Potential Lubricating Mechanism of Hyaluronic Acid for a Reduction of Albumin-Mediated Friction in the Artificial Joint System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Solutions
2.2. Friction Testing
2.3. Measurement of the Structural Changes of Thermally Processed Albumin
2.4. Adsorption of Fluorescent-Labeled HSA on CoCrMo Alloy Disc
2.5. Viscosity Analysis of Different Solutions
2.6. Statistical Analysis
3. Results
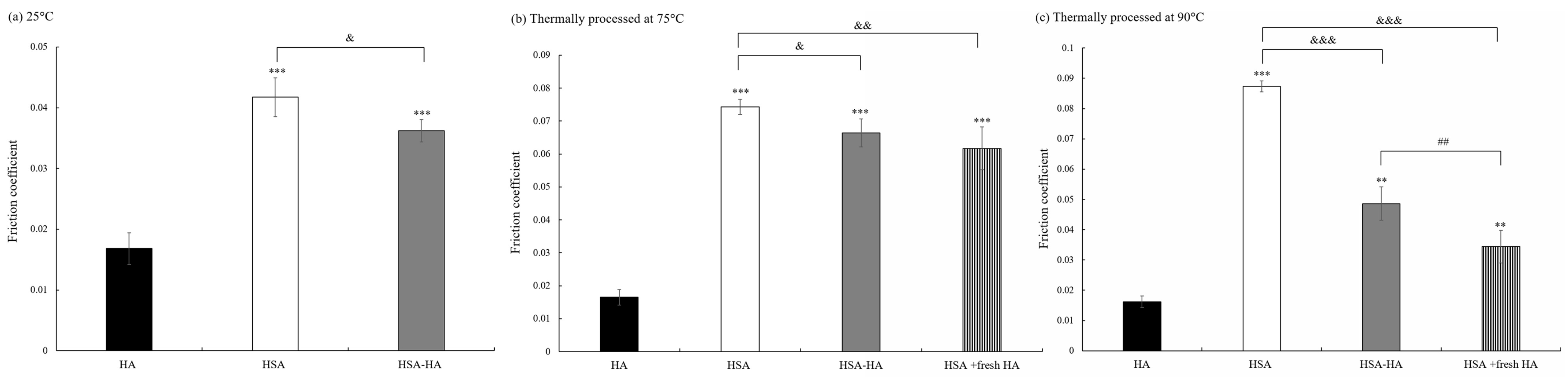
3.1. Thee Effect of Thermally Processed Albumin with or without HA on Friction Coefficient
3.2. Thermally Processed Albumin Reduces Its α-Helix Content
3.3. Thermally Processed Albumin Is More Easily Adsorbed on the Surface of CoCrMo Alloy Disc
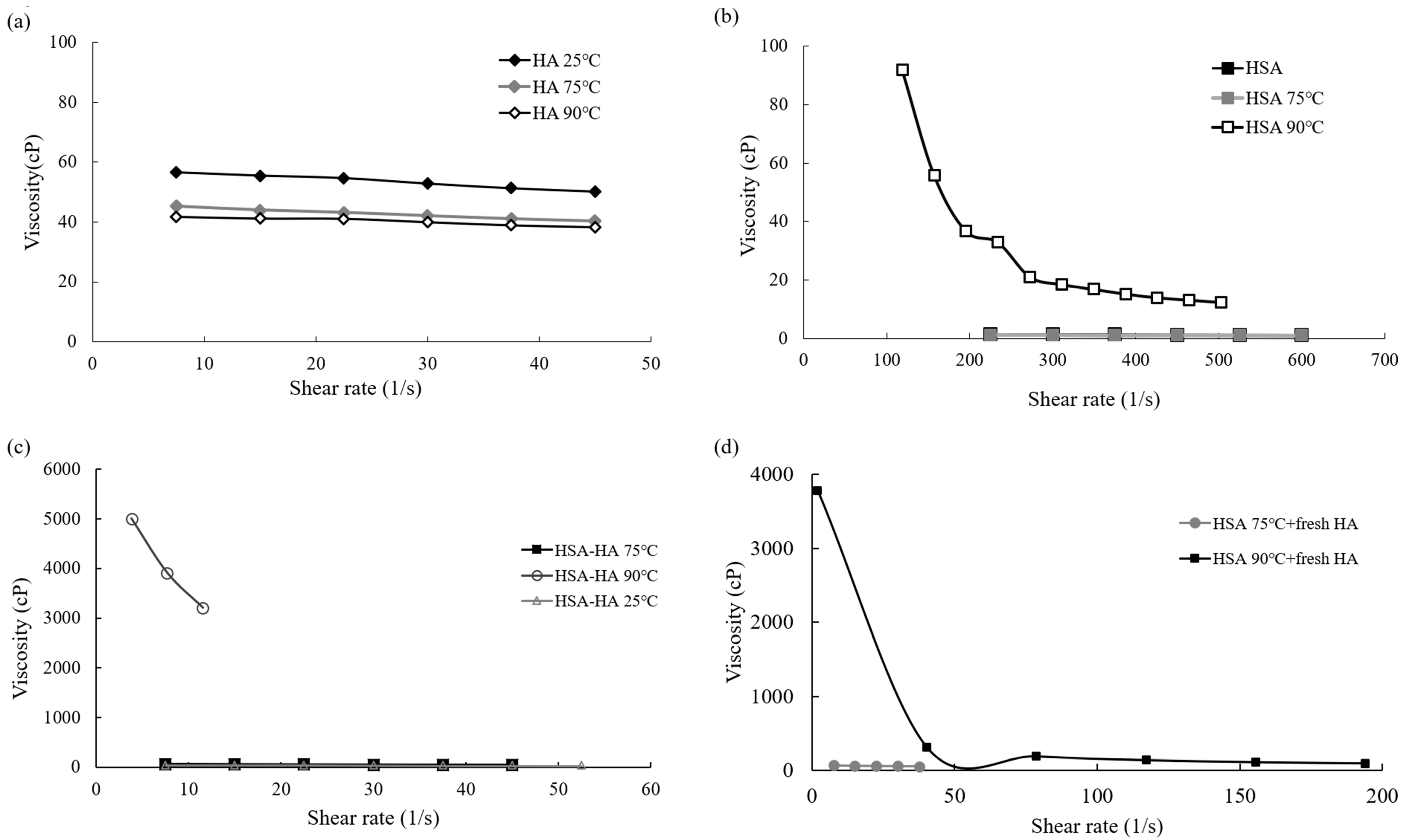
3.4. Viscosity Characteristics of HSA and HA after Being Thermally Processed
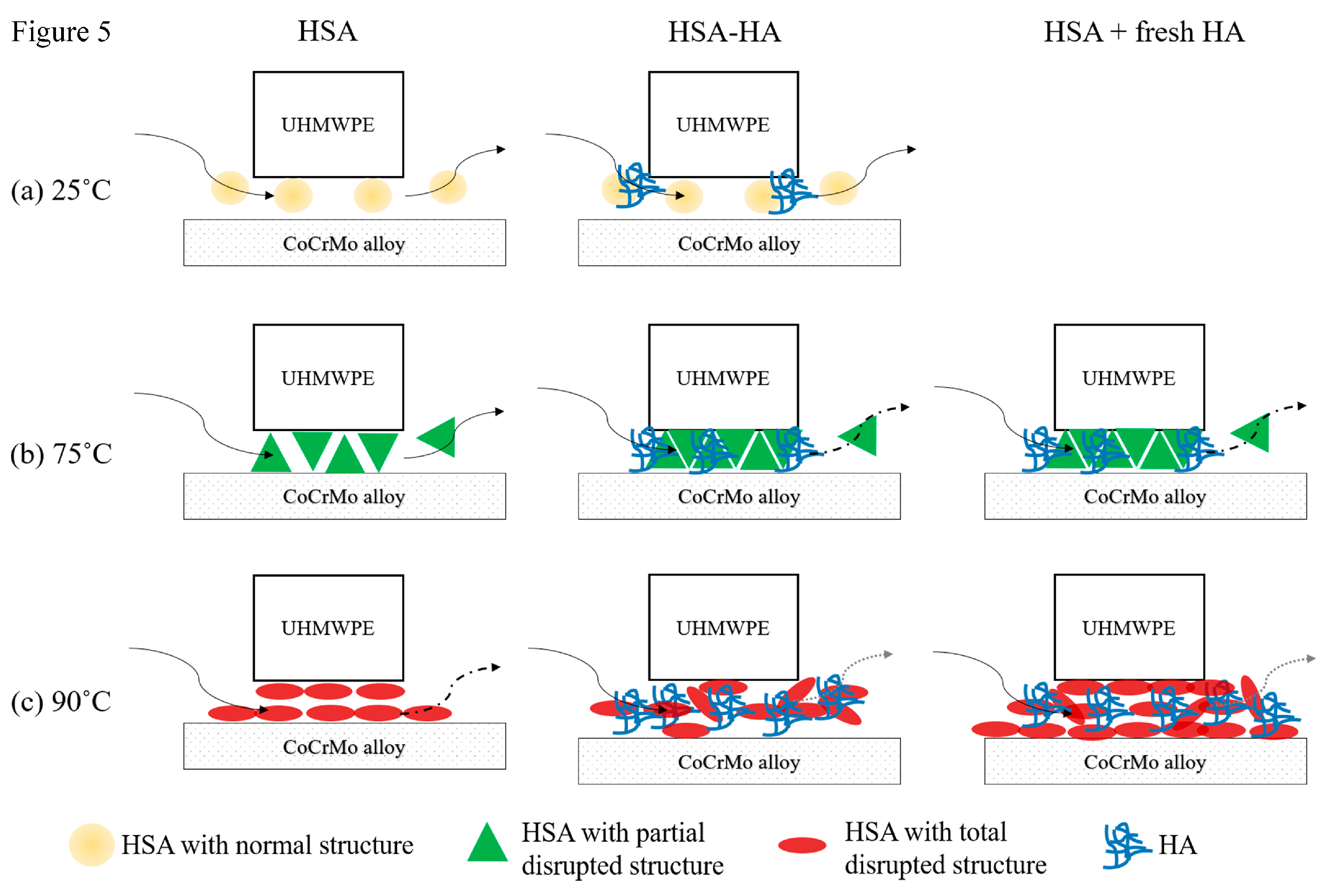
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van der Woude, J.A.; Nair, S.C.; Custers, R.J.; van Laar, J.M.; Kuchuck, N.O.; Lafeber, F.P.; Welsing, P.M. Knee joint distraction compared to total knee arthroplasty for treatment of end stage osteoarthritis: Simulating long-term outcomes and cost-effectiveness. PLoS ONE 2016, 11, e0155524. [Google Scholar] [CrossRef]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Sabatini, L.; Aprato, A.; Bracco, P.; Bellare, A. Ultra-high molecular weight polyethylene (uhmwpe) for hip and knee arthroplasty: The present and the future. J. Orthop. 2021, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Kandahari, A.M.; Yang, X.; Laroche, K.A.; Dighe, A.S.; Pan, D.; Cui, Q. A review of uhmwpe wear-induced osteolysis: The role for early detection of the immune response. Bone Res. 2016, 4, 16014. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Choudhury, D.; Roy, T.; Moradi, A.; Masjuki, H.H.; Pingguan-Murphy, B. Tribological performance of the biological components of synovial fluid in artificial joint implants. Sci. Technol. Adv. Mater. 2015, 16, 045002. [Google Scholar] [CrossRef] [PubMed]
