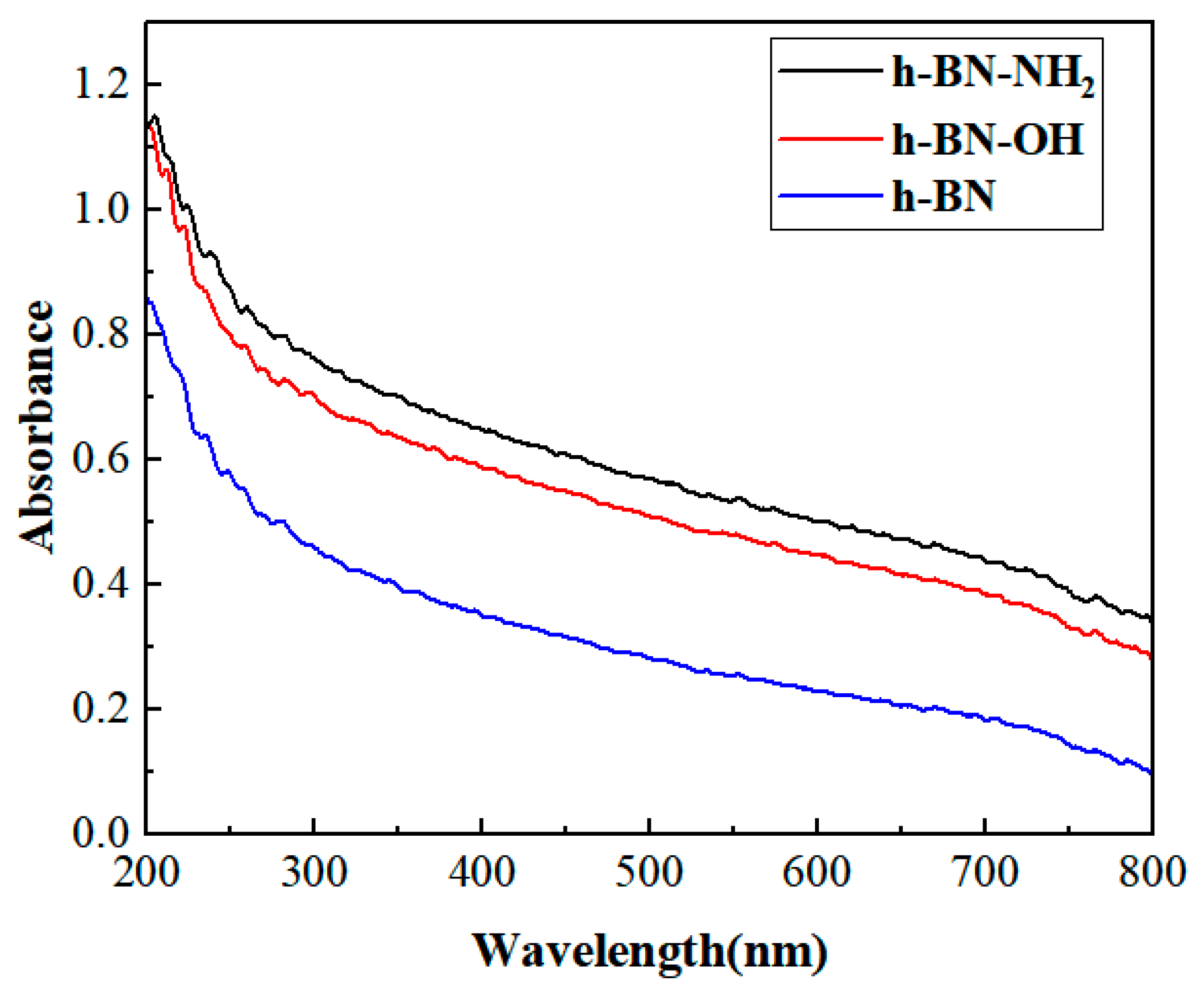
3.6. UV-Visible Analysis
The UV-Vis spectra of h-BN, h-BN-OH, and h-BN-NH2 samples are shown in Figure 7. The three samples were dispersed in water to form a dispersion solution of 1 mg mL−1, which was then left to settle at room temperature for more than 48 h and centrifuged three times (1500 rpm, 15 min each time). After centrifugation, the solution was left to stand for 24 h to allow the insoluble substances to settle. By studying the UV-Visible spectra, we investigated the difference between the UV-Visible spectra of h-BN-OH, h-BN-NH2, and h-BN. The h-BN had poor dispersibility in water, but functionalized h-BN was found to improve its solubility in water through UV-Visible spectroscopy [36]. The absorbance values at a wavelength of 300 nm for h-BN, h-BN-OH, and h-BN-NH2 were 0.458, 0.701, and 0.759, respectively. Thus, it can be concluded that under the same preparation and testing conditions, the dispersion solubility of h-BN-OH and h-BN-NH2 in water was higher than that of h-BN [37].
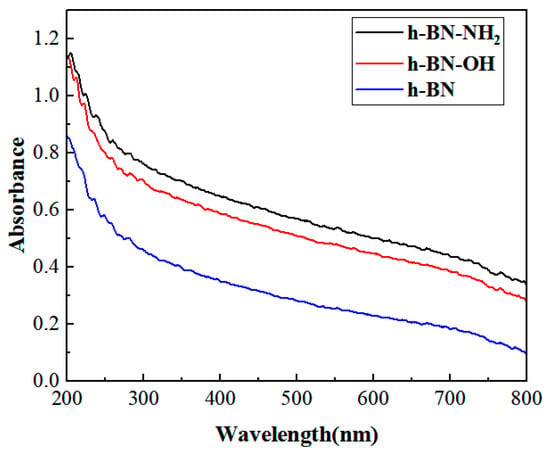
Figure 7.
