Impact of Temperature Variation on Friction Behaviour of Rare Earth-Doped Diamond-like Carbon Coatings with Ionic Liquid Lubricants
Abstract
1. Introduction
2. Materials and Methods
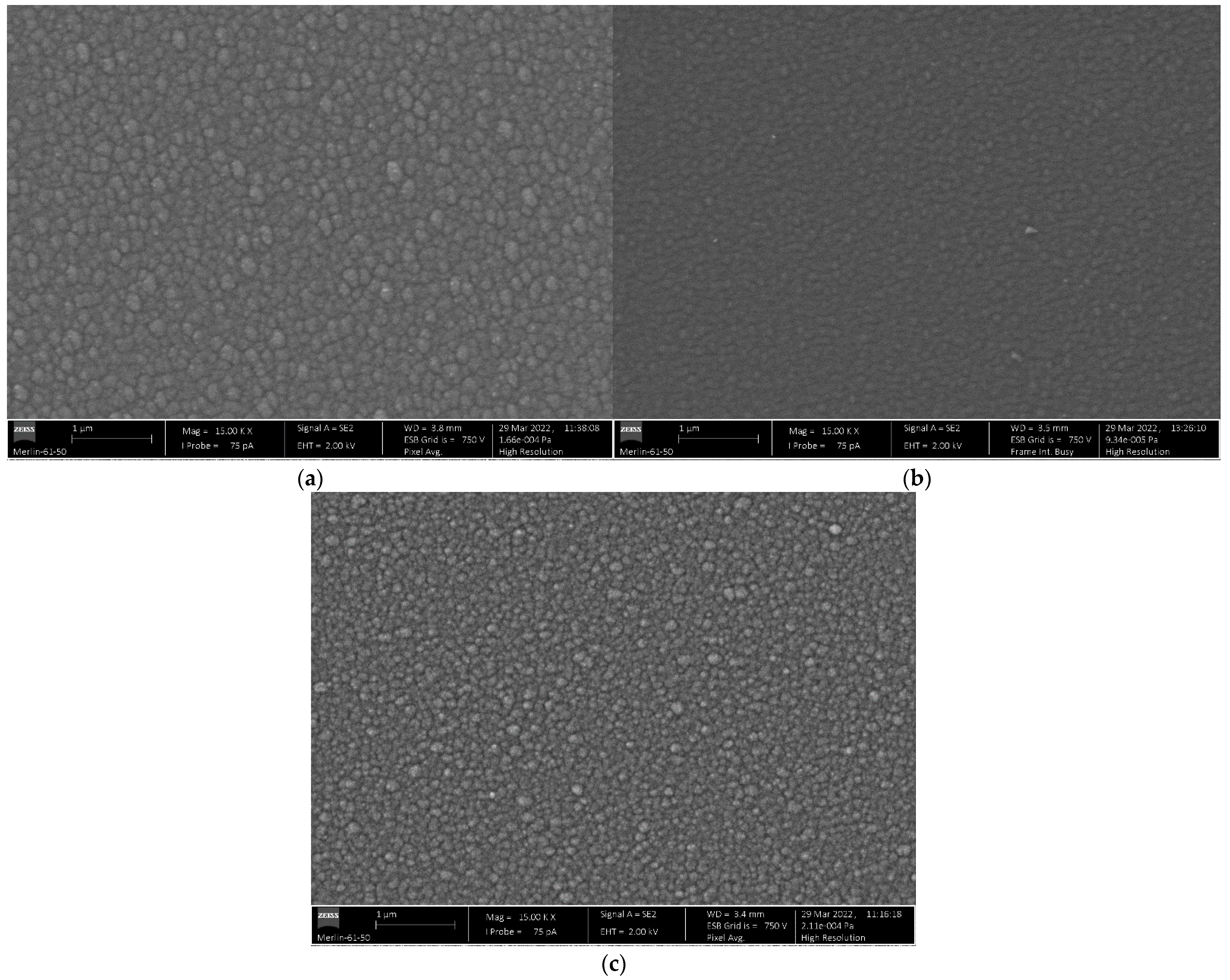
2.1. Specimens
2.2. Lubricants
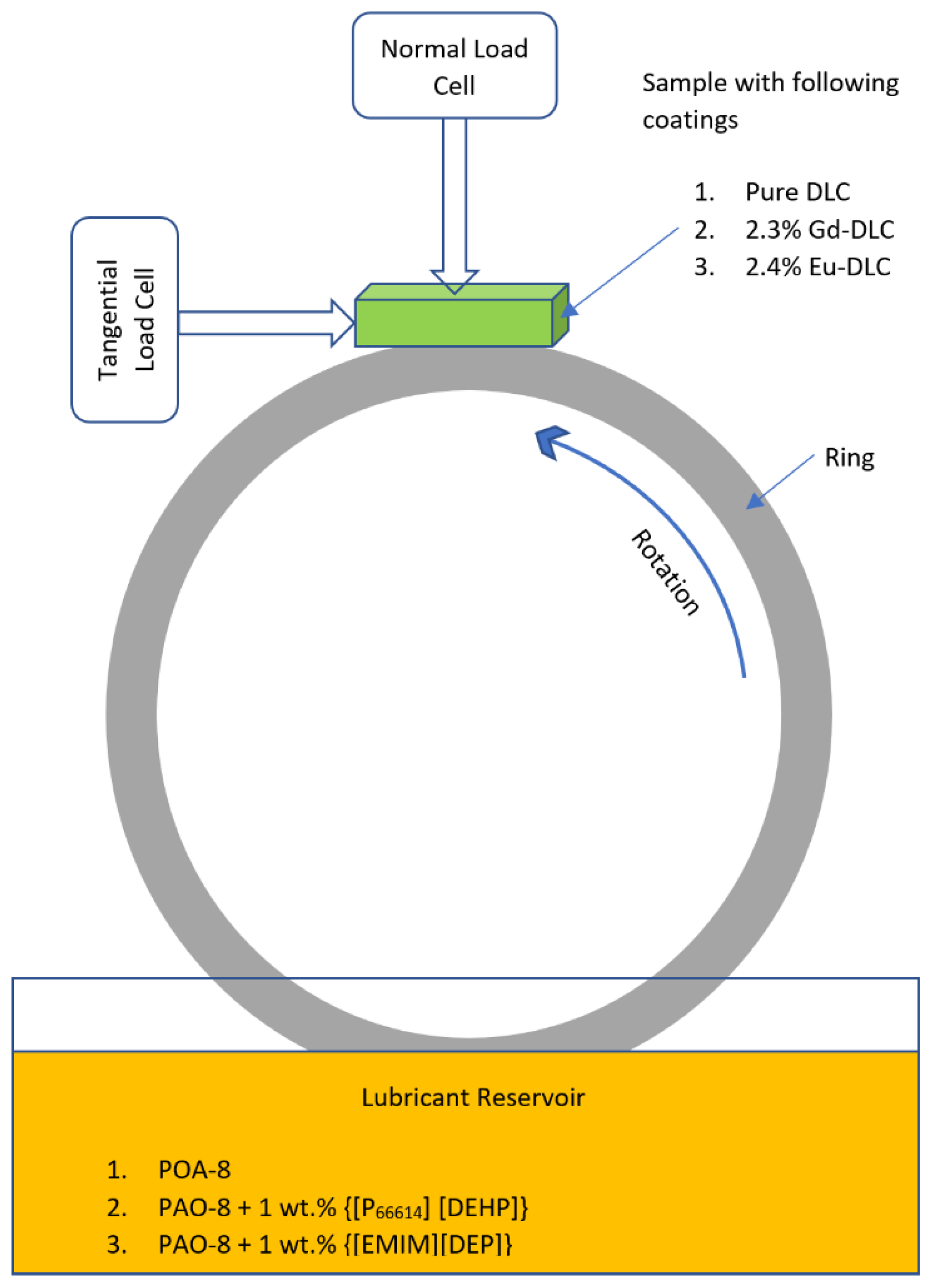
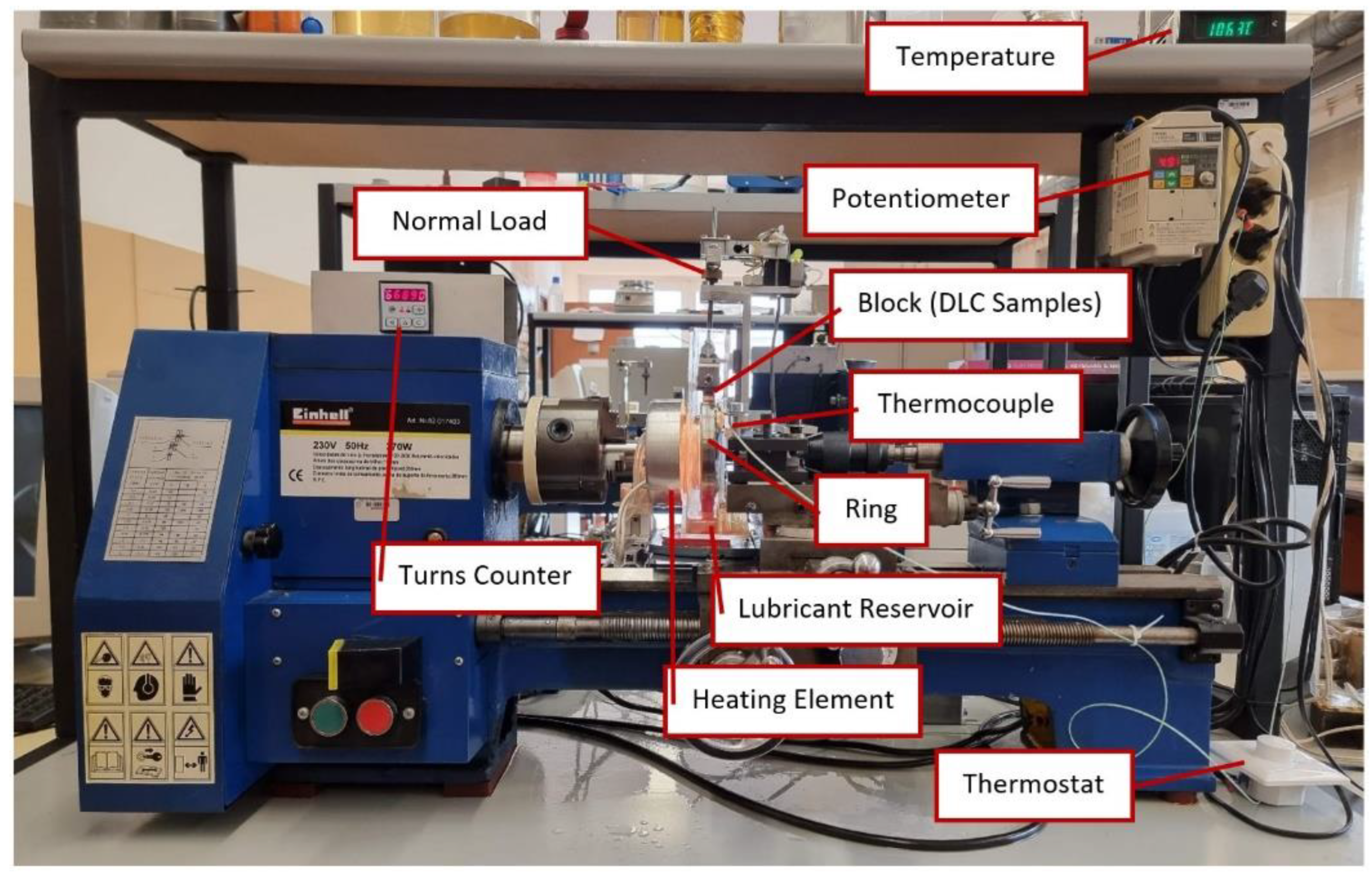
2.3. Tribological Tests
- λ > 5: Complete hydrodynamic lubrication
- ○
- This regime indicates a lubrication condition where the lubricant film thickness is sufficient to entirely separate the surfaces, resulting in minimal wear and friction.
- 3 < λ < 5: Asperity smoothing, minimum wear
- ○
- In this regime, the lubricant film thickness is moderate, allowing some contact between the surfaces. However, the pressure is high enough to deform the asperities, reducing wear and promoting smoother operation.
- 1.5 < λ < 3: Asperity smoothing
- ○
- This regime represents a lubrication condition with a relatively thin lubricant film, allowing some contact between the surfaces. However, the pressure is insufficient to prevent direct contact between the asperities.
- 1 < λ < 1.5: Asperity smoothing, delamination
- ○
- Similar to the previous regime, this regime involves asperity smoothing but with an additional factor of delamination. Delamination refers to the separation of thin surface layers under specific conditions.
- λ < 1: Macro-Plastic deformation
- ○
- The regime indicates a lubrication condition where the lubricant film thickness is extremely thin or absent, leading to direct contact between the surfaces. This regime is characterized by significant plastic deformation of the contacting asperities.
