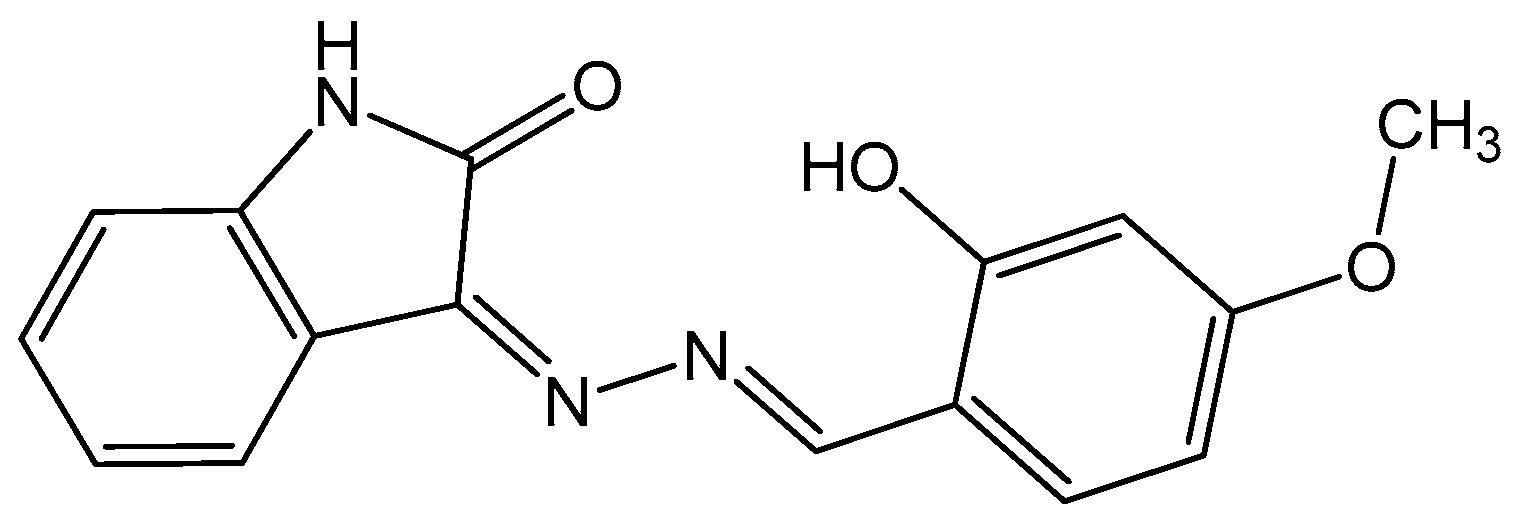
Exploring the Effectiveness of Isatin–Schiff Base as an Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid
Abstract
1. Introduction
2. Social and Economic Effects of Corrosion
3. Materials and Methods
3.1. Materials
3.2. Test Solutions