UV-Vis spectra of h-BN, h-BN-OH, and h-BN-NH2.
3.7. Thermo Gravimetric Analysis
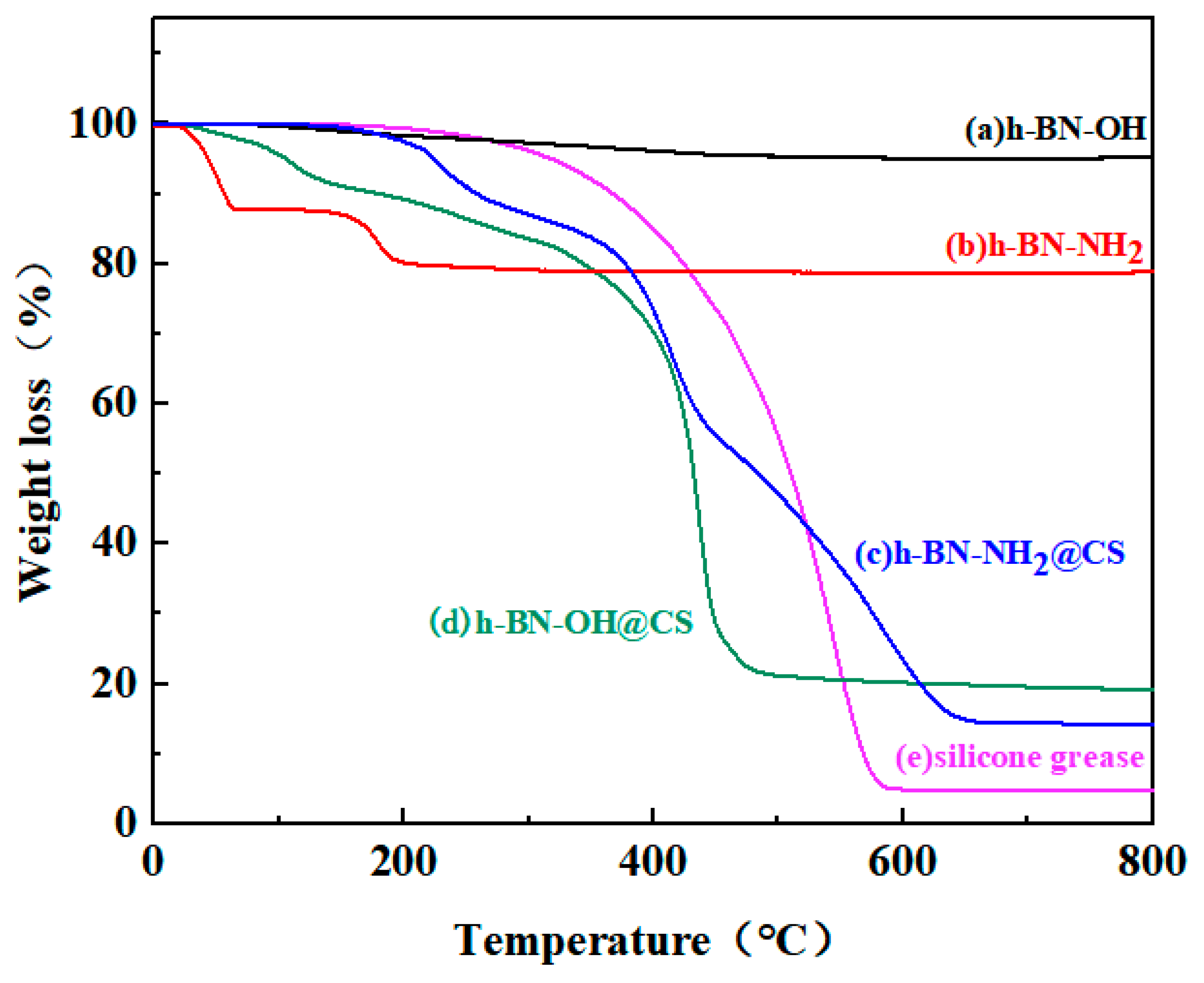
The thermogravimetric analysis of h-BN-OH, h-BN-NH2, and grafted products is shown in Figure 8. The TG curve for h-BN-OH shows a slight decrease in the 0–800 °C range, with a total weight loss of approximately 5 wt%, which can be traced back to the grafting of –OH on the h-BN surface because generally pure h-BN is highly thermal and stable below 800 °C [38]. The base silicone decomposes rapidly at 300 °C, and the decomposition rate slows down to a temperature of 600 °C. When h-BN-OH (20 wt%) was grafted with the CS, no weight loss occurred between 130 and 200 °C for h-BN-OH@CS, and no evaporation of carboxyl groups was present in the sample. As shown in the TG curve, there is a mass loss in the range of 25–50 °C and 180–200 °C for h-BN-NH2, with a total weight loss of approximately 20 wt%, which was attributed to the grafting of the –NH2 on the h-BN surface. h-BN-NH2 (20 wt%) grafted with CS resulted in a smaller mass loss in the 0–200 °C range for h-BN-NH2@CS. The thermal stability of the silicone is improved by the grafted hBN-NH2. Most significantly, the presence of h-BN-NH2 significantly improved the thermal stability of the CS and retarded the thermal decomposition process [39]. In summary, the h-BN-grafted thermally conductive filler can effectively enhance the thermal stability of the base silicone grease. h-BN-OH/h-BN-NH2 has good compatibility with CS and forms closed chains in the matrix, limiting the thermal movement of the CS segments [40].