- Watterson, J.R.; Esdaile, J.M. Viscosupplementation: Therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J. Am. Acad. Orthop. Surg. 2000, 8, 277–284. [Google Scholar] [CrossRef]
- Forsey, R.W.; Fisher, J.; Thompson, J.; Stone, M.H.; Bell, C.; Ingham, E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials 2006, 27, 4581–4590. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, K.; Tsukamoto, Y.; Obara, T.; Yamaguchi, T. The effect of additive hyaluronic acid on animal joints with experimentally reduced lubricating ability. J. Biomed. Mater. Res. 1994, 28, 865–870. [Google Scholar] [CrossRef]
- Huang, H.T.; Fang, H.W. Lubricating characteristics of hyaluronic acid molecules on the albumin-mediated tribological processes. Biomed. Eng. 2012, 24, 557–562. [Google Scholar] [CrossRef]
- Serro, A.P.; Colaco, R.; Saramago, B. Effect of albumin adsorption on biotribological properties of artificial joint materials. In Proteins at Interfaces III State of the Art; Acs Symposium Series; American Chemical Society: Washington, DC, USA, 2012; pp. 497–523. [Google Scholar]
- Lu, Z.; McKellop, H. Frictional heating of bearing materials tested in a hip joint wear simulator. Proc. Inst. Mech. Eng. H 1997, 211, 101–108. [Google Scholar] [CrossRef]
- Das, N.K.; Ghosh, N.; Kale, A.P.; Mondal, R.; Anand, U.; Ghosh, S.; Tiwari, V.K.; Kapur, M.; Mukherjee, S. Temperature induced morphological transitions from native to unfolded aggregated states of human serum albumin. J. Phys. Chem. B 2014, 118, 7267–7276. [Google Scholar] [CrossRef]