3.3. Gravimetric Analysis
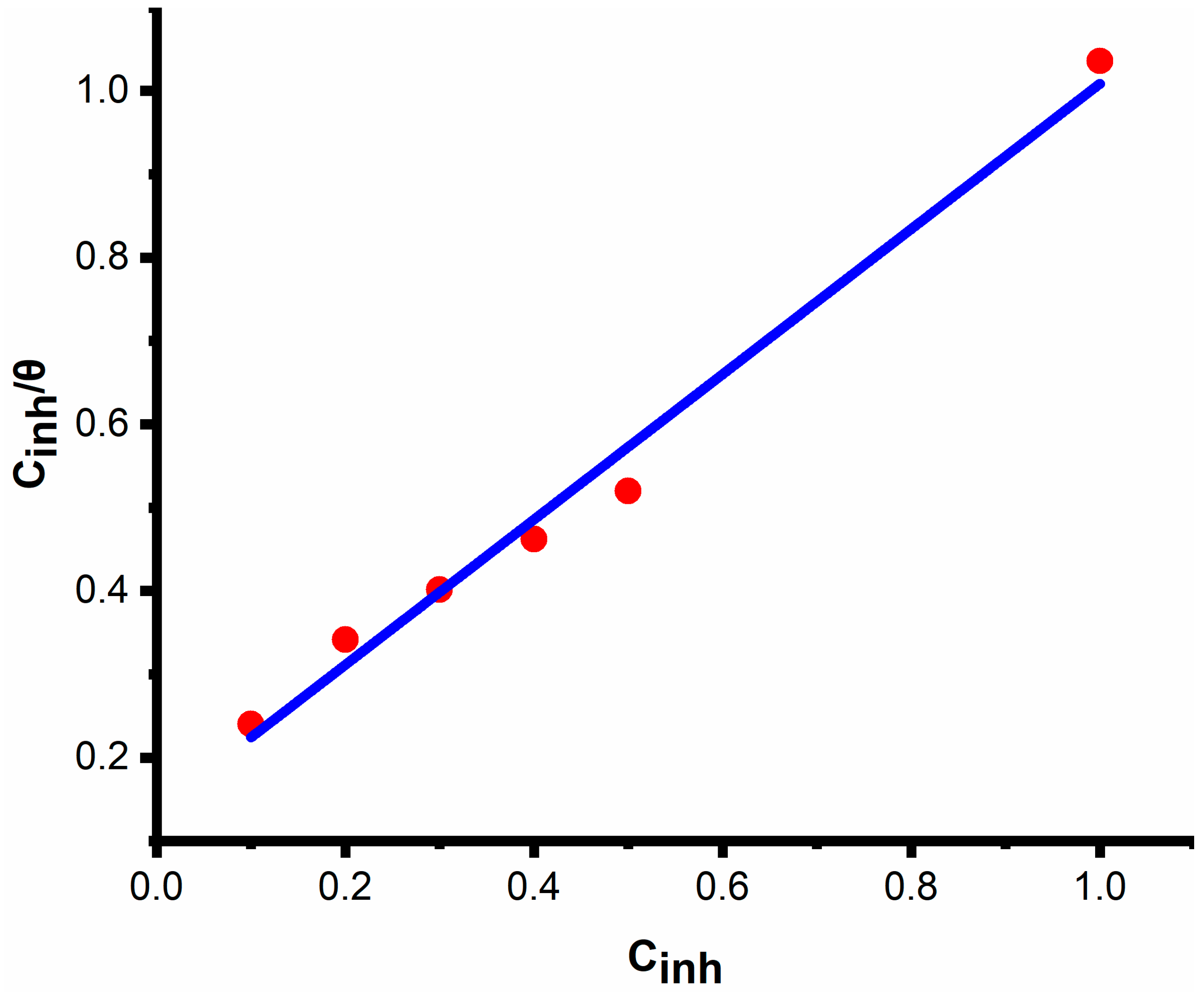
3.4. Adsorption Isotherms
3.5. Electrochemical Method
3.6. SEM
3.7. Computational Investigations
4. Results and Discussion
4.1. Gravimetrical Measurements
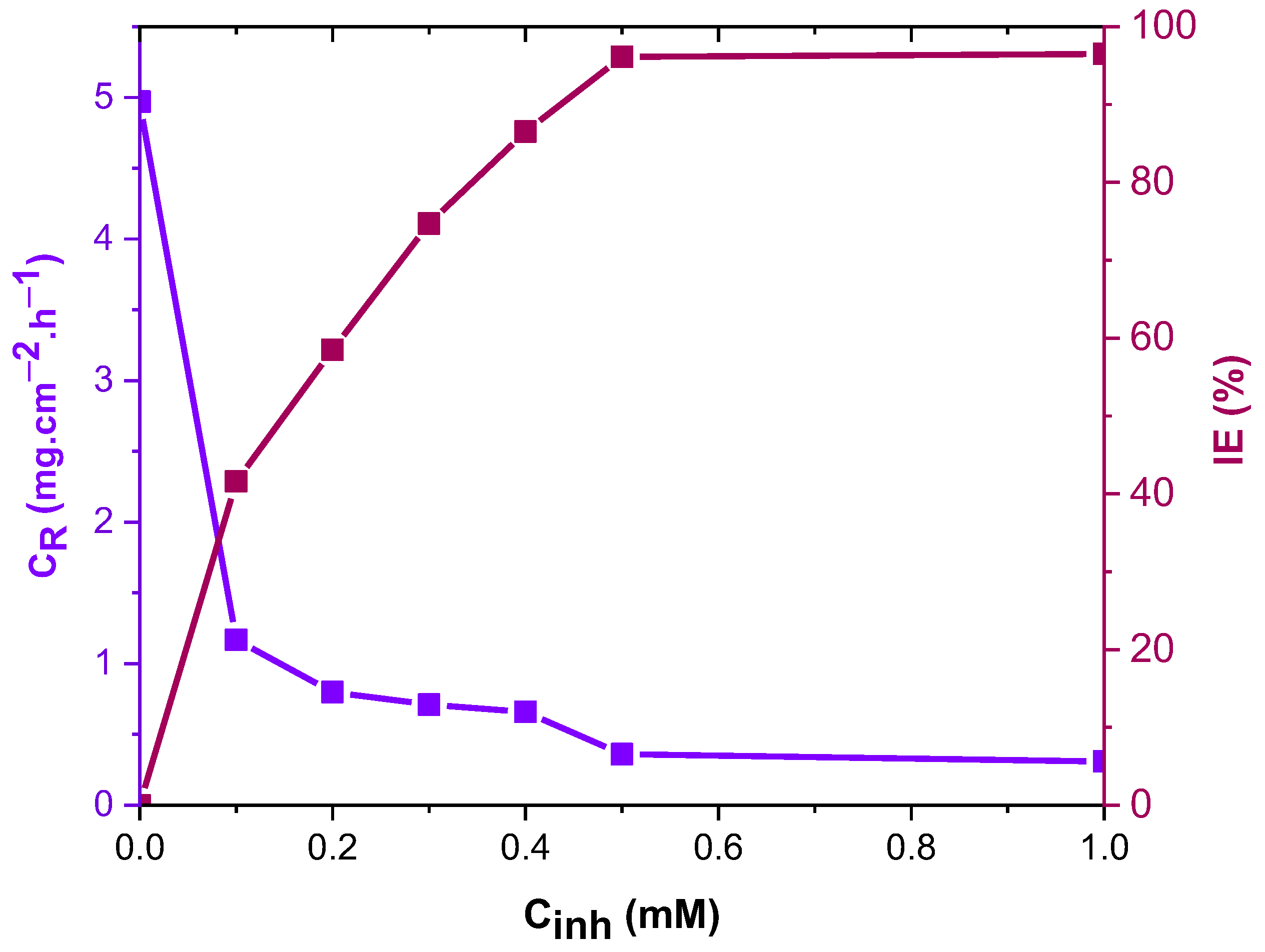