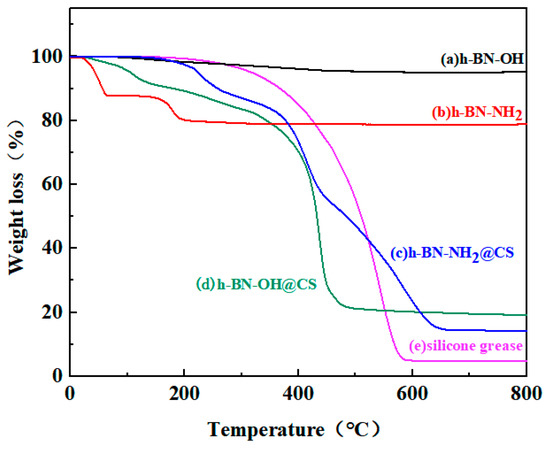
Figure 8.
TG curves of (a) h-BN-OH, (b) h-BN-NH2, (c) h-BN-NH2@CS, (d) h-BN-OH@CS, and (e) silicone grease.
3.8. Viscosity Analysis
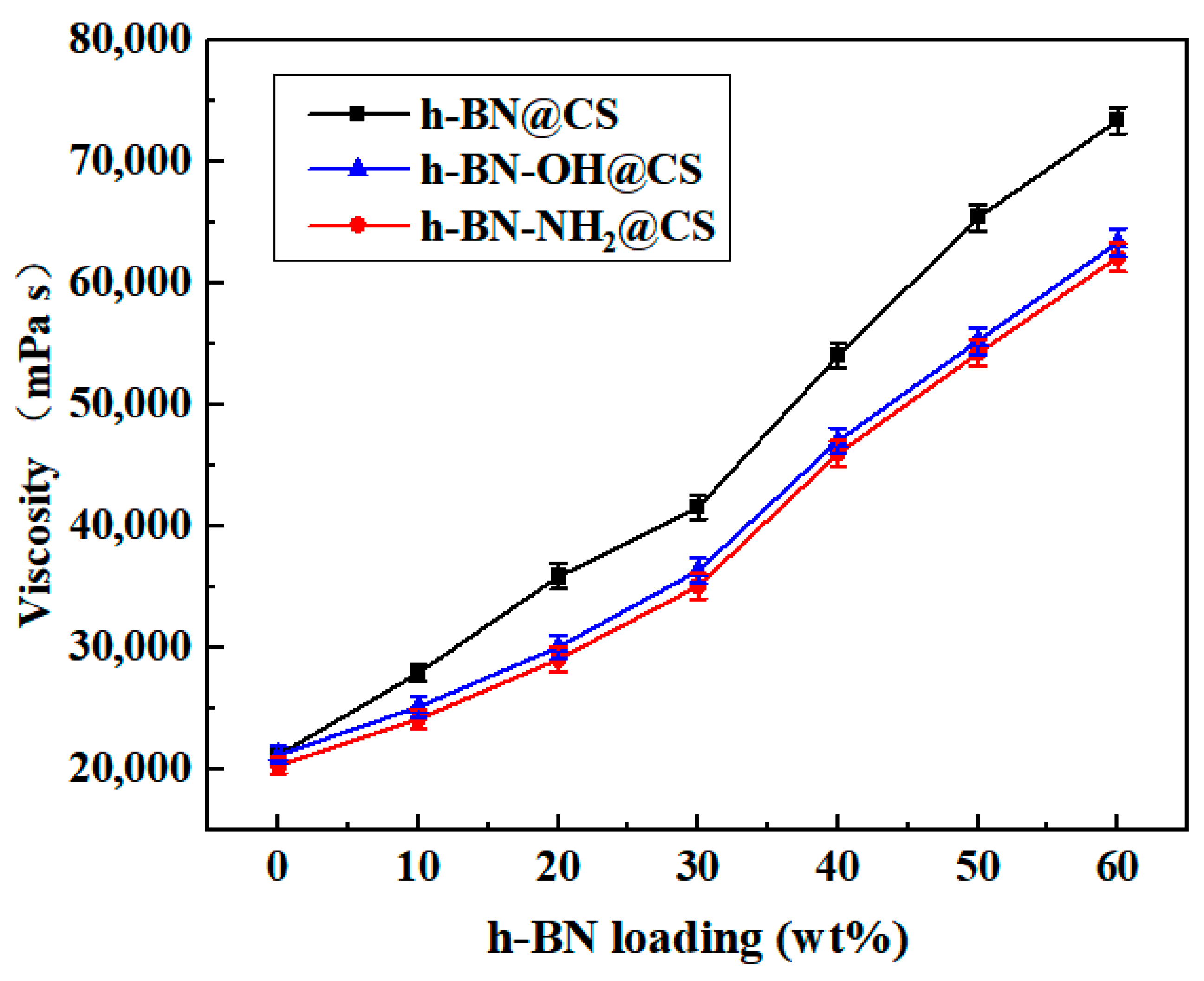
The variation of the viscosity of the silicone grease with the amount of filler is shown in Figure 9. The viscosity of base silicone grease is 21,220 mPa s, h-BN (10–60 wt%) was filled with the CS (h-BN@CS), and the viscosity is 27,980–73,460 mPa s when the amount of 30 wt% h-BN @CS is in the base silicone grease. h-BN-OH (10–60 wt%) was grafted with the CS (h-BN-OH@CS), and the viscosity is 25,180–63,400 mPa s when the amount of 30 wt% h-BN-OH @CS is in the base silicone grease. Compared to the unmodified h-BN, the h-BN modification reduced the viscosity of the silicone grease by 10.01–13.7%. A total of 10–60 wt% of h-BN-NH2 was grafted with a CS to form a thermally conductive filler h-BN-NH2@CS, and the viscosity was 24,180–62,180 mPa s when the amount of 30 wt% h-BN-NH2@CS was in the base silicone grease. This indicates that the viscosity of the silicone grease is reduced by the modified h-BN, and h-BN-NH2@CS had a higher viscosity reduction than h-BN-OH@CS. It can be due to the fact that the modified grafting improved the IR of the silicone grease and increased the thermal conductivity of the base silicone grease. h-BN or modified h-BN grafting products filled into the base silicone grease showed a significant increase in viscosity, which can be due to the presence of the filler significantly changing the rheology of the base silicone grease [41]. When loaded with thermally conductive fillers, there are mutual frictional and chemical forces between the h-BN particles that can cause rheological distortions in the base silicone grease, thus increasing the viscosity of the base silicone grease [42]. The viscosity of the silicone grease is reduced by the filling of the modified h-BN. This is mainly due to the covalent bonds formed between the modified h-BN and the CS, and the forces of the covalent bonds are larger than the van der Waals gravitational forces, making it easier for the h-BN particles to be dispersed evenly in the base silicone grease, after which a reduction in IR can be obtained [43].