3. Results and Discussion
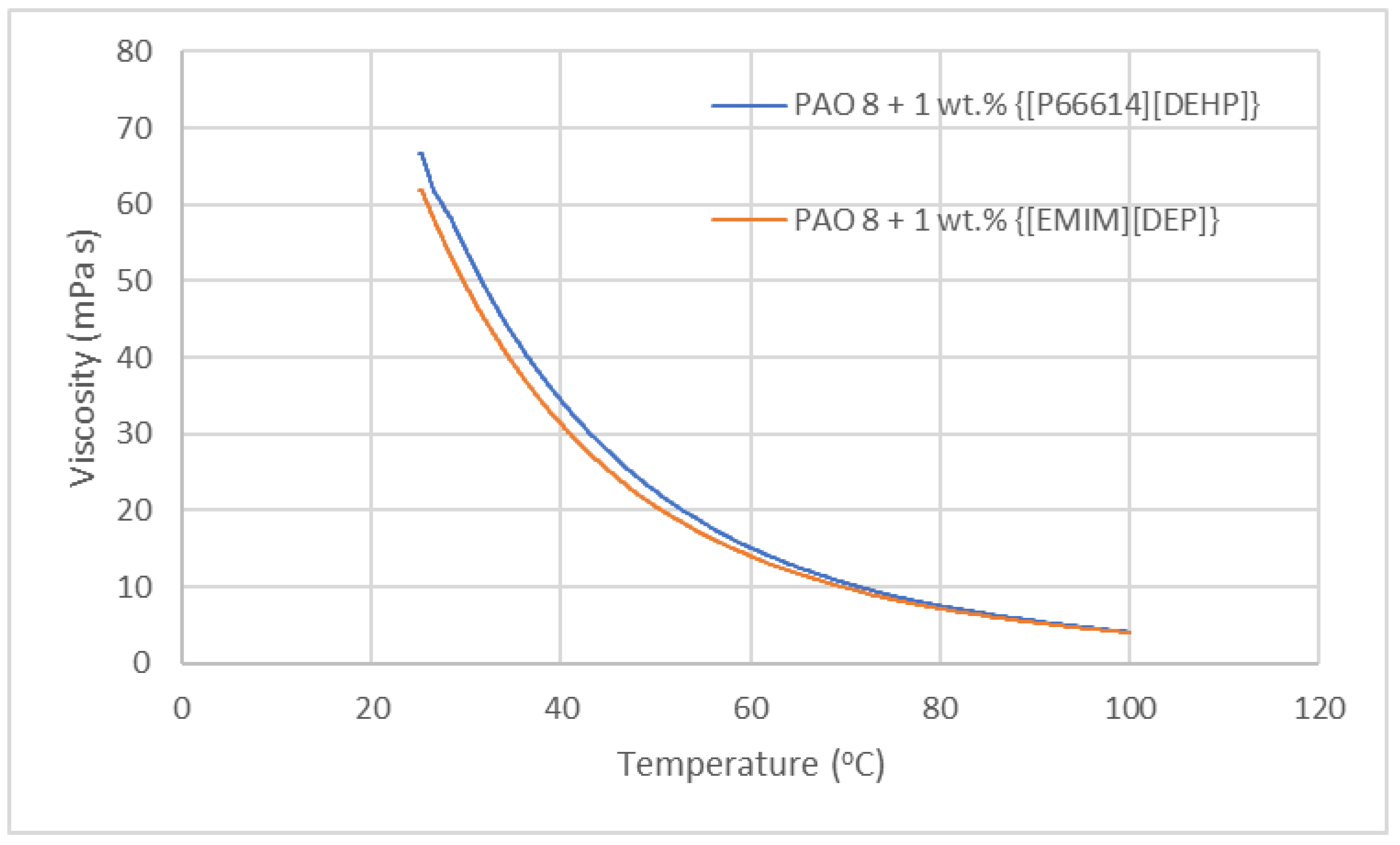
3.1. Viscosity of Lubricants
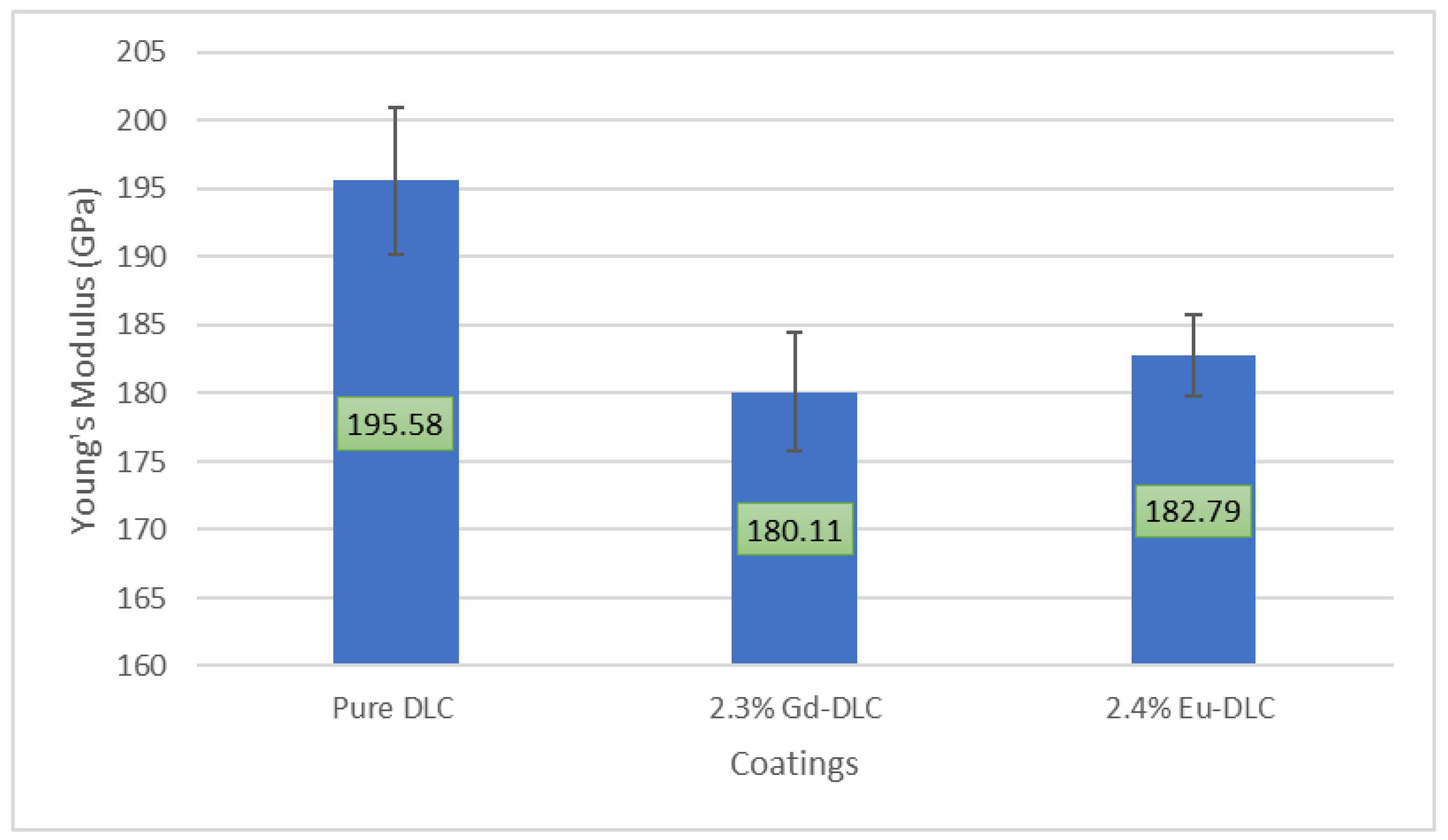
3.2. Mechanical Characterization (Hardness and Young’s Modulus)
3.3. Tribological Test (Influence of the Temperature)
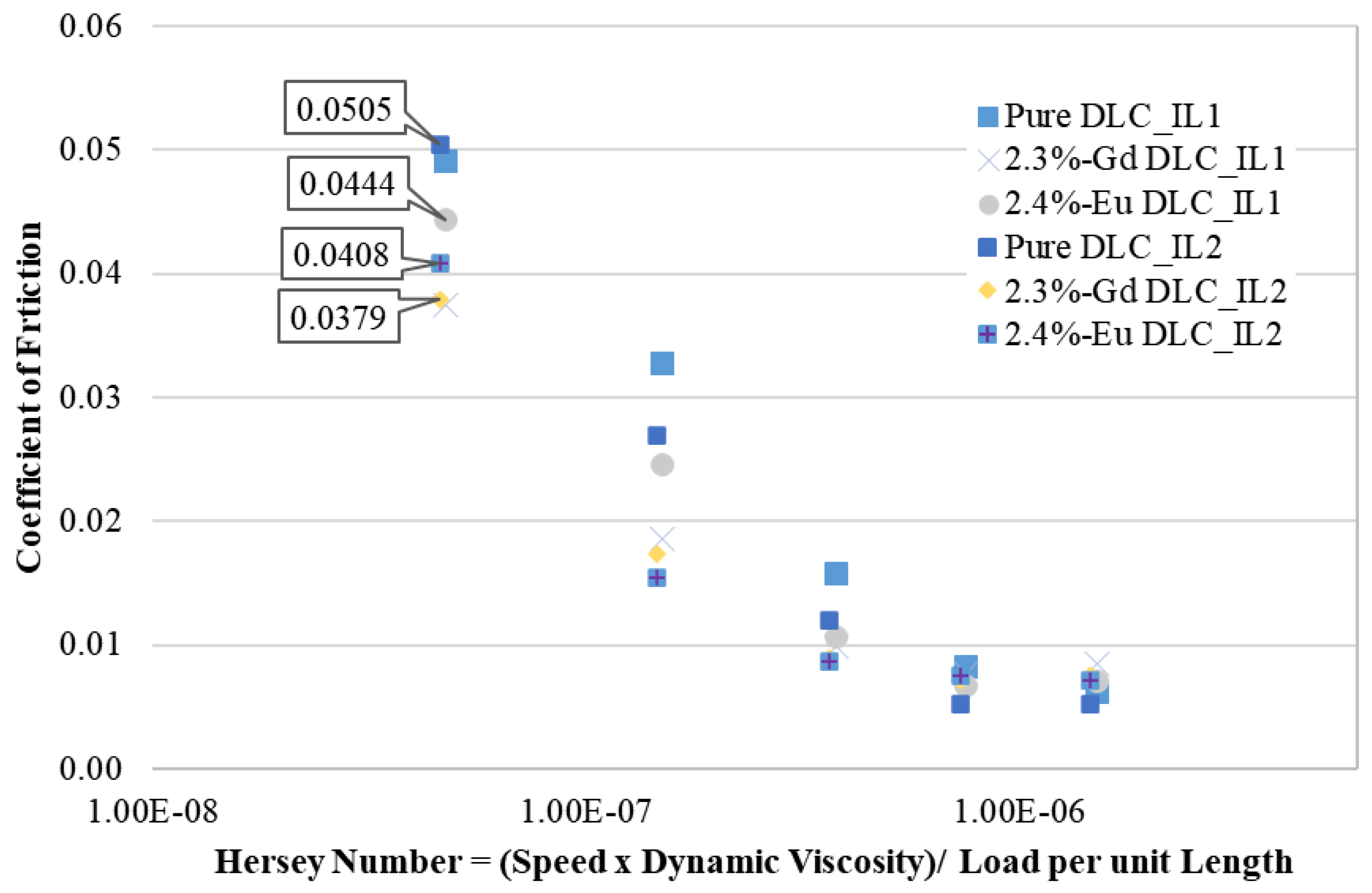
3.3.1. Temperature of 60 °C
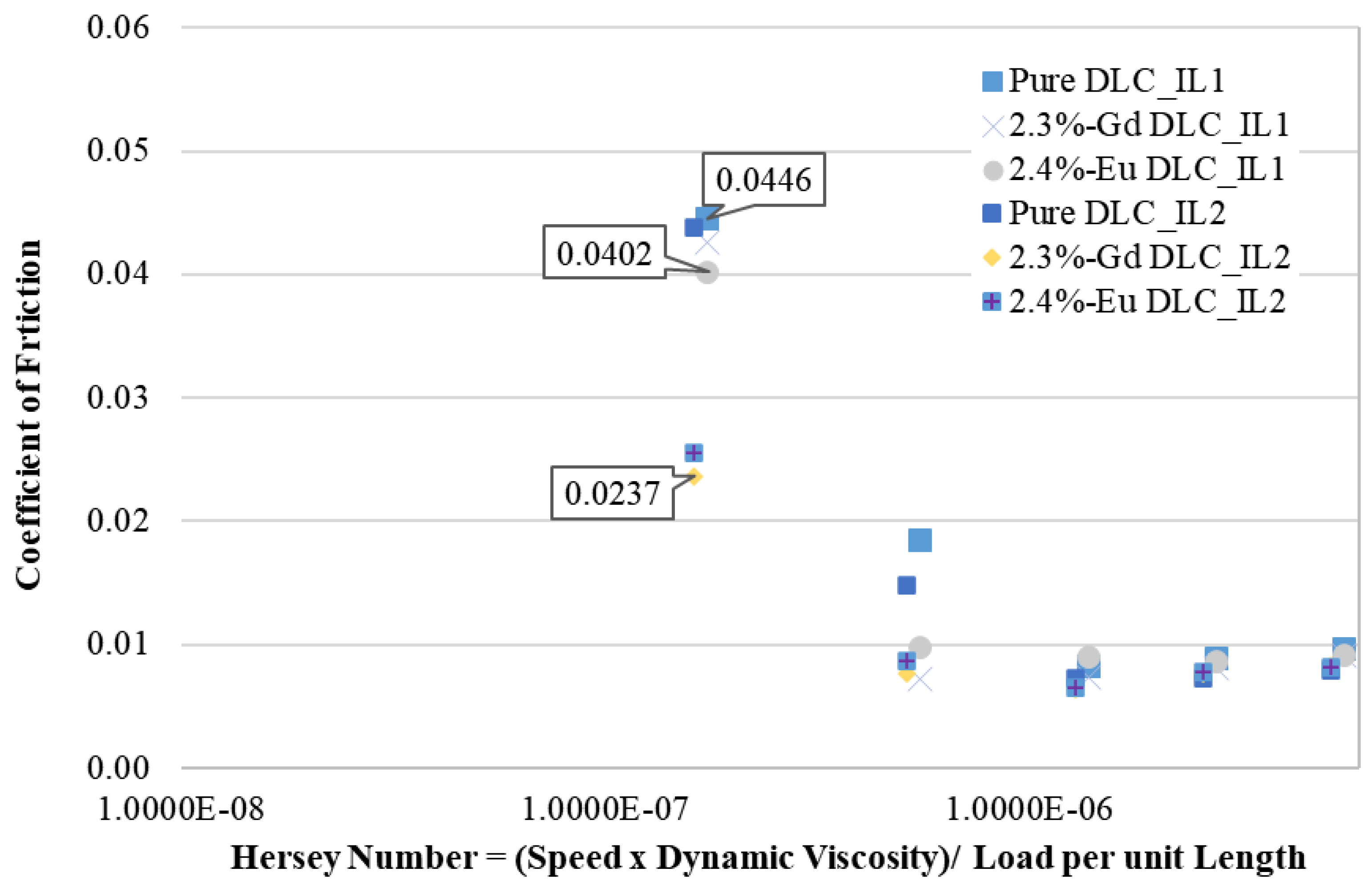
3.3.2. Temperature of 80 °C
3.3.3. Temperature of 100 °C
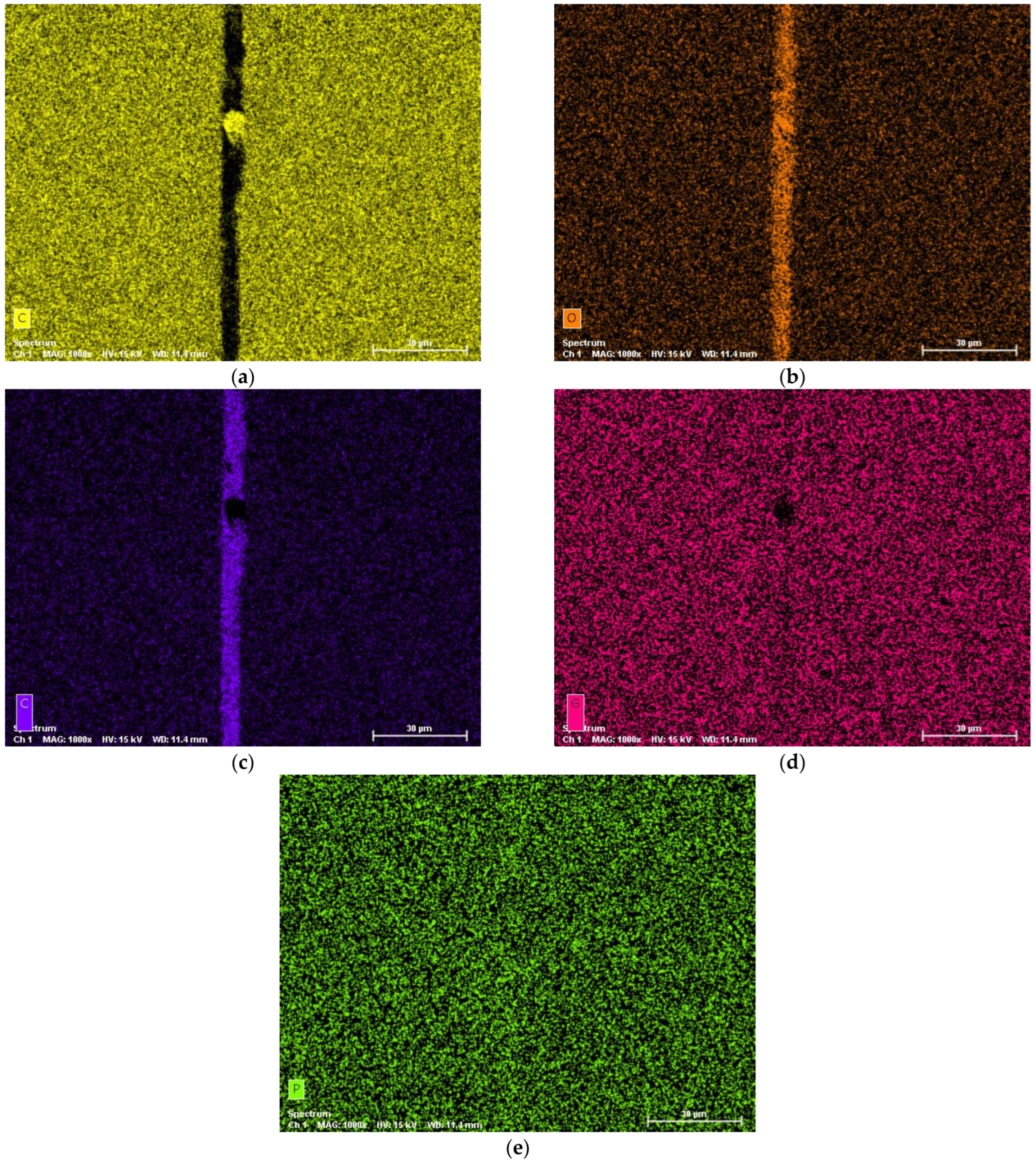
3.4. Wear Mechanism Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. The Impact of Tribology on Energy Use and CO2 Emission Globally and in Combustion Engine and Electric Cars. Tribol. Int. 2019, 135, 389–396. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global Energy Consumption Due to Friction in Passenger Cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Zichao, L.; Bin, S.; Fanghong, S.; Zhiming, Z.; Songshou, G. Diamond-Coated Tube Drawing Die Optimization Using Finite Element Model Simulation and Response Surface Methodology. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2014, 228, 1432–1441. [Google Scholar] [CrossRef]
- Santiago, J.A.; Fernández-Martínez, I.; Sánchez-López, J.C.; Rojas, T.C.; Wennberg, A.; Bellido-González, V.; Molina-Aldareguia, J.M.; Monclús, M.A.; González-Arrabal, R. Tribomechanical Properties of Hard Cr-Doped DLC Coatings Deposited by Low-Frequency HiPIMS. Surf. Coat. Technol. 2020, 382, 124899. [Google Scholar] [CrossRef]
- Martins, P.S.; Pires, S.S.; da Silva, E.R.; Vieira, V.F.; Ba, E.C.T.; Dias, C.A.R. Tribological Aspects of the Diamond-like Carbon Film Applied to Different Surfaces of AISI M2 Steel. Wear 2022, 506, 204469. [Google Scholar] [CrossRef]
- Yue, Z.; Fan, X.; Wang, Y.; Li, H.; Zhang, J.; Zhu, M. Fretting Behaviors of Self-Mated Diamond-like Carbon Films: The Evolution of Fretting Regime and Transfer Film. Carbon N. Y. 2023, 203, 695–705. [Google Scholar] [CrossRef]
- Santhosh, N.; Shankar, G.; Nunthavarawong, P. Mechanical and Tribological Performance of Diamond-like Carbon Coatings: An Overview. Diam.-Like Carbon Coat. 2022, 255–274. [Google Scholar]
- Tasdemir, H.A.; Tokoroyama, T.; Kousaka, H.; Umehara, N.; Mabuchi, Y. Friction and Wear Performance of Boundary-Lubricated DLC/DLC Contacts in Synthetic Base Oil. Procedia Eng. 2013, 68, 518–524. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Tailor, S. Self-Lubricating Composite Coatings: A Review of Deposition Techniques and Material Advancement. Mater Today Proc. 2023. [Google Scholar] [CrossRef]
- Zia, A.W.; Zhou, Z.; Li, L.K.-Y. Structural, Mechanical, and Tribological Characteristics of Diamond-like Carbon Coatings. In Nanomaterials-Based Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–194. [Google Scholar]
- ISO 20523:2017; Carbon Based Films—Classification and Designations. The International Organization for Standardization, Vernier: Geneva, Switzerland, 2017.