4.1.1. Effect of Concentration
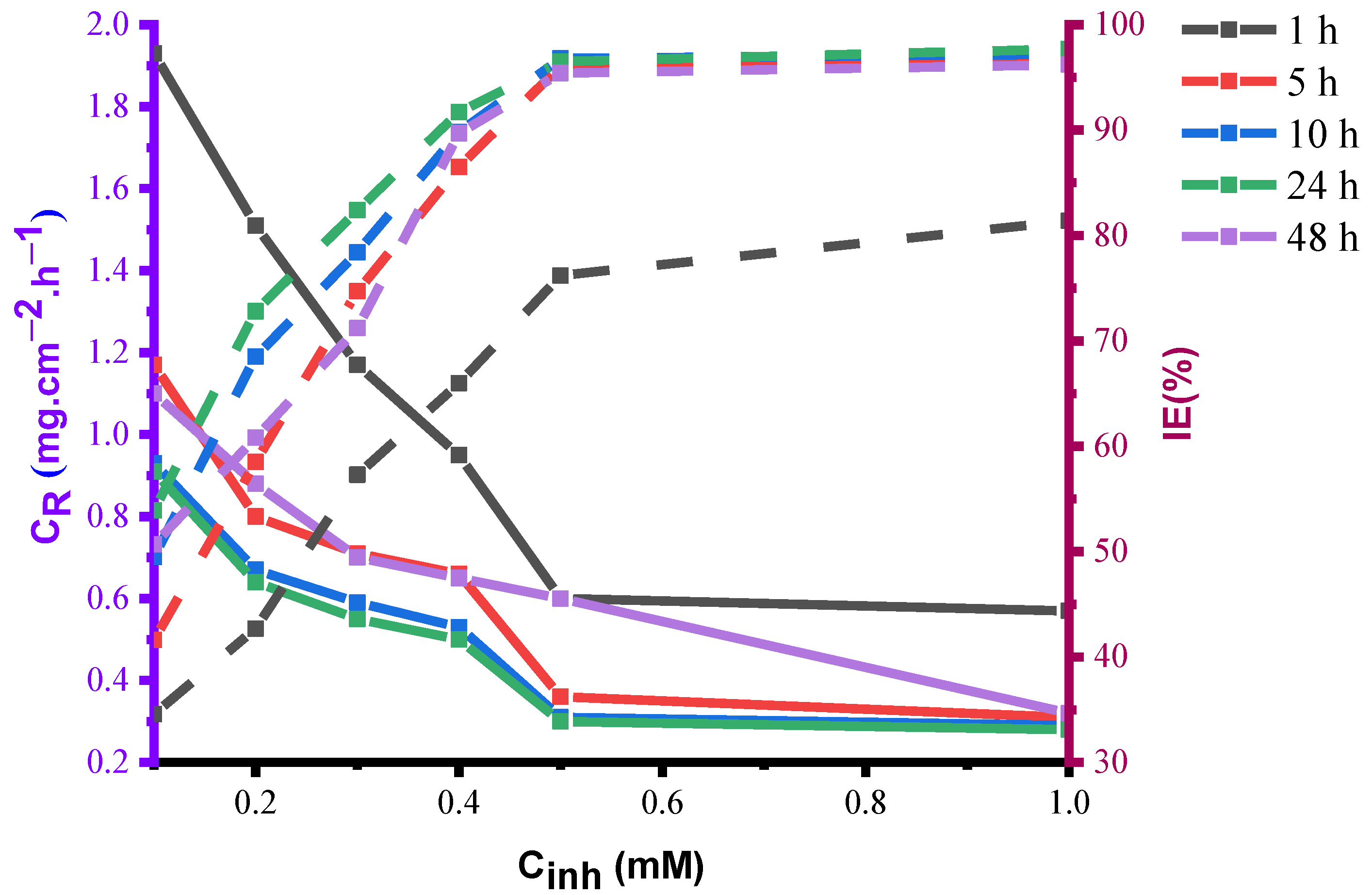
4.1.2. Effect of Immersion Periods
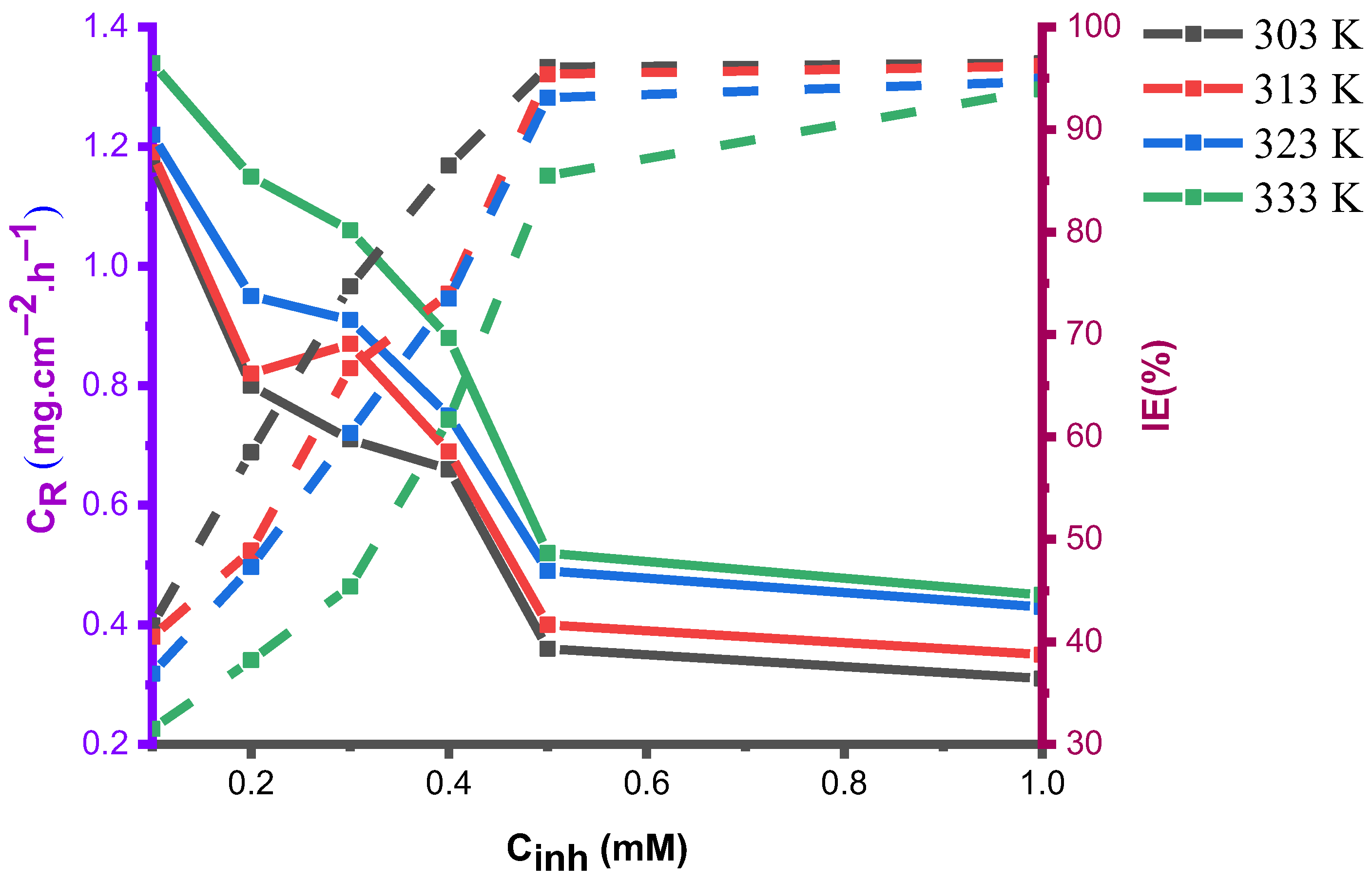
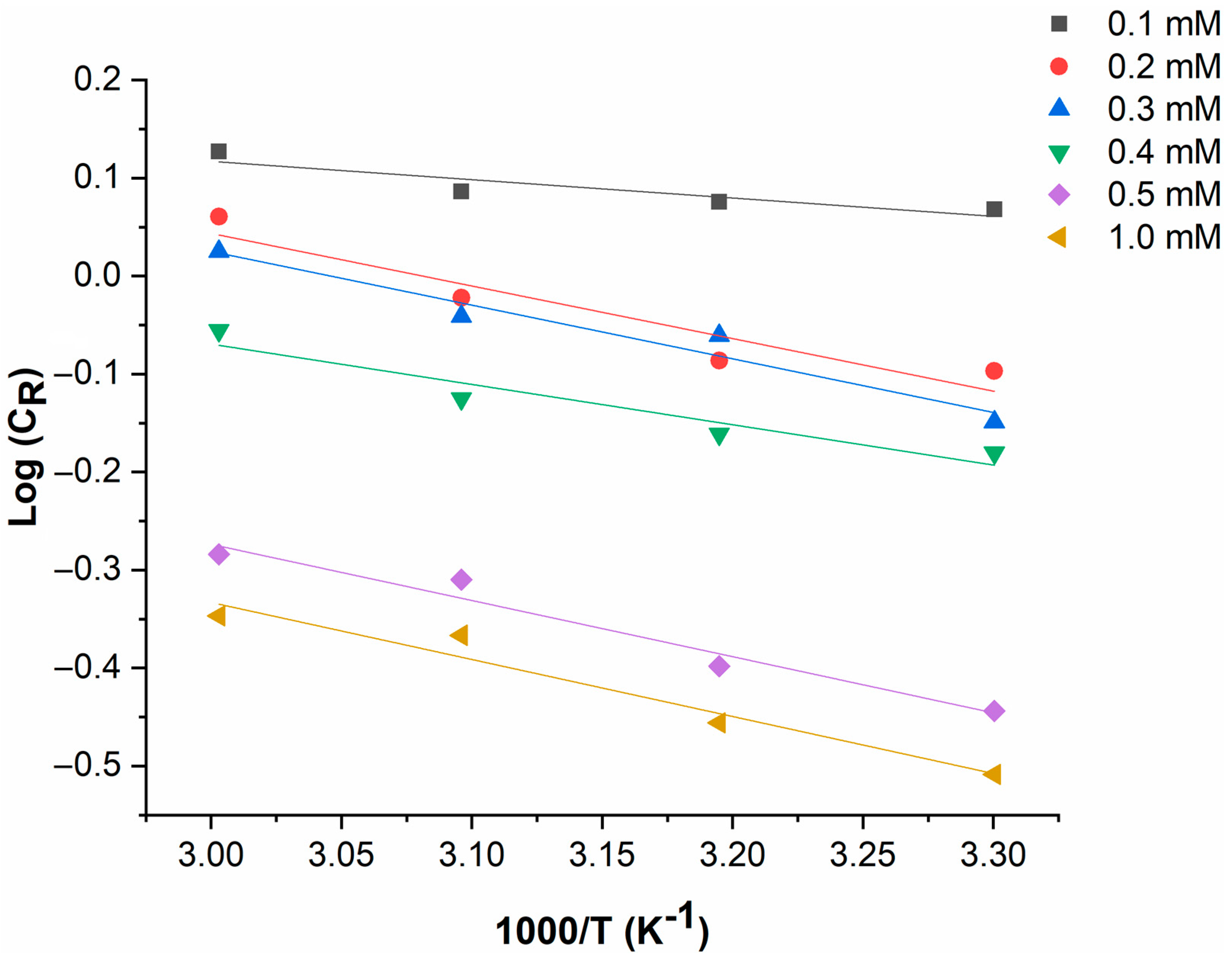
4.1.3. Effect of Temperature
4.1.4. Adsorption Isotherm
4.2. Electrochemical Measurements