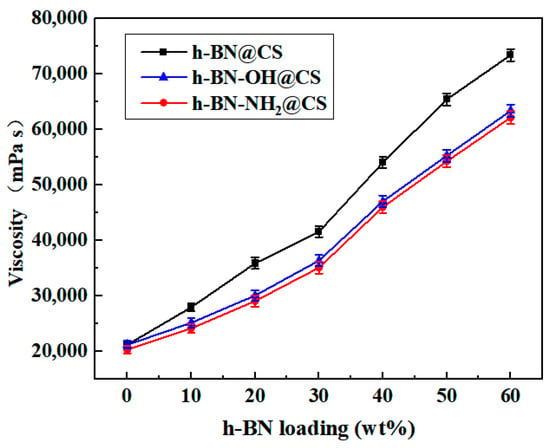
Figure 9.
The viscosity of thermally conductive silicone grease varies with the h-BN@CS, h-BN-OH@CS, and h-BN-NH2@CS loadings.
3.9. Thermal Conductivity and Interface Thermal Resistance
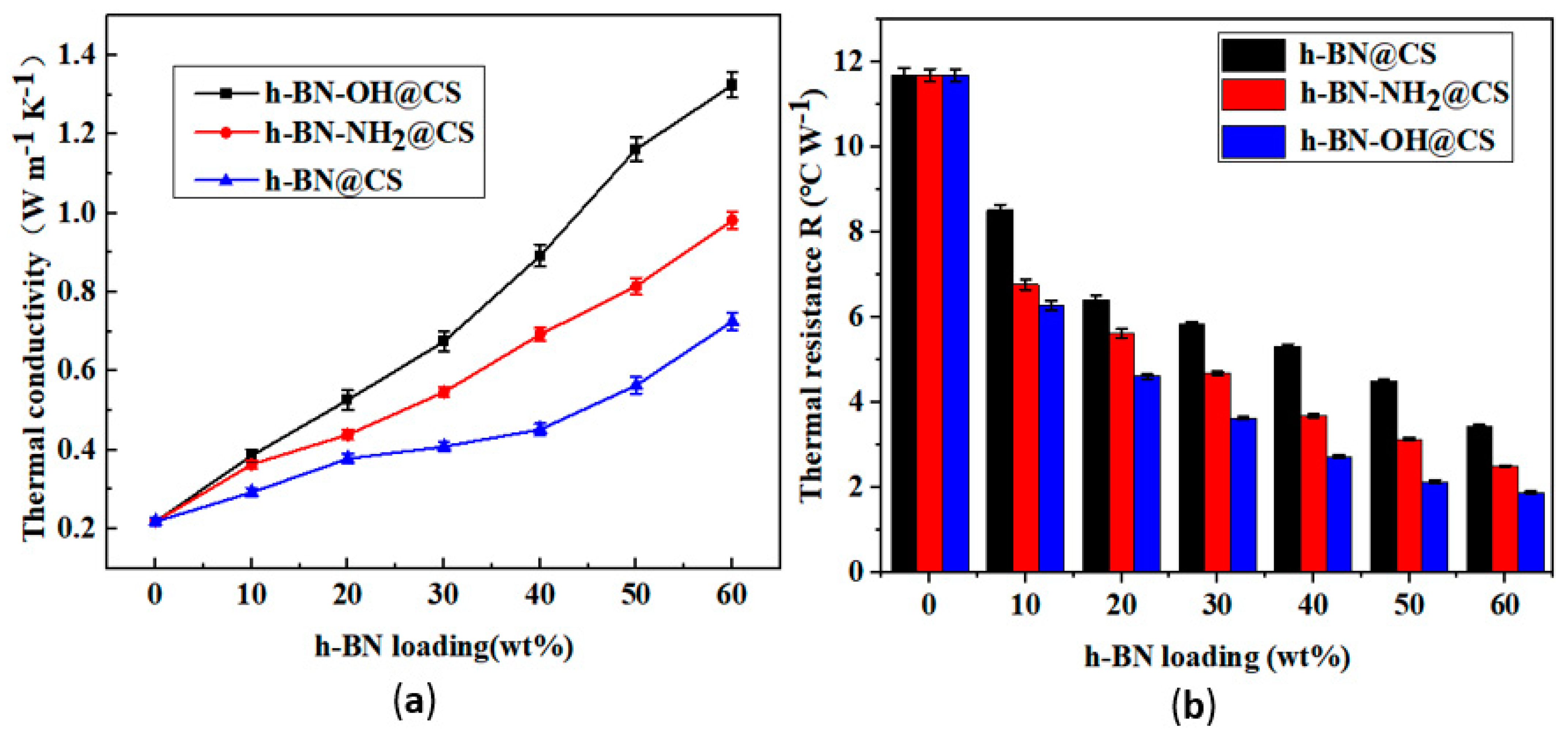
The TC and IR of h-BN@CS, h-BN-OH@CS, and h-BN-NH2@CS are shown in Figure 10. The TC of the base silicone grease was 0.219 W m−1 K−1, and 10–60 wt% of h-BN-OH was grafted with CS to form a thermally conductive filler. After the modification, the TC of the silicone grease was increased from 0.219 W m−1 K−1 to 1.324 W m−1 K−1, and the IR was reduced from 11.699 °C W−1 to 1.889 °C W−1. When 10–60 wt% of h-BN-NH2 was grafted with a CS to form a thermally conductive filler that is filled in silicone grease, the TC of the silicone grease was increased from 0.219 W m−1 K−1 to 0.982 W m−1 K−1, and the IR was reduced from 11.699 °C W−1 to 2.514 °C W−1. The TC of the modified grafted silicone grease was improved, and the IR was reduced [44]. Notably, when 60 wt% of h-BN-OH was grafted with CS, the TC of the nanocomposite reached 1.324 W m−1 K−1, which is 6.05 times that of the pure silicone grease, and the IR was reduced from 11.699 °C W−1 to 1.889 °C W−1. This can be attributed to the strong chemical bonding between the modified thermally conductive filler, and h-BN can be evenly dispersed in silicone grease [45]. When the content of modified thermally conductive fillers in the silicone grease increases, the thermal conductivity increases [46]. However, as the amount of h-BN filler increases, the van der Waals forces between the h-BN and the matrix material become greater [47]. Being filled directly with h-BN in thermally conductive silicone, the resulting silicone is unstable. The ester and amide bonds are able to create thermally conductive channels in the silicone grease, which is beneficial to heat transfer [48].