- Erdemir, A. Review of Engineered Tribological Interfaces for Improved Boundary Lubrication. Tribol. Int. 2005, 38, 249–256. [Google Scholar] [CrossRef]
- Neville, A.; Morina, A.; Haque, T.; Voong, M. Compatibility between Tribological Surfaces and Lubricant Additives—How Friction and Wear Reduction Can Be Controlled by Surface/Lube Synergies. Tribol. Int. 2007, 40, 1680–1695. [Google Scholar] [CrossRef]
- Velkavrh, I.; Kalin, M.; Vizintin, J. The Performance and Mechanisms of DLC-Coated Surfaces in Contact with Steel in Boundary-Lubrication Conditions: A Review. Stroj. Vestn. 2008, 54, 189–206. [Google Scholar]
- Hsu, S.M.; Gates, R.S. Boundary Lubricating Films: Formation and Lubrication Mechanism. Tribol. Int. 2005, 38, 305–312. [Google Scholar] [CrossRef]
- Suzuki, A.; Shinka, Y.; Masuko, M. Tribological Characteristics of Imidazolium-Based Room Temperature Ionic Liquids under High Vacuum. Tribol. Lett. 2007, 27, 307–313. [Google Scholar] [CrossRef]
- Bermúdez, M.-D.; Jiménez, A.-E.; Sanes, J.; Carrión, F.-J. Ionic Liquids as Advanced Lubricant Fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef]
- Somers, A.E.; Khemchandani, B.; Howlett, P.C.; Sun, J.; MacFarlane, D.R.; Forsyth, M. Ionic Liquids as Antiwear Additives in Base Oils: Influence of Structure on Miscibility and Antiwear Performance for Steel on Aluminum. ACS Appl. Mater. Interfaces 2013, 5, 11544–11553. [Google Scholar] [CrossRef]
- Zhou, Y.; Dyck, J.; Graham, T.W.; Luo, H.; Leonard, D.N.; Qu, J. Ionic Liquids Composed of Phosphonium Cations and Organophosphate, Carboxylate, and Sulfonate Anions as Lubricant Antiwear Additives. Langmuir 2014, 30, 13301–13311. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Qu, J.; Luo, H.; Meyer III, H.M.; Ma, C.; Chi, M.; Papke, B.L. Phosphonium-Organophosphate Ionic Liquids as Lubricant Additives: Effects of Cation Structure on Physicochemical and Tribological Characteristics. ACS Appl. Mater. Interfaces 2014, 6, 22585–22593. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M.; Papke, B.L.; Qu, J. Tertiary and Quaternary Ammonium-Phosphate Ionic Liquids as Lubricant Additives. Tribol. Lett. 2016, 63, 22. [Google Scholar] [CrossRef]
- Minami, I.; Inada, T.; Sasaki, R.; Nanao, H. Tribo-Chemistry of Phosphonium-Derived Ionic Liquids. Tribol. Lett. 2010, 40, 225–235. [Google Scholar] [CrossRef]
- Somers, A.E.; Biddulph, S.M.; Howlett, P.C.; Sun, J.; MacFarlane, D.R.; Forsyth, M. A Comparison of Phosphorus and Fluorine Containing IL Lubricants for Steel on Aluminium. Phys. Chem. Chem. Phys. 2012, 14, 8224–8231. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Doerr, N.; Aswath, P.B. Chemical–Mechanical Properties of Tribofilms and Their Relationship to Ionic Liquid Chemistry. RSC Adv. 2016, 6, 22341–22356. [Google Scholar] [CrossRef]
- Verma, D.K.; Dewangan, Y.; Singh, A.K.; Mishra, R.; Susan, M.A.B.H.; Salim, R.; Taleb, M.; El Hajjaji, F.; Berdimurodov, E. Ionic Liquids as Green and Smart Lubricant Application: An Overview. Ionics 2022, 28, 4923–4932. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.-Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef]
- Groover, M.P. Fundamentals of Modern Manufacturing: Materials, Processes, and Systems; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 1119722012. [Google Scholar]
- Tu, W.; Chat, K.; Szklarz, G.; Laskowski, L.; Grzybowska, K.; Paluch, M.; Richert, R.; Adrjanowicz, K. Dynamics of Pyrrolidinium-Based Ionic Liquids under Confinement. II. The Effects of Pore Size, Inner Surface, and Cationic Alkyl Chain Length. J. Phys. Chem. C 2019, 124, 5395–5408. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L. Uncovering the Underlying Mechanisms Governing the Solidlike Layering of Ionic Liquids (ILs) on Mica. Langmuir 2020, 36, 2743–2756. [Google Scholar] [CrossRef]
- Kong, Q.; Zheng, S.S.; Liu, T.Q.; Nie, Y.; Song, K.D. Evaluation of Ionic Liquid “Greenness”-Cytotoxicity of Ionic Liquids. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 10–12 October 2019; IOP Publishing: Bristol, UK, 2019; Volume 592, p. 012031. [Google Scholar]
- Xiao, H.; Guo, D.; Liu, S.; Pan, G.; Lu, X. Film Thickness of Ionic Liquids under High Contact Pressures as a Function of Alkyl Chain Length. Tribol. Lett. 2011, 41, 471–477. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic Liquid Lubricants: Designed Chemistry for Engineering Applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-Alkyl-3-Methylimidazolium Ionic Liquids as Neat Lubricants and Lubricant Additives in Steel–Aluminium Contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Qu, J.; Truhan, J.J.; Dai, S.; Luo, H.; Blau, P.J. Ionic Liquids with Ammonium Cations as Lubricants or Additives. Tribol. Lett. 2006, 22, 207–214. [Google Scholar] [CrossRef]
- Minami, I.; Kita, M.; Kubo, T.; Nanao, H.; Mori, S. The Tribological Properties of Ionic Liquids Composed of Trifluorotris (Pentafluoroethyl) Phosphate as a Hydrophobic Anion. Tribol. Lett. 2008, 30, 215–223. [Google Scholar] [CrossRef]
- Shah, F.U.; Glavatskih, S.; MacFarlane, D.R.; Somers, A.; Forsyth, M.; Antzutkin, O.N. Novel Halogen-Free Chelated Orthoborate–Phosphonium Ionic Liquids: Synthesis and Tribophysical Properties. Phys. Chem. Chem. Phys. 2011, 13, 12865–12873. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.E.; Howlett, P.C.; Sun, J.; MacFarlane, D.R.; Forsyth, M. Phosphonium Ionic Liquids as Lubricants for Aluminium-Steel. Tribol. Des. 2010, 66, 273–283. [Google Scholar]
- Itoh, T.; Watanabe, N.; Inada, K.; Ishioka, A.; Hayase, S.; Kawatsura, M.; Minami, I.; Mori, S. Design of Alkyl Sulfate Ionic Liquids for Lubricants. Chem. Lett. 2009, 38, 64–65. [Google Scholar] [CrossRef]
- Dupont, J.; Suarez, P.A.Z.; Umpierre, A.P.; de Souza, R.F. Pd (II)-Dissolved in Ionic Liquids: A Recyclable Catalytic System for the Selective Biphasic Hydrogenation of Dienes to Monoenes. J. Braz. Chem. Soc. 2000, 11, 293–297. [Google Scholar] [CrossRef]
- Naveed, T.; Zahid, R.; Mufti, R.A.; Waqas, M.; Hanif, M.T. A Review on Tribological Performance of Ionic Liquids as Additives to Bio Lubricants. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 235, 1782–1806. [Google Scholar] [CrossRef]
- Jelínek, M.; Smetana, K.; Kocourek, T.; Dvořánková, B.; Zemek, J.; Remsa, J.; Luxbacher, T. Biocompatibility and Sp3/Sp2 Ratio of Laser Created DLC Films. Mater. Sci. Eng. B 2010, 169, 89–93. [Google Scholar] [CrossRef]