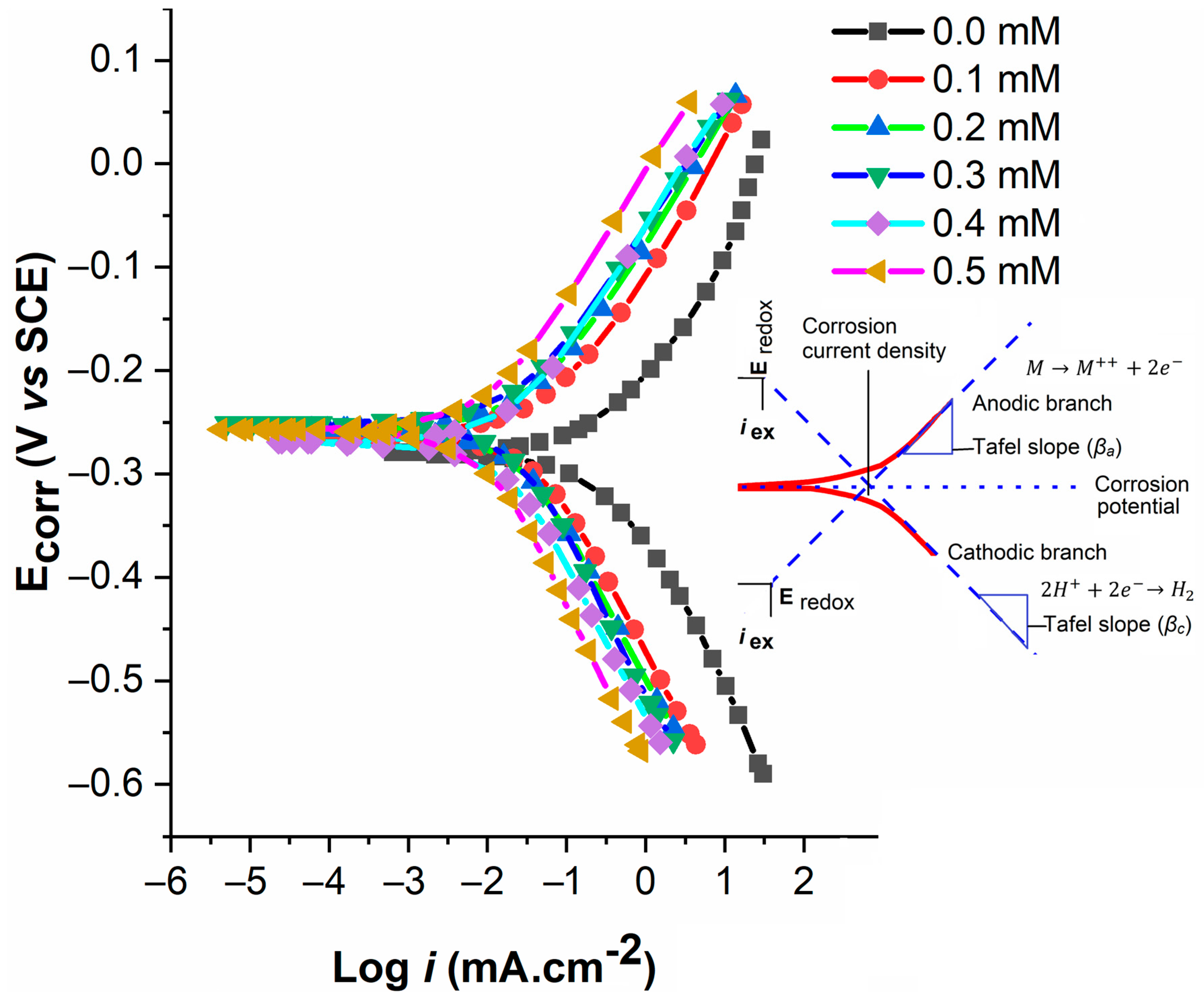
4.2.1. PDP
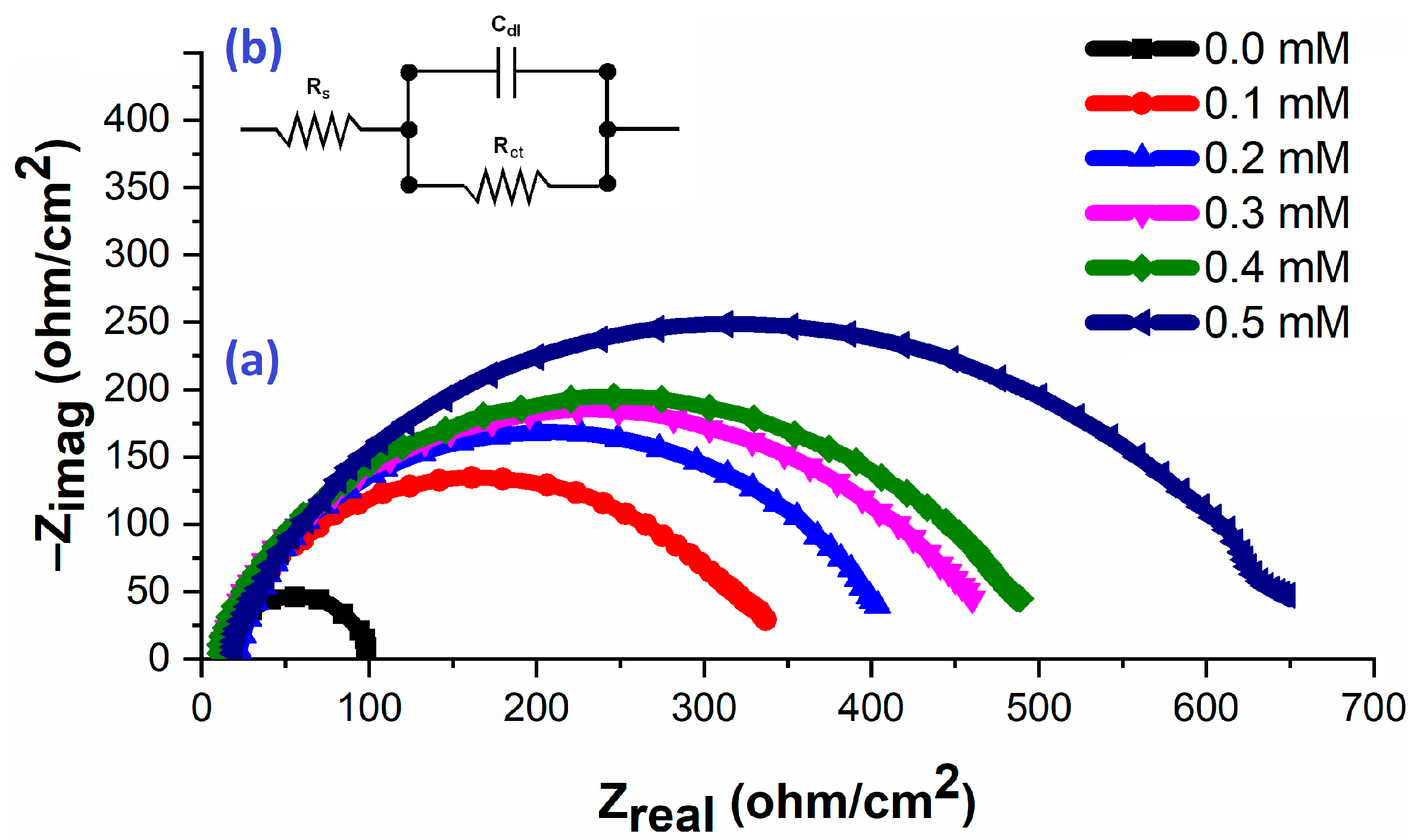
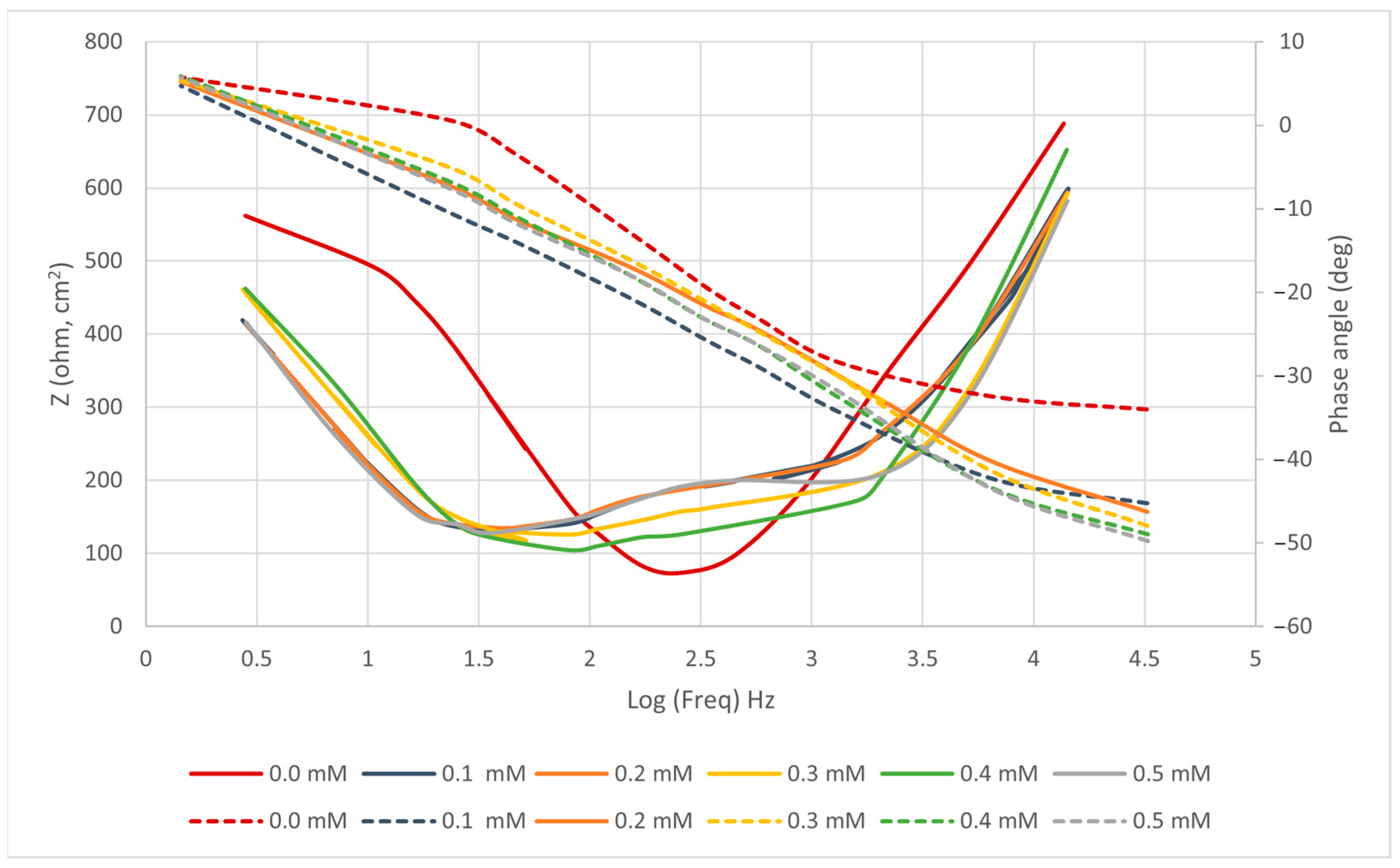
4.2.2. EIS
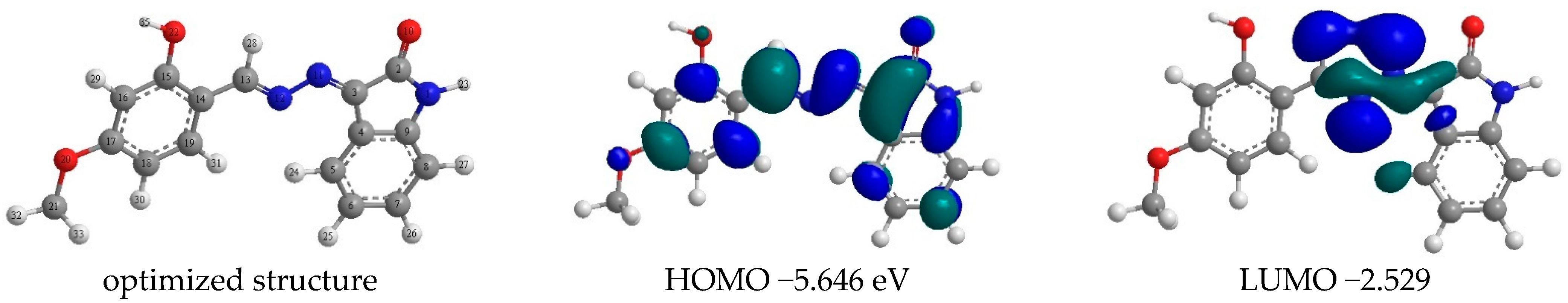
4.3. DFT
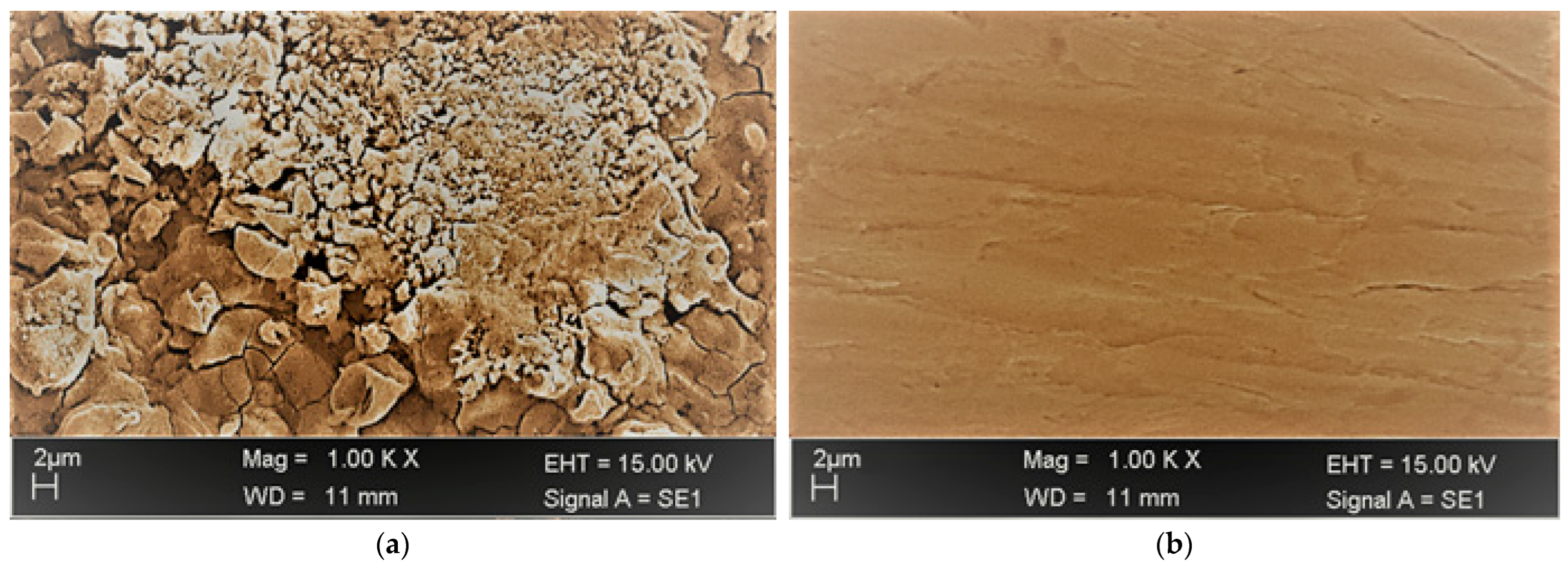
4.4. Surface Analysis