- Fang, H.W.; Hsieh, M.C.; Huang, H.T.; Tsai, C.Y.; Chang, M.H. Conformational and adsorptive characteristics of albumin affect interfacial protein boundary lubrication: From experimental to molecular dynamics simulation approaches. Colloids Surf. B Biointerfaces 2009, 68, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mishina, H.; Kojima, M. Changes in human serum albumin on arthroplasty frictional surfaces. Wear 2008, 265, 655–663. [Google Scholar] [CrossRef]
- Yoon, J.; Ha, S.; Lee, S.; Chae, S.-W. Analysis of contact pressure at knee cartilage during gait with respect to foot progression angle. Int. J. Precis. Eng. Manuf. 2018, 19, 761–766. [Google Scholar] [CrossRef]
- Li, G.; Kozanek, M.; Hosseini, A.; Liu, F.; Van de Velde, S.K.; Rubash, H.E. New fluoroscopic imaging technique for investigation of 6dof knee kinematics during treadmill gait. J. Orthop. Surg. Res. 2009, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.W. An interaction of albumin with hyaluronic acid and chondroitin sulfate: A study of affinity chromatography and circular dichroism. Biopolymers 1980, 19, 1407–1414. [Google Scholar] [CrossRef]
- Jamaledin, R.; Sartorius, R.; Di Natale, C.; Vecchione, R.; De Berardinis, P.; Netti, P.A. Recombinant filamentous bacteriophages encapsulated in biodegradable polymeric microparticles for stimulation of innate and adaptive immune responses. Microorganisms 2020, 8, 650. [Google Scholar] [CrossRef]
- Serro, A.P.; Gispert, M.P.; Martins, M.C.; Brogueira, P.; Colaco, R.; Saramago, B. Adsorption of albumin on prosthetic materials: Implication for tribological behavior. J. Biomed. Mater. Res. A 2006, 78, 581–589. [Google Scholar] [CrossRef]
- Gleghorn, J.P.; Bonassar, L.J. Lubrication mode analysis of articular cartilage using stribeck surfaces. J. Biomech. 2008, 41, 1910–1918. [Google Scholar] [CrossRef]