- Jelínek, M.; Kocourek, T.; Remsa, J.; Mikšovský, J.; Zemek, J.; Smetana, K.; Dvořánková, B.; Luxbacher, T. Diamond/Graphite Content and Biocompatibility of DLC Films Fabricated by PLD. Appl. Phys. A 2010, 101, 579–583. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Wijanarko, W.; Cruz, S.; Evaristo, M.; Espallargas, N. Triboelectrochemical Friction Control of W-and Ag-Doped DLC Coatings in Water–Glycol with Ionic Liquids as Lubricant Additives. RSC Adv. 2022, 12, 3573–3583. [Google Scholar] [CrossRef]
- Qu, J.; Bansal, D.G.; Yu, B.; Howe, J.Y.; Luo, H.; Dai, S.; Li, H.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G. Antiwear Performance and Mechanism of an Oil-Miscible Ionic Liquid as a Lubricant Additive. ACS Appl. Mater. Interfaces 2012, 4, 997–1002. [Google Scholar] [CrossRef]
- Qu, J.; Luo, H.; Chi, M.; Ma, C.; Blau, P.J.; Dai, S.; Viola, M.B. Comparison of an Oil-Miscible Ionic Liquid and ZDDP as a Lubricant Anti-Wear Additive. Tribol. Int. 2014, 71, 88–97. [Google Scholar] [CrossRef]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.; Luo, H.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-Miscible and Non-Corrosive Phosphonium-Based Ionic Liquids as Candidate Lubricant Additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Li, Z.; Dolocan, A.; Morales-Collazo, O.; Sadowski, J.T.; Celio, H.; Chrostowski, R.; Brennecke, J.F.; Mangolini, F. Lubrication Mechanism of Phosphonium Phosphate Ionic Liquid in Nanoscale Single-asperity Sliding Contacts. Adv. Mater. Interfaces 2020, 7, 2000426. [Google Scholar] [CrossRef]
- Okubo, H.; Watanabe, S.; Tadokoro, C.; Sasaki, S. Effects of Concentration of Zinc Dialkyldithiophosphate on the Tribological Properties of Tetrahedral Amorphous Carbon Films in Presence of Organic Friction Modifiers. Tribol. Int. 2016, 94, 446–457. [Google Scholar] [CrossRef]
- Okubo, H.; Watanabe, S.; Tadokoro, C.; Sasaki, S. Effects of Structure of Zinc Dialkyldithiophosphates on Tribological Properties of Tetrahedral Amorphous Carbon f Ilm under Boundary Lubrication. Tribol. Int. 2016, 98, 26–40. [Google Scholar] [CrossRef]
- Okubo, H.; Tadokoro, C.; Sasaki, S. Tribological Properties of a Tetrahedral Amorphous Carbon (Ta-C) Film under Boundary Lubrication in the Presence of Organic Friction Modifiers and Zinc Dialkyldithiophosphate (ZDDP). Wear 2015, 332, 1293–1302. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Tadokoro, C.; Sasaki, S. Effects of Alkyl Chain Length of Sulfate and Phosphate Anion-Based Ionic Liquids on Tribochemical Reactions. Tribol. Lett. 2018, 66, 8. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Sasaki, S.; Miyatake, M. Tribochemical Reactions of Halogen-Free Ionic Liquids on Nascent Steel Surface. Recent Adv. Ion. Liq. IntechOpen 2018, 47–65. [Google Scholar] [CrossRef]
- Taokaew, S.; Kriangkrai, W. Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides 2022, 3, 671–691. [Google Scholar] [CrossRef]
- Hu, D.; Xiao, L.; Li, L.; Zhong, C.; Ju, X.; Yan, L.; Wu, T.; Qing, M.; Hu, Z. Effects of Ionic Liquid 1-Ethyl-3-Methylimidazolium Diethylphosphate on Cellulase Produced by Paenibacillus Sp. LLZ1. ACS Sustain. Chem. Eng. 2016, 4, 4922–4926. [Google Scholar] [CrossRef]
- Dubey, S.; Bharmoria, P.; Gehlot, P.S.; Agrawal, V.; Kumar, A.; Mishra, S. 1-Ethyl-3-Methylimidazolium Diethylphosphate Based Extraction of Bioplastic “Polyhydroxyalkanoates” from Bacteria: Green and Sustainable Approach. ACS Sustain. Chem. Eng. 2018, 6, 766–773. [Google Scholar] [CrossRef]
- Zeng, Q. Superlow Friction and Diffusion Behaviors of a Steel-Related System in the Presence of Nano Lubricant Additive in PFPE Oil. J. Adhes Sci. Technol. 2019, 33, 1001–1018. [Google Scholar] [CrossRef]
- Forsberg, P.; Gustavsson, F.; Renman, V.; Hieke, A.; Jacobson, S. Performance of DLC Coatings in Heated Commercial Engine Oils. Wear 2013, 304, 211–222. [Google Scholar] [CrossRef]
- Kosarieh, S.; Morina, A.; Lainé, E.; Flemming, J.; Neville, A. The Effect of MoDTC-Type Friction Modifier on the Wear Performance of a Hydrogenated DLC Coating. Wear 2013, 302, 890–898. [Google Scholar] [CrossRef]
- Kalin, M.; Kogovšek, J.; Remškar, M. Nanoparticles as Novel Lubricating Additives in a Green, Physically Based Lubrication Technology for DLC Coatings. Wear 2013, 303, 480–485. [Google Scholar] [CrossRef]
- Velkavrh, I.; Kalin, M. Effect of Base Oil Lubrication in Comparison with Non-Lubricated Sliding in Diamond-like Carbon Contacts. Tribol.-Mater. Surf. Interfaces 2011, 5, 53–58. [Google Scholar] [CrossRef]
- Kalin, M.; Velkavrh, I.; Vižintin, J.; Ožbolt, L. Review of Boundary Lubrication Mechanisms of DLC Coatings Used in Mechanical Applications. Meccanica 2008, 43, 623–637. [Google Scholar] [CrossRef]
- Kalin, M.; Velkavrh, I. Non-Conventional Inverse-Stribeck-Curve Behaviour and Other Characteristics of DLC Coatings in All Lubrication Regimes. Wear 2013, 297, 911–918. [Google Scholar] [CrossRef]
- Balestra, R.M.; Castro, A.M.G.; Evaristo, M.; Escudeiro, A.; Mutafov, P.; Polcar, T.; Cavaleiro, A. Carbon-Based Coatings Doped by Copper: Tribological and Mechanical Behavior in Olive Oil Lubrication. Surf. Coat. Technol. 2011, 205, S79–S83. [Google Scholar] [CrossRef]
- Manninen, N.K.; Ribeiro, F.; Escudeiro, A.; Polcar, T.; Carvalho, S.; Cavaleiro, A. Influence of Ag Content on Mechanical and Tribological Behavior of DLC Coatings. Surf. Coat Technol. 2013, 232, 440–446. [Google Scholar] [CrossRef]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-Doped Nanocomposite Carbon Coatings (Ag-DLC) for Biomedical Applications–Physiochemical and Biological Evaluation. Appl. Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Capano, M.A.; Laube, S.J.P.; Donley, M.S.; Zabinski, J.S. Design of a Ti/TiC/DLC Functionally Gradient Coating Based on Studies of Structural Transitions in Ti–C Thin Films. Thin. Solid Film. 1997, 298, 107–115. [Google Scholar] [CrossRef]
- Corbella, C.; Vives, M.; Pinyol, A.; Bertran, E.; Canal, C.; Polo, M.C.; Andújar, J.L. Preparation of Metal (W, Mo, Nb, Ti) Containing AC: H Films by Reactive Magnetron Sputtering. Surf. Coat. Technol. 2004, 177, 409–414. [Google Scholar] [CrossRef]
- Zhao, F.; Li, H.; Ji, L.; Wang, Y.; Zhou, H.; Chen, J. Ti-DLC Films with Superior Friction Performance. Diam Relat. Mater. 2010, 19, 342–349. [Google Scholar] [CrossRef]
- Evaristo, M.; Polcar, T.; Cavaleiro, A. Tribological Behaviour of W-alloyed Carbon-based Coatings in Dry and Lubricated Sliding Contact. Lubr. Sci. 2014, 26, 428–439. [Google Scholar] [CrossRef]
- Ming, M.Y.; Jiang, X.; Piliptsou, D.G.; Zhuang, Y.; Rogachev, A.V.; Rudenkov, A.S.; Balmakou, A. Chromium-Modified AC Films with Advanced Structural, Mechanical and Corrosive-Resistant Characteristics. Appl. Surf. Sci. 2016, 379, 424–432. [Google Scholar] [CrossRef]