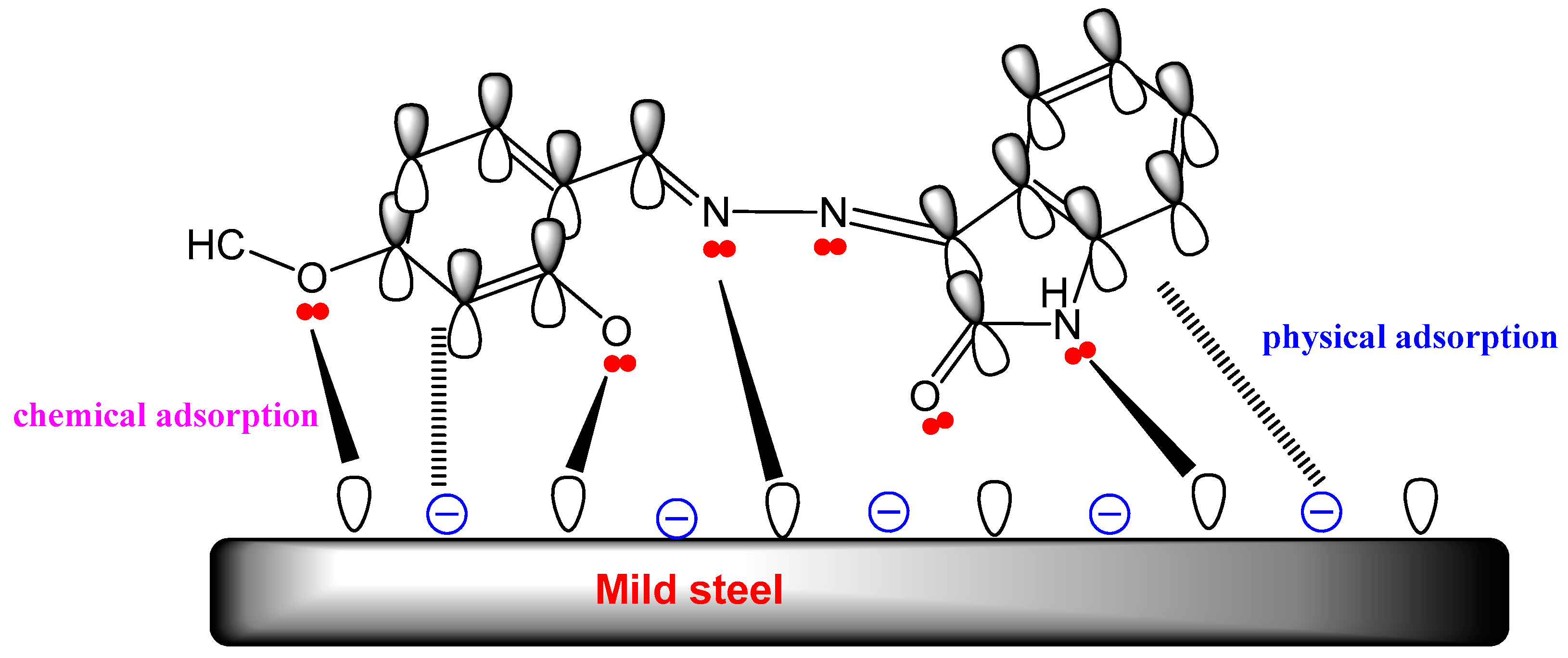
4.5. Suggested Mechanism
- Protonated molecules (ions) of the OHMHI are present in corrosive media. Through electrostatic contact, these ions are deposited on the mild steel interface where had earlier been adsorbed (physical adsorption).