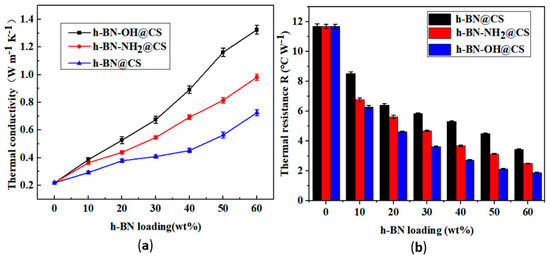
Figure 10.
(a) TC of silicone grease variations with h-BN@CS, h-BN-OH@CS, and h-BN-NH2@CS loadings; (b) IR of silicone grease with h-BN@CS, h-BN-OH@CS, and h-BN-NH2@CS loadings.
The thermal conductivity of filled-high TC silicone grease is mainly influenced by hydrodynamics, mutual friction between filler particles, and mutual attraction between molecules [49]. Because of the hydrodynamic interaction between h-BN and silicone oil molecules, the inorganic filler h-BN interferes with the silicone grease, which leads to the distortion of the flow lines. Spherical fillers lead to less distortion of the flow lines than flake fillers. Therefore, the grafting of h-BN results in a thermally conductive filler with fewer angles and smoother edges. Furthermore, TC is also influenced by the viscosity of the thermally conductive silicone grease and the forces between the thermally conductive filler and the silicone grease molecules [50]. It was found that the formation of microscopic routes that transport heat contributes to the filling of the materials [51]. At low content, the thermally conductive filler spreads in the silicone grease, and the TC is not significantly enhanced owing to the influence of the interfacial layer among the filler that is thermally conductive and due to the heat transfer being weak [52]. When the quantity of filling is raised to a specified limit, the thermally conductive fillers commence making contact, forming a thermally conductive pathway or thermally conductive network chain. The heat flow is transferred along the path with the lowest thermal resistance, resulting in a substantial increase in the thermal conductivity of the silicone grease.
In summary, h-BN-OH and h-BN-NH2 at 10–60 wt% were chemically grafted with CS, and h-BN-OH@CS and h-BN-NH2@CS were used as thermally conductive fillers. When they were filled with 30 wt% into the base silicone, the TC of the silicone grease is 0.386–1.324 W m−1 K−1 and 0.364–0.982 W m−1 K−1, respectively, which is higher than the base silicone grease (0.219 W m−1 K−1). The IR of the base silicone grease drops from 11.699 °C W−1 to 1.889 °C W−1 and 2.514 °C W−1. By analyzing the viscosity of the lubricant, it was demonstrated that there is an interaction between the modified h-BN and the base silicone grease molecules. The dispersion of the filler can be effectively improved, and thermal conductivity pathways can be formed. Then the IR was reduced, and the TC of the silicone grease was improved.
4. Conclusions
In this work, two types of thermally conductive fillers were prepared by grafting carboxylic acid silicone oil onto 10–60 wt% hydroxyl and amino-functionalized h-BN. The effect of h-BN modification on the TC of the base grease was studied, and the thermal mechanism of the thermally conductive fillers on the grease was explored. After thermal stability testing, the modified thermally conductive fillers effectively enhanced the thermal stability of base grease. The h-BN-OH@CS has better thermal stability than h-BN-NH2@CS. The addition of h-BN or modified boron nitride graft products significantly increased the viscosity of the silicone grease. When the modified thermal conductive filler was added to the silicone grease, the viscosity was smaller than when unmodified h-BN particles were directly added to the grease. The viscosity-reducing effect of the h-BN-NH2@CS was more significant than that of the h-BN-OH@CS. The modified thermal conductive fillers, h-BN-NH2@CS and h-BN-OH@CS, effectively improved the thermal conductivity of the grease and reduced the IR. When there is less filling, the filling separates in the grease, which is not conducive to heat transfer. As the content of the modified thermally conductive filler increased, the thermal conductivity increased. However, with increasing h-BN content, the formed grease became unstable and did not easily form thermal transfer paths. By forming ester bonds and amide bonds, h-BN could form thermal conductivity paths in the silicone grease, thereby promoting heat transfer.