- Fang, H.W.; Su, Y.C.; Huang, H.T.; Tsai, W.B.; Liu, H.L. Investigation of the friction induced conformational change of protein and wear of uhmwpe by a wear process with microfabricated surfaces. Mater. Sci. Eng. C 2009, 29, 1118–1123. [Google Scholar] [CrossRef]
- Flannery, M.; Jones, E.; Birkinshaw, C. Analysis of wear and friction of total knee replacements part ii: Friction and lubrication as a function of wear. Wear 2008, 265, 1009–1016. [Google Scholar] [CrossRef]
- Flannery, M.; McGloughlin, T.; Jones, E.; Birkinshaw, C. Analysis of wear and friction of totak knee replacements: Part i. Wear assessment of a three station wear simulator. Wear 2008, 265, 999–1008. [Google Scholar] [CrossRef]
- Murakami, T.; Yarimitsu, S.; Nakashima, K.; Sawae, Y.; Sakai, N. Influence of synovia constituents on tribological behaviors of articular catilage. Friction 2013, 1, 150–162. [Google Scholar] [CrossRef]
- Oates, K.M.; Krause, W.E.; Jones, R.L.; Colby, R.H. Rheopexy of synovial fluid and protein aggregation. J. R Soc. Interface 2006, 3, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mirea, D.A.; Trunfio-Sfarghiu, A.-M.; Matei, C.I.; Munteanu, B.; Piednoir, A.; Rieu, J.P.; Blanchin, M.G.; Berthier, Y. Role of the biomolecular interactions in the structure and tribological properties of synovial fluid. Tribol. Int. 2013, 59, 302–311. [Google Scholar] [CrossRef]
- Kruszewska, N.; Mazurkiewicz, A.; Szala, G.; Slomion, M. Characterization of synovial fluid components: Albumin-chondroitin sulfate interactions seen through molecular dynamics. Materials 2022, 15, 6935. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.B.; Huang, H.T.; Chen, C.C.; Lai, Y.H.; Huang, C.H.; Lu, Y.C.; Fang, H.W. The role of synovial biomolecules nano-tribology in the articulation between artificial joint materials. Curr. Nanosci. 2014, 10, 179–184. [Google Scholar] [CrossRef]