- Through the chemical adsorption process, OHMHI can be adsorbed on the metallic surface by donating lone pairs of electrons from nitrogen and oxygen atoms to the vacant orbital of iron atoms.
- Pi-electrons from benzene ring and the unoccupied d-orbital of iron atoms interact as donors and acceptors [75].
5. Conclusions
6. Compared with Other Published Materials, What Does Ours Bring to the Field?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Materials 2020, 13, 740. [Google Scholar] [CrossRef]
- Yesudass, S.; Olasunkanmi, L.O.; Bahadur, I.; Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Experimental and Theoretical Studies on Some Selected Ionic Liquids with Different Cations/Anions as Corrosion Inhibi-tors for Mild Steel in Acidic Medium. J. Taiwan Inst. Chem. Eng. 2016, 64, 252. [Google Scholar] [CrossRef]
- Mansoor, B.; Majid, R.; Sina, R.; Mahdieh, B. Anti-corrosion behavior of 2-(((4-((2-morpholinoethyl)(pyridin-2-ylmethyl)amino)butyl)imino)methyl)naphthalen-1-ol on Mild Steel in Hydrochloric Acid solution: Experimental and theoretical studies. Thin Solid Films 2022, 762, 139558. [Google Scholar] [CrossRef]
- Desai, P.D.; Pawar, C.P.; Avhad, M.S.; More, A.P. Corrosion inhibitors for carbon steel: A review. Vietnam J. Chem. 2023, 61, 15. [Google Scholar]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef]
- Singh, A.K.; Thakur, S.; Pani, B.; Ebenso, E.E.; Quraishi, M.A.; Pandey, A.K. 2-Hydroxy-N-((Thiophene-2-yl) methylene) benzohydrazide: Ultrasound-Assisted Synthesis and Corrosion Inhibition Study. ACS Omega 2018, 3, 4695–4705. [Google Scholar] [CrossRef] [PubMed]
- Mwakalesi, A.J.; Nyangi, M. Effective Corrosion Inhibition of Mild Steel in an Acidic Environment Using an Aqueous Extract of Macadamia Nut Green Peel Biowaste. Eng. Proc. 2023, 31, 41. [Google Scholar] [CrossRef]
- Ali, N.; Yusof, M.S.; Khairul, W.M.; Rahamathullah, R.; Isa, M.I.; Nik, W.B. The Effect of Concentration of Lawsonia inermis as a Corrosion Inhibitor for Aluminum Alloy in Seawater. Adv. Phys. Chem. 2017, 2017, 8521623. [Google Scholar] [CrossRef]
- Haris, N.I.N.; Sobri, S.; Yusof, Y.A.; Kassim, N.K. An Overview of Molecular Dynamic Simulation for Corrosion Inhibition of Ferrous Metals. Metals 2021, 11, 46. [Google Scholar] [CrossRef]
- Youssefi, Y.; Ansari, A.; Ou-ani, O.; Oucheikh, L.; Oubair, A.; Lgaz, H.; Hammouti, B.; Chaouiki, A.; Ko, Y.G.; Znini, M. Insights into the Corrosion Inhibition Performance of Three 2-Isoxazoline-γ-Lactones for Carbon Steel in Acidic Medium: Linking Molecular and Experimental-Level Information with Microscopic-Scale Modeling. Lubricants 2023, 11, 141. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Zhang, Q.; Zhao, C.; Zhou, X.; Zheng, H.; Zhang, R.; Sun, Y.; Yan, Z. Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review. Molecules 2023, 28, 2832. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Cielecka-Piontek, J.; Nosal-Wiercińska, A.; Pietrzak, R. Adsorption of Organic Compounds on Adsorbents Obtained with the Use of Microwave Heating. Materials 2022, 15, 5664. [Google Scholar] [CrossRef]
- Sanni, O.; Iwarere, S.A.; Daramola, M.O. Investigation of Eggshell Agro-Industrial Waste as a Potential Corrosion Inhibitor for Mild Steel in Oil and Gas Industry. Sustainability 2023, 15, 6155. [Google Scholar] [CrossRef]
- Tan, B.; Xiang, B.; Zhang, S.; Qiang, Y.; Xu, L.; Chen, S.; He, J. Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid Interface Sci. 2021, 582, 918–931. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, B.; Yang, W.; Yin, X.; Liu, Y.; Chen, Y. Halogen-Substituted Imidazoline Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2015, 90, 284–295. [Google Scholar] [CrossRef]
- Farag, A.A.; Toghan, A.; Mostafa, M.S.; Lan, C.; Ge, G. Environmental Remediation through Catalytic Inhibition of Steel Corrosion by Schiff’s Bases: Electrochemical and Biological Aspects. Catalysts 2022, 12, 838. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study. Int. J. Mol. Sci. 2022, 23, 9360. [Google Scholar] [CrossRef]
- Inada, Y.; Mochizuki, K.; Tsuchiya, T.; Tsuji, H.; Funahashi, S. Equilibrium and kinetics of the dinuclear complex formation between N, N0 -ethylenebis (salicylideneiminato) copper (II) and metal (II, I) ions in acetonitrile. Inorg. Chim. Acta 2005, 358, 3009–3014. [Google Scholar] [CrossRef]
- Sheng, Y.; Hou, R.; Liu, C.; Xue, Z.; Zhang, K.; Li, J.; Guan, S. Tailoring of Biodegradable Magnesium Alloy Surface with Schiff Base Coating via Electrostatic Spraying for Better Corrosion Resistance. Metals 2022, 12, 471. [Google Scholar] [CrossRef]
- Maqbool, A.; Khan, N.Z.; Siddiquee, A.N. Towards Mg Based Light Materials of Future: Properties, Applications, Problems, and Their Mitigation. J. Manuf. Sci. Eng. 2022, 144, 030801. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Song, J.F.; She, J.; Chen, D.L.; Pan, F.S. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Azzawi, W.K.; Isahak, W.N.R.W. Isatin Schiff base is an effective corrosion inhibitor for mild steel in hydrochloric acid solution: Gravimetrical, electrochemical, and computational investigation. Sci. Rep. 2021, 12, 17773. [Google Scholar] [CrossRef]
- Ju, H.; Kai, Z.-P.; Li, Y. Aminic Nitrogen-Bearing Polydentate Schiff Base Compounds as Corrosion Inhibitors for Iron in Acidic Media: A Quantum Chemical Calculation. Corros. Sci. 2008, 50, 865–871. [Google Scholar] [CrossRef]
- Singh, A.K.; Quraishi, M.A. The Effect of Some Bis-Thiadiazole Derivatives on the Corrosion of Mild Steel in Hydrochloric Acid. Corros. Sci. 2010, 52, 1373–1385. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Zeng, X. Revealing the Correlation between Molecular Structure and Corrosion Inhibition Characteristics of N-Heterocycles in Terms of Substituent Groups. Materials 2023, 16, 2148. [Google Scholar] [CrossRef]
- Guo, L.; Kaya, S.; Obot, I.B.; Zheng, X.; Qiang, Y. Toward Understanding the Anticorrosive Mechanism of Some Thiourea Derivatives for Carbon Steel Corrosion: A Combined DFT and Molecular Dynamics Investigation. J. Colloid Interface Sci. 2017, 506, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.S.; Quraishi, M.A.; Nik, W.B.; Srivastava, V. Triazines as a Potential Class of Corrosion Inhibitors: Present Scenario, Challenges and Future Perspectives. J. Mol. Liq. 2021, 321, 114747. [Google Scholar] [CrossRef]
- Munawar, H.S.; Ullah, F.; Shahzad, D.; Heravi, A.; Qayyum, S.; Akram, J. Civil Infrastructure Damage and Corrosion Detection: An Application of Machine Learning. Buildings 2022, 12, 156. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. The Economic Impact of Corrosion. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/corrosion.pdf (accessed on 1 March 2023).