Author Contributions
Conceptualization, Y.W., J.Y. and S.H.; methodology, Y.W., J.Y. and S.H.; data curation, Y.W.; writing—original draft preparation, Y.W.; writing—review and editing, Y.W., M.L., J.Y. and N.S.; project administration, S.H. and J.Y.; funding acquisition, S.H. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of the National Natural Science Foundation of China (Nos. 22075183, 22008155, 22278269, and 21975161), Industrial Collaborative Innovation Project of Shanghai (Grant Nos. 2021-cyxt1-kj37 and XTCX-KJ-2022-70), Natural Science Foundation Project of Shanghai (Grant No. 22ZR1426100), Leading Talents Program of Shanghai (Grant No. 053), Foundation of Science and Technology Commission of Shanghai Municipality (Grant No. 22010503900), Foundation of Department of Education of Guangdong Province (Grant No. 2018KTSCX219), Foundation of Guangdong Basic and Applied Research (Grant No. 2020A1515011102).
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Liu, C.; Wu, W.; Wang, Y.; Liu, X.R.; Chen, Q.M.; Xia, S.X. Silver Nanoparticle-Enhanced Three-Dimensional Boron Nitride/Reduced Graphene Oxide Skeletons for Improving Thermal Conductivity of Polymer Composites. ACS Appl. Polym. Mater. 2021, 3, 3334–3343. [Google Scholar] [CrossRef]
- Jiang, Z.-H.; Xue, C.-H.; Guo, X.-J.; Liu, B.-Y.; Wang, H.-D.; Fan, T.-T.; Jia, S.-T.; Deng, F.-Q. Thermally Conductive, Superhydrophobic, and Flexible Composite Membrane of Polyurethane and Boron Nitride Nanosheets by Ultrasonic Assembly for Thermal Management. ACS Appl. Polym. Mater. 2023, 5, 1264–1275. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Huang, J.H.; Cao, M.; Jiang, G.W.; Hu, J.; Chen, Q. Preparation of Binary Thermal Silicone Grease and Its Application in Battery Thermal Management. Materials 2020, 13, 4763. [Google Scholar] [CrossRef]
- Swamy, M.C.K.; Satyanarayan. A Review of the Performance and Characterization of Conventional and Promising Thermal Interface Materials for Electronic Package Applications. J. Electron. Mater. 2019, 48, 7623–7634. [Google Scholar] [CrossRef]
- Wu, T.T.; Hu, Y.X.; Liu, X.Q.; Wang, C.H. Effect Analysis on Thermal Management of Power Batteries Utilizing a Form-Stable Silicone Grease/Composite Phase Change Material. ACS Appl. Energy Mater. 2021, 4, 6233–6244. [Google Scholar] [CrossRef]
- Zhang, R.C.; Huang, Z.R.; Huang, Z.H.; Zhong, M.L.; Zang, D.M.; Lu, A.; Lin, Y.F.; Millar, B.; Garet, G.; Turner, J.; et al. Uniaxially stretched polyethylene/boron nitride nanocomposite films with metal-like thermal conductivity. Compos. Sci. Technol. 2020, 196, 108154. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, C.; Tang, Y.L.; Zhang, X.; Zheng, K.; Tian, X.Y. Preparation and properties of boron nitride nanosheets/cellulose nanofiber shear-oriented films with high thermal conductivity. Ceram. Int. 2019, 45, 12965–12974. [Google Scholar] [CrossRef]
- Yang, S.Y.; Huang, Y.F.; Lei, J.; Zhu, L.; Li, Z.M. Enhanced thermal conductivity of polyethylene/boron nitride multilayer sheets through annealing. Compos. Part A Appl. Sci. Manuf. 2018, 107, 135–143. [Google Scholar] [CrossRef]
- Zheng, X.R.; Kim, S.; Park, C.W. Enhancement of thermal conductivity of carbon fiber-reinforced polymer composite with copper and boron nitride particles. Compos. Part A Appl. Sci. Manuf. 2019, 121, 449–456. [Google Scholar] [CrossRef]
- Kusunose, T.; Sekino, T. Thermal conductivity of hot-pressed hexagonal boron nitride. Scr. Mater. 2016, 124, 138–141. [Google Scholar] [CrossRef]
- Ren, J.W.; Li, Q.H.; Yan, L.; Jia, L.C.; Huang, X.L.; Zhao, L.H.; Ran, Q.C.; Fu, M.L. Enhanced thermal conductivity of epoxy composites by introducing graphene@boron nitride nanosheets hybrid nanoparticles. Mater. Des. 2020, 191, 108663. [Google Scholar] [CrossRef]
- Chakraborty, P.; Xiong, G.P.; Cao, L.; Wang, Y. Lattice thermal transport in superhard hexagonal diamond and wurtzite boron nitride: A comparative study with cubic diamond and cubic boron nitride. Carbon 2018, 139, 85–93. [Google Scholar] [CrossRef]
- Zheng, J.C.; Zhang, L.; Kretinin, A.V.; Morozov, S.V.; Wang, Y.B.; Wang, T.; Li, X.J.; Ren, F.; Zhang, J.Y.; Lu, C.Y.; et al. High thermal conductivity of hexagonal boron nitride laminates. 2D Mater. 2016, 3, 011004. [Google Scholar] [CrossRef]
- Lewis, J.S.; Barani, Z.; Magana, A.S.; Kargar, F.; Balandin, A.A. Thermal and electrical conductivity control in hybrid composites with graphene and boron nitride fillers. Mater. Res. Express 2019, 6, 8. [Google Scholar] [CrossRef]
- Ou, X.H.; Chen, S.S.; Lu, X.M.; Lu, Q.H. Enhancement of thermal conductivity and dimensional stability of polyimide/boron nitride films through mechanochemistry. Compos. Commun. 2021, 23, 100549. [Google Scholar] [CrossRef]