- Necas, D.; Vrbka, M.; Marian, M.; Rothammer, B.; Tremmel, S.; Wartzack, S.; Galandakova, A.; Gallo, J.; Wimmer, M.A.; Krupka, I.; et al. Towards the understanding of lubrication mechanisms in total knee replacements—Part i: Experimental investigation. Tribol. Int. 2021, 156, 106874. [Google Scholar] [CrossRef]
- Poliakov, A.; Pakhaliuk, V.; Popov, V.L. Current trends in improving of artificial joints design and technologies for their arthroplasty. Front. Mech. Eng. 2020, 6, 4. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-Y.; Lu, Y.-F.; Lu, Y.-C.; Huang, C.-H.; Fang, H.-W. Potential Lubricating Mechanism of Hyaluronic Acid for a Reduction of Albumin-Mediated Friction in the Artificial Joint System. Lubricants 2023, 11, 210. https://doi.org/10.3390/lubricants11050210
Su C-Y, Lu Y-F, Lu Y-C, Huang C-H, Fang H-W. Potential Lubricating Mechanism of Hyaluronic Acid for a Reduction of Albumin-Mediated Friction in the Artificial Joint System. Lubricants. 2023; 11(5):210. https://doi.org/10.3390/lubricants11050210
Chicago/Turabian StyleSu, Chen-Ying, Yi-Fang Lu, Yung-Chang Lu, Chang-Hung Huang, and Hsu-Wei Fang. 2023. "Potential Lubricating Mechanism of Hyaluronic Acid for a Reduction of Albumin-Mediated Friction in the Artificial Joint System" Lubricants 11, no. 5: 210. https://doi.org/10.3390/lubricants11050210
APA StyleSu, C.-Y., Lu, Y.-F., Lu, Y.-C., Huang, C.-H., & Fang, H.-W. (2023). Potential Lubricating Mechanism of Hyaluronic Acid for a Reduction of Albumin-Mediated Friction in the Artificial Joint System. Lubricants, 11(5), 210. https://doi.org/10.3390/lubricants11050210