- ASTM G 31-72; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. American Society for Testing and Materials: Philadelphia, PA, USA, 1990.
- TM0169/G31-12a; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. NACE International: Houston, TX, USA, 2012.
- Talat, R.; Asghar, M.A.; Tariq, I.; Akhter, Z.; Liaqat, F.; Nadeem, L.; Haider, A.; Ali, S. Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media. Coatings 2022, 12, 1701. [Google Scholar] [CrossRef]
- Duke, B.J.; O’Leary, B. The Gaussian programs as a teaching tool: A case study on molecular hydrogen calculations. J. Chem. Educ. 1992, 69, 529. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian; Gaussian, Inc.: Wallingford, UK, 2013. [Google Scholar]
- Koopmans, T. Ordering of wave functions and eigenenergy’s to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS Investigation of the Corrosion Behavior of Steel Bars Embedded into Modified Concretes with Eggshell Contents. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Gómez-Sánchez, G.; Olivares-Xometl, O.; Arellanes-Lozada, P.; Likhanova, N.V.; Lijanova, I.V.; Arriola-Morales, J.; Díaz-Jiménez, V.; López-Rodríguez, J. Temperature Effect on the Corrosion Inhibition of Carbon Steel by Polymeric Ionic Liquids in Acid Medium. Int. J. Mol. Sci. 2023, 24, 6291. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Irfan, O.M.; Djavanroodi, F.; Asad, M. Development of Sustainable Inhibitors for Corrosion Control. Sustainability 2022, 14, 9502. [Google Scholar] [CrossRef]
- Pi, J.; Chen, M.; Chen, T.; Wang, Q.; Cheng, S.; Fu, C. Corrosion inhibition effect of 1-phenyl-5-mercaptotetrazole on nickel-aluminum bronze in seawater: A combined experi-mental and theoretical study. Colloids Surf. A: Physicochem. Eng. Asp. 2023, 666, 131354. [Google Scholar] [CrossRef]
- Kohl, M.; Alafid, F.; Bouška, M.; Krejčová, A.; Raycha, Y.; Kalendová, A.; Hrdina, R.; Burgert, L. New Corrosion Inhibitors Based on Perylene Units in Epoxy Ester Resin Coatings. Coatings 2022, 12, 923. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Thakur, S.; Pani, B.; Pandey, A.K.; Lgaz, H.; Chung, I.-M.; Ebenso, E.E. An Exploration about the Interaction of Mild Steel with Hydrochloric Acid in the Presence of N-(Benzo[d] Thiazole-2-yl)-1-Phenylethan-1-Imines. J. Phys. Chem. C 2019, 123, 22897. [Google Scholar] [CrossRef]
- Xie, S.-W.; Liu, Z.; Han, G.-C.; Li, W.; Liu, J.; Chen, Z. Molecular Dynamics Simulation of Inhibition Mechanism of 3,5-Dibromo Salicylaldehyde Schiff’s Base. Comput. Theor. Chem. 2015, 1063, 50–62. [Google Scholar] [CrossRef]
- Obot, I.B.; Haruna, K.; Saleh, T.A. Atomistic Simulation: A Unique and Powerful Computational Tool for Corrosion Inhibition Research. Arabian J. Sci. Eng. 2019, 44, 1. [Google Scholar] [CrossRef]
- Lgaz, H.; Chung, I.-M.; Salghi, R.; Ali, I.H.; Chaouiki, A.; El Aoufir, Y.; Khan, M.I. On the Understanding of the Adsorption of Fenugreek Gum on Mild Steel in an Acidic Medium: Insights from Experimental and Computational Studies. Appl. Surf. Sci. 2019, 463, 647–658. [Google Scholar] [CrossRef]
- Verma, C.; Lgaz, H.; Verma, D.; Ebenso, E.E.; Bahadur, I.; Quraishi, M. Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: A review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Zhu, L.; Li, S.; Li, X.; Zhang, W. Evaluation of Organic Corrosion Inhibitors in HCl Solution for Mild Steel Protection. Materials 2020, 13, 3176. [Google Scholar]
- Mousa, H.; Al-Mobarak, N. Effect of Pomegranate Peel Extract on Mild Steel Corrosion in HCl Solution: Experimental and Theoretical Studies. Materials 2020, 13, 2573. [Google Scholar]
- Olasunkanmi, L.O.; Fayomi, O.S.I.; Abdulwahab, M. Influence of Castor Oil Concentration on the Corrosion Inhibition of Mild Steel in 1 M HCl Solution. Materials 2018, 11, 1101. [Google Scholar]
- Pham, T.H.; Lee, W.-H.; Son, G.-H.; Tran, T.T.; Kim, J.-G. Synthesis and Corrosion Inhibition Potential of Cerium/Tetraethylenepentamine Dithiocarbamate Complex on AA2024-T3 in 3.5% NaCl. Materials 2022, 15, 6631. [Google Scholar] [CrossRef]
- Betti, N.; Al-Amiery, A.A.; Al-Azzawi, W.K. Experimental and Quantum Chemical Investigations on the Anticorrosion Efficiency of a Nicotinehydrazide Derivative for Mild Steel in HCl. Molecules 2022, 27, 6254. [Google Scholar] [CrossRef]
- Mahdi, B.S.; Abbass, M.K.; Mohsin, M.K.; Al-azzawi, W.K.; Hanoon, M.M.; Al-kaabi, M.H.H.; Shaker, L.M.; Al-amiery, A.A.; Isahak, W.N.R.W.; Kadhum, A.A.H.; et al. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Environment Using Terephthaldehyde Based on Schiff Base: Gravimetric, Thermodynamic, and Computational Studies. Molecules 2022, 27, 4857. [Google Scholar] [CrossRef]
- Aziz, I.A.A.; Abdulkareem, M.H.; Annon, I.A.; Hanoon, M.M.; Al-Kaabi, M.H.H.; Shaker, L.M.; Alamiery, A.A.; Isahak, W.N.R.W.; Takriff, M.S. Weight Loss, Thermodynamics, SEM, and Electrochemical Studies on N-2-Methylbenzylidene-4-antipyrineamine as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Lubricants 2022, 10, 23. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M.; Gandomi, A. Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- Cao, C. On electrochemical techniques for interface inhibitor research. Corros. Sci. 1996, 38, 2073–2082. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci. 2010, 52, 657–660. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Khayatkashani, M.; Jalali, M.R.; Mosavizade, A. Green Approach to Corrosion Inhibition of 304 Stainless Steel in Hydrochloric Acid Solution by the Extract of Salvia officinalis Leaves. Corros. Sci. 2012, 62, 122–135. [Google Scholar] [CrossRef]
- Bentiss, F.; Mernari, B.; Traisnel, M.; Vezin, H.; Lagrenée, M. On the Relationship between Corrosion Inhibiting Effect and Molecular Structure of 2,5-bis(n-pyridyl)-1,3,4-thiadiazole Derivatives in Acidic Media: AC Impedance and DFT Studies. Corros. Sci. 2011, 53, 487–495. [Google Scholar] [CrossRef]