- Wang, S.; Xue, H.Q.; Araby, S.; Demiral, M.; Han, S.S.; Cui, C.; Zhang, R.; Meng, Q.S. Thermal conductivity and mechanical performance of hexagonal boron nitride nanosheets-based epoxy adhesives. Nanotechnology 2021, 32, 355707. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Moradi, S. Thermal Conductivity and Cure Kinetics of Epoxy-Boron Nitride Composites—A Review. Materials 2020, 13, 3634. [Google Scholar] [CrossRef]
- Zhong, B.; Zou, J.X.; An, L.L.; Ji, C.Y.; Huang, X.X.; Liu, W.; Yu, Y.L.; Wang, H.T.; Wen, G.W.; Zhao, K.; et al. The effects of the hexagonal boron nitride nanoflake properties on the thermal conductivity of hexagonal boron nitride nanoflake/silicone rubber composites. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105629. [Google Scholar] [CrossRef]
- Sun, N.; Sun, J.J.; Zeng, X.L.; Chen, P.; Qian, J.S.; Xia, R.; Sun, R. Hot-pressing induced orientation of boron nitride in polycarbonate composites with enhanced thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2018, 110, 45–52. [Google Scholar] [CrossRef]
- Yu, C.P.; Zhang, J.; Li, Z.; Tian, W.; Wang, L.J.; Luo, J.; Li, Q.L.; Fan, X.D.; Yao, Y.G. Enhanced through-plane thermal conductivity of boron nitride/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 25–31. [Google Scholar] [CrossRef]
- Ahn, H.J.; Cha, S.H.; Lee, W.S.; Kim, E.S. Effects of amphiphilic agent on thermal conductivity of boron nitride/poly(vinyl butyral) composites. Thermochim. Acta 2014, 591, 96–100. [Google Scholar] [CrossRef]
- Yu, J.H.; Mo, H.L.; Jiang, P.K. Polymer/boron nitride nanosheet composite with high thermal conductivity and sufficient dielectric strength. Polym. Adv. Technol. 2015, 26, 514–520. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhan, C.H.; You, Y.; Tong, L.F.; Wei, R.B.; Liu, X.B. Preparation and thermal conductivity of copper phthalocyanine grafted boron nitride nanosheets. Mater. Lett. 2018, 227, 33–36. [Google Scholar] [CrossRef]
- Han, W.F.; Chen, M.Y.; Song, W.; Ge, C.H.; Zhang, X.D. Construction of hexagonal boron nitride@polystyrene nanocomposite with high thermal conductivity for thermal management application. Ceram. Int. 2020, 46, 7595–7601. [Google Scholar] [CrossRef]
- Guan, J.W.; Ashrafi, B.; Martinez-Rubi, Y.; Jakubinek, M.B.; Rahmat, M.; Kim, K.S.; Simard, B. Epoxy resin nanocomposites with hydroxyl (OH) and amino (NH2) functionalized boron nitride nanotubes. Nanocomposites 2018, 4, 10–17. [Google Scholar] [CrossRef]
- Terao, T.; Bando, Y.; Mitome, M.; Zhi, C.; Tang, C.; Golberg, D. Thermal Conductivity Improvement of Polymer Films by Catechin-Modified Boron Nitride Nanotubes. J. Phys. Chem. C 2009, 113, 13605–13609. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Li, J.Q.; Leng, J.; Zhang, J. Extrusion-Based Additive Manufacturing Samples with Desirable Thermal Conductivities Prepared by Incorporating Hybrid Hexagonal Boron Nitride(h-BN) and Novel Process Strategy. Macromol. Mater. Eng. 2022, 307, 2100715. [Google Scholar] [CrossRef]
- Yang, N.; Ji, H.F.; Jiang, X.X.; Qu, X.W.; Zhang, X.J.; Zhang, Y.; Liu, B.Y. Preparation of Boron Nitride Nanoplatelets via Amino Acid Assisted Ball Milling: Towards Thermal Conductivity Application. Nanomaterials 2020, 10, 1652. [Google Scholar] [CrossRef]
- Kim, S.M.; Hsu, A.; Park, M.H.; Chae, S.H.; Yun, S.J.; Lee, J.S.; Cho, D.H.; Fang, W.J.; Lee, C.; Palacios, T.; et al. Synthesis of large-area multilayer hexagonal boron nitride for high material performance. Nat. Commun. 2015, 6, 8662. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, Y.; Yao, Y.; Pan, G.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.-B.; Song, B.; Wong, C.-P. Polymer Composite with Improved Thermal Conductivity by Constructing a Hierarchically Ordered Three-Dimensional Interconnected Network of BN. ACS Appl. Mater. Interfaces 2017, 9, 13544–13553. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Gao, X.; Wang, J.L.; He, W.; Silberschmidt, V.V.; Wang, S.X.; Tao, Z.H.; Xu, H. Properties and application of polyimide-based composites by blending surface functionalized boron nitride nanoplates. J. Appl. Polym. Sci. 2015, 132, 41889. [Google Scholar] [CrossRef]
- Ren, J.K.; Stagi, L.; Carbonaro, C.M.; Malfatti, L.; Casula, M.F.; Ricci, P.C.; Castillo, A.E.D.; Bonaccorso, F.; Calvillo, L.; Granozzi, G.; et al. Defect-assisted photoluminescence in hexagonal boron nitride nanosheets. 2D Mater. 2020, 7, 045023. [Google Scholar] [CrossRef]
- Burghaus, U. Surface chemistry of CO2—Adsorption of carbon dioxide on clean surfaces at ultrahigh vacuum. Prog. Surf. Sci. 2014, 89, 161–217. [Google Scholar] [CrossRef]