- Dasami, P.M.; Parameswari, K.; Chitra, S. Corrosion Inhibition of Mild Steel in 1 M H2SO4 by Thiadiazole Schiff Bases. Measurement 2015, 69, 195–201. [Google Scholar] [CrossRef]
- Anupama, K.K.; Ramya, K.; Shainy, K.M.; Joseph, A. A Study on the Synthesis, Characterization and Electrochemical Properties of Poly(aniline-co-o-toluidine)/ZnO Nanocomposite. Mater. Chem. Phys. 2015, 167, 28–41. [Google Scholar] [CrossRef]
- Shaban, S.M.; Aiad, I.; El-Sukkary, M.M.; Soliman, E.; ElAwady, M.Y. Experimental and Theoretical Studies on Some Novel 1,2,4-Triazole Derivatives as Corrosion Inhibitors for Mild Steel in Acidic Media. J. Mol. Liq. 2015, 203, 20–28. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Quraishi, M. Synthesis, Characterization and Application of Nanostructured Polyaniline for the Adsorption of Hg(II) Ions. J. Taiwan Inst. Chem. Eng. 2015, 60, 561–588. [Google Scholar] [CrossRef]
- Zarrok, H.; Assouag, M.; Zarrouk, A.; Oudda, H.; Hallaoui, A.; Touzani, R.; Allali, M.; Hammouti, B.; El Hezzat, M.; Bouachrine, M. Quantum chemical study on the corrosion inhibition of some bipyrazoles. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1853–1860. [Google Scholar]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Camacho-Mendoza, R.L.; Gutierrez-Moreno, E.; Guzman-Percastegui, E.; Aquino-Torres, E.; Cruz-Borbolla, J.; Rodríguez-Avila, J.A.; Alvarado-Rodríguez, J.G.; Olvera-Neria, O.; Thangarasu, P.; Medina-Franco, J.L. Density Functional Theory and Electrochemical Studies: Structure-Efficiency Relationship on Corrosion Inhibition. J. Chem. Inf. Model. 2015, 55, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N. Quantum chemical approach of corrosion inhibition. Electrochim. Acta 2003, 48, 2635–2640. [Google Scholar] [CrossRef]
- Deng, S.; Li, X.; Xie, X. Hydroxymethyl urea and 1,3- bis(hydroxymethyl) urea as corrosion inhibitors for steel in HCl solution. Corros. Sci. 2014, 80, 276–289. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Isabirye, D.A.; Eddy, N.O. Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. Int. J. Mol. Sci. 2010, 11, 2473–2498. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Ibrahim, T.; Gomes, E.; Obot, I.B.; Khamis, M.; Abou Zour, M. Corrosion inhibition of mild steel by Calotropisprocera leaves extract in a CO2 saturated sodium chloride solution. J. Adhes. Sci. Technol. 2016, 30, 2523–2543. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, M.; Quraishi, M.A.; Haque, J.; Singh, G. Bispyranopyrazoles as Green Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Approach. ACS Omega 2018, 3, 11151–11162. [Google Scholar] [CrossRef]
- Yadav, D.K.; Chauhan, D.S.; Ahamad, I.; Quraishi, M.A. Adsorption and Inhibition Effect of Oligomeric Aniline at the Steel/Acid Interface: An Electrochemical Study. RSC Adv. 2013, 3, 632–646. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Electrochemical, Surface and Quantum Chemical Studies of Pyridine Derivatives as Corrosion Inhibitors for N80 Steel in 15% HCl. Measurement 2015, 76, 136–147. [Google Scholar] [CrossRef]

| C (mM) | |||
|---|---|---|---|
| 0.0 | 63.11 | 60.43 | 57.58 |
| 0.1 | 55.36 | 53.73 | 117.09 |
| 0.2 | 50.66 | 51.79 | 120.06 |
| 0.3 | 47.04 | 48.37 | 123.79 |
| 0.4 | 42.81 | 45.64 | 129.47 |
| 0.5 | 40.47 | 42.54 | 138.92 |
| 1.0 | 38.25 | 40.46 | 147.76 |
| Cinh (mM) | Ecorr (mV vs. SCE) | βa (mV/dec) | −βc (mV/dec) | i (μA/cm2) | IE (%) |
|---|---|---|---|---|---|
| 0.0 | −435 | 118.4 | 144.6 | 590 | 58.3 |
| 0.1 | −424 | 92.8 | 117.5 | 140 | 69.5 |
| 0.2 | −422 | 71.7 | 162.5 | 95 | 79.2 |
| 0.3 | −405 | 66.9 | 183.2 | 60 | 84.7 |
| 0.4 | −425 | 53.1 | 171.8 | 88 | 90.2 |
| 0.5 | −410 | 51.8 | 190.4 | 55 | 93.4 |
| n | ||||||
|---|---|---|---|---|---|---|
| 0.0 | 0.311 | 291 | 0.89 | 241 | 105 | - |
| 0.1 | 0.416 | 420 | 0.87 | 190 | 69 | 60.8 |
| 0.2 | 0.383 | 388 | 0.78 | 118 | 59 | 69.8 |
| 0.3 | 0.512 | 476 | 0.88 | 98 | 50 | 77.4 |
| 0.4 | 0.595 | 541 | 0.77 | 80 | 41 | 87.3 |
| 0.5 | 0.653 | 636 | 0.89 | 58 | 31 | 94.3 |
| Atoms | Charges | Atoms | Charges | Atoms | Charges | Atoms | Charges |
|---|---|---|---|---|---|---|---|
| 0.353 | −0.139 | −0.229 | −0.154 | ||||
| 0.276 | −0.142 | −0.055 | −0.233 | ||||
| −0.110 | 0.095 | 0.190 | 0.078 | ||||
| −0.075 | −0.928 | −0.185 | −0.277 | ||||
| −0.118 | 0.599 | 0.138 | 0.078 | ||||
| −0.132 | 0.665 | −0.146 | 0.026 |
| 5.646 eV | 2.529 eV | −5.646 eV | −2.529 eV | −3.117 eV | 4.0875 eV | 1.5585 eV | 0.93433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Amiery, A.A.; Betti, N.; Isahak, W.N.R.W.; Al-Azzawi, W.K.; Wan Nik, W.M.N. Exploring the Effectiveness of Isatin–Schiff Base as an Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Lubricants 2023, 11, 211. https://doi.org/10.3390/lubricants11050211
Al-Amiery AA, Betti N, Isahak WNRW, Al-Azzawi WK, Wan Nik WMN. Exploring the Effectiveness of Isatin–Schiff Base as an Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Lubricants. 2023; 11(5):211. https://doi.org/10.3390/lubricants11050211
Chicago/Turabian StyleAl-Amiery, Ahmed A., Nadia Betti, Wan Nor Roslam Wan Isahak, Waleed Khalid Al-Azzawi, and Wan Mohd Norsani Wan Nik. 2023. "Exploring the Effectiveness of Isatin–Schiff Base as an Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid" Lubricants 11, no. 5: 211. https://doi.org/10.3390/lubricants11050211
APA StyleAl-Amiery, A. A., Betti, N., Isahak, W. N. R. W., Al-Azzawi, W. K., & Wan Nik, W. M. N. (2023). Exploring the Effectiveness of Isatin–Schiff Base as an Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Lubricants, 11(5), 211. https://doi.org/10.3390/lubricants11050211