- Soong, Y.C.; Chiu, C.W. Multilayered graphene/boron nitride/thermoplastic polyurethane composite films with high thermal conductivity, stretchability, and washability for adjustable-cooling smart clothes. J. Colloid Interface Sci. 2021, 599, 611–619. [Google Scholar] [CrossRef]
- Guo, F.H.; Zhao, J.; Li, F.X.; Kong, D.Y.; Guo, H.G.; Wang, X.; Hu, H.Q.; Zong, L.B.; Xu, J.T. Polar crystalline phases of PVDF induced by interaction with functionalized boron nitride nanosheets. CrystEngComm 2020, 22, 6207–6215. [Google Scholar] [CrossRef]
- Sainsbury, T.; Satti, A.; May, P.; Wang, Z.; McGovern, I.; Gun’ko, Y.K.; Coleman, J. Oxygen Radical Functionalization of Boron Nitride Nanosheets. J. Am. Chem. Soc. 2012, 134, 18758–18771. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Stagi, L.; Innocenzi, P. Hydroxylated boron nitride materials: From structures to functional applications. J. Mater. Sci. 2021, 56, 4053–4079. [Google Scholar] [CrossRef]
- Bhang, J.; Ma, H.; Yim, D.; Galli, G.; Seo, H. First-Principles Predictions of Out-of-Plane Group IV and V Dimers as High-Symmetry, High-Spin Defects in Hexagonal Boron Nitride. Acs Appl. Mater. Interfaces 2021, 13, 45768–45777. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.-Y.; Jung, H.-B.; Kim, M.-K.; Lim, J.-H.; Kim, J.-Y.; Ryu, J.; Jeong, D.-Y. Enhanced Energy Storage Performance of Polymer/Ceramic/Metal Composites by Increase of Thermal Conductivity and Coulomb-Blockade Effect. ACS Appl. Mater. Interfaces 2021, 13, 27343–27352. [Google Scholar] [CrossRef]
- de los Reyes, C.A.; Hernández, K.; Martínez-Jiménez, C.; Walz Mitra, K.L.; Ginestra, C.; Smith McWilliams, A.D.; Pasquali, M.; Martí, A.A. Tunable Alkylation of White Graphene (Hexagonal Boron Nitride) Using Reductive Conditions. J. Phys. Chem. C 2019, 123, 19725–19733. [Google Scholar] [CrossRef]
- Ren, L.L.; Zeng, X.L.; Sun, R.; Xu, J.B.; Wong, C.P. Spray-assisted assembled spherical boron nitride as fillers for polymers with enhanced thermally conductivity. Chem. Eng. J. 2019, 370, 166–175. [Google Scholar] [CrossRef]
- Yazdan, A.; Wang, J.Z.; Nan, C.W.; Li, L.L. Rheological Behavior and Thermal Conductivities of Emulsion-Based Thermal Pastes. J. Electron. Mater. 2020, 49, 2100–2109. [Google Scholar] [CrossRef]
- Terao, T.; Zhi, C.; Bando, Y.; Mitome, M.; Tang, C.; Golberg, D. Alignment of Boron Nitride Nanotubes in Polymeric Composite Films for Thermal Conductivity Improvement. J. Phys. Chem. C 2010, 114, 4340–4344. [Google Scholar] [CrossRef]
- Huang, X.; Iizuka, T.; Jiang, P.; Ohki, Y.; Tanaka, T. Role of Interface on the Thermal Conductivity of Highly Filled Dielectric Epoxy/AlN Composites. J. Phys. Chem. C 2012, 116, 13629–13639. [Google Scholar] [CrossRef]
- Jiang, Y.; Yilmaz, N.E.D.; Barker, K.P.; Baek, J.; Xia, Y.; Zheng, X.L. Enhancing Mechanical and Combustion Performance of Boron/Polymer Composites via Boron Particle Functionalization. ACS Appl. Mater. Interfaces 2021, 13, 28908–28915. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.F.; Li, Y.; Gao, C.; Tian, X.J.; Wu, N.; Geng, Z.S.; Che, S.; Yang, F.; Li, Y.F. Three-dimensional skeleton assembled by carbon nanotubes/boron nitride as filler in epoxy for thermal management materials with high thermal conductivity and electrical insulation. Compos. Part B Eng. 2021, 224, 109168. [Google Scholar] [CrossRef]
- Xu, C.Y.; Miao, M.; Jiang, X.F.; Wang, X.B. Thermal conductive composites reinforced via advanced boron nitride nanomaterials. Compos. Commun. 2018, 10, 103–109. [Google Scholar] [CrossRef]
- Xu, C.K.; Wei, C.M.; Li, Q.H.; Li, Z.H.; Zhang, Z.X.; Ren, J.W. Robust Biomimetic Nacreous Aramid Nanofiber Composite Films with Ultrahigh Thermal Conductivity by Introducing Graphene Oxide and Edge-Hydroxylated Boron Nitride Nanosheet. Nanomaterials 2021, 11, 2544. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, Z.; Hu, Q.; Zhang, Y.; Wang, F.; Wang, H.; Shu, Y. Proportional Optimization Model of Multiscale Spherical BN for Enhancing Thermal Conductivity. ACS Appl. Electron. Mater. 2022, 4, 4659–4667. [Google Scholar] [CrossRef]
- Li, C.N.; Cao, X.W.; Tong, Y.Z.; Yang, Z.T.; Gao, D.L.; Ru, Y.; He, G.J. Hybrid Filler with Nanoparticles Grown in Situ on the Surface for the Modification of Thermal Conductive and Insulating Silicone Rubber. ACS Appl. Polym. Mater. 2022, 4, 7152–7161. [Google Scholar] [CrossRef]
- Christensen, G.; Lou, D.; Hong, H.P.; Peterson, G.P. Improved thermal conductivity of fluids and composites using boron nitride (BN) nanoparticles through hydrogen bonding. Thermochim. Acta 2021, 700, 178927. [Google Scholar] [CrossRef]
- Wei, Q.G.; Yang, D. Formation of Thermally Conductive Network Accompanied by Reduction of Interface Resistance for Thermal Conductivity Enhancement of Silicone Rubber. ACS Appl. Electron. Mater. 2022, 4, 3503–3511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).