- Wongpanya, P.; Silawong, P.; Photongkam, P. Adhesion and Corrosion of Al–N Doped Diamond-like Carbon Films Synthesized by Filtered Cathodic Vacuum Arc Deposition. Ceram Int. 2022, 48, 20743–20759. [Google Scholar] [CrossRef]
- Fontes, M.A.; Serra, R.G.H.; Fernandes, F.D.; Cavaleiro Rodrigues de Carvalho, A.A.; Ferreira, F.E.d.S. Comparison of Mechanical and Tribological Properties of Diamond-like Carbon Coatings Doped with Europium and Gadolinium Produced by HiPIMS. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2022, 09544054221136528. [Google Scholar] [CrossRef]
- Omiya, T.; Fontes, M.; Vuchkov, T.; Cruz, S.; Cavaleiro, A.; Ferreira, F. Tribological Performance of Gd-DLC and Eu-DLC Coatings in the Presence of Synthetic Oils Containing Ionic Liquid Additives. Tribol. Lett. 2023, 71, 65. [Google Scholar] [CrossRef]
- Sadeghi, M.; Omiya, T.; Fernandes, F.; Vilhena, L.; Ramalho, A.; Ferreira, F. Tribological Behavior of Doped DLC Coatings in the Presence of Ionic Liquid Additive under Different Lubrication Regimes. Coatings 2023, 13, 891. [Google Scholar] [CrossRef]
- Valente, A.J.M.; Burrows, H.D.; Cruz, S.M.A.; Pereira, R.F.P.; Ribeiro, A.C.F.; Lobo, V.M.M. Aggregation and Micellization of Sodium Dodecyl Sulfate in the Presence of Ce (III) at Different Temperatures: A Conductometric Study. J. Colloid Interface Sci. 2008, 323, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Turanov, A.N.; Karandashev, V.K.; Boltoeva, M. Solvent Extraction of Intra-Lanthanides Using a Mixture of TBP and TODGA in Ionic Liquid. Hydrometallurgy 2020, 195, 105367. [Google Scholar] [CrossRef]
- Mishra, B.B.; Niharbala, D. Application of Bifunctional Ionic Liquids for Extraction and Separation of Eu3+ from Chloride Medium. Trans. Nonferrous Met. Soc. China 2022, 32, 2061–2070. [Google Scholar] [CrossRef]
- Stoy, L.; Xu, J.; Kulkarni, Y.; Huang, C.-H. Ionic Liquid Recovery of Rare-Earth Elements from Coal Fly Ash: Process Efficiency and Sustainability Evaluations. ACS Sustain. Chem. Eng. 2022, 10, 11824–11834. [Google Scholar] [CrossRef]
- Rout, A.; Kumar, S.; Ramanathan, N. Probing the Coordination of Europium (III) in a Functionalized Ionic Liquid Using Luminescence Spectroscopy. J. Mol. Liq. 2021, 323, 115109. [Google Scholar] [CrossRef]
- Yan, M.; Wang, X.; Zhang, S.; Zhang, S.; Sui, X.; Li, W.; Hao, J.; Liu, W. Friction and Wear Properties of GLC and DLC Coatings under Ionic Liquid Lubrication. Tribol. Int. 2020, 143, 106067. [Google Scholar] [CrossRef]
- Bociąga, D.; Jakubowski, W.; Komorowski, P.; Sobczyk-Guzenda, A.; Jędrzejczak, A.; Batory, D.; Olejnik, A. Surface Characterization and Biological Evaluation of Silver-Incorporated DLC Coatings Fabricated by Hybrid RF PACVD/MS Method. Mater. Sci. Eng. C 2016, 63, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Ashiq, M.N.; Bonn, G.K. Chemically Modified Diamond-like Carbon (DLC) for Protein Enrichment and Profiling by MALDI-MS. Amino Acids 2012, 43, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, X.; Ren, L.; Wang, T.; Qi, X.; Yang, Y. Improving the Tribological Properties of Spark-Anodized Titanium by Magnetron Sputtered Diamond-like Carbon. Coatings 2018, 8, 83. [Google Scholar] [CrossRef]
- Ze, S.; Dejun, K. Effect of Load on the Friction-Wear Behavior of Magnetron Sputtered DLC Film at High Temperature. Mater. Res. Express 2017, 4, 016404. [Google Scholar] [CrossRef]
- Ghosh, S.; Choudhury, D.; Roy, T.; Mamat, A.B.; Masjuki, H.H.; Pingguan-Murphy, B. Tribological Investigation of Diamond-like Carbon Coated Micro-Dimpled Surface under Bovine Serum and Osteoarthritis Oriented Synovial Fluid. Sci. Technol. Adv. Mater. 2015, 16, 35002. [Google Scholar] [CrossRef] [PubMed]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.D.; Laurindo, C.; Soares, P. Tribocorrosion Behavior of DLC-Coated Ti-6Al-4V Alloy Deposited by PIID and PEMS+ PIID Techniques for Biomedical Applications. Surf. Coat. Technol. 2017, 332, 223–232. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Mahboubi, F.; Oskouie, A. Structural Evolution and Tribological Behavior of Nitrogen-Doped DLC Coatings Deposited by Pulsed DC PACVD Method. Diam. Relat. Mater. 2019, 91, 74–83. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Lee, P.; Wei, R. Thick Diamond like Carbon Coatings Deposited by Deep Oscillation Magnetron Sputtering. Surf. Coat. Technol. 2017, 315, 294–302. [Google Scholar] [CrossRef]
- Hamrock, B.J.; Dowson, D. Ball Bearing Lubrication: The Elastohydrodynamics of Elliptical Contacts; NASA Lewis Research Center: Cleveland, OH, USA, 1981. [Google Scholar]
- Hamrock, B.J.; Dowson, D. Isothermal Elastohydrodynamic Lubrication of Point Contacts: Part III—Fully Flooded Results. J. Lubr. Technol. 1977, 99, 264–275. [Google Scholar] [CrossRef]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology; Butterworth-heinemann: Oxford, UK, 2013; ISBN 0123977762. [Google Scholar]
- Tallian, T.E. On Competing Failure Modes in Rolling Contact. ASLE Trans. 1967, 10, 418–439. [Google Scholar] [CrossRef]
- Balan, M.R.; Tufescu, A.; Cretu, S.S. A Case Study on Relation between Roughness, Lubrication and Fatigue Life of Rolling Bearings. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 147, p. 012013. [Google Scholar]
- Harris, T.A.; Kotzalas, M.N. Essential Concepts of Bearing Technology; CRC Press: Boca Raton, FL, USA, 2006; ISBN 1420006592. [Google Scholar]
- Mu, L.; Wu, J.; Matsakas, L.; Chen, M.; Vahidi, A.; Grahn, M.; Rova, U.; Christakopoulos, P.; Zhu, J.; Shi, Y. Lignin from Hardwood and Softwood Biomass as a Lubricating Additive to Ethylene Glycol. Molecules 2018, 23, 537. [Google Scholar] [CrossRef]
- Song, J. Research Progress of Ionic Liquids as Lubricants. ACS Omega 2021, 6, 29345–29349. [Google Scholar] [CrossRef]
- Yoshida, Y.; Baba, O.; Saito, G. Ionic Liquids Based on Dicyanamide Anion: Influence of Structural Variations in Cationic Structures on Ionic Conductivity. J. Phys. Chem. B 2007, 111, 4742–4749. [Google Scholar] [CrossRef]
- Clarke, C.J.; Bui-Le, L.; Hallett, J.P.; Licence, P. Thermally-Stable Imidazolium Dicationic Ionic Liquids with Pyridine Functional Groups. ACS Sustain. Chem. Eng. 2020, 8, 8762–8772. [Google Scholar] [CrossRef]
- Zorębski, E.; Musiał, M.; Dzida, M. Relation between Temperature–Pressure Dependence of Internal Pressure and Intermolecular Interactions in Ionic Liquids–Comparison with Molecular Liquids. J. Chem. Thermodyn. 2019, 131, 347–359. [Google Scholar] [CrossRef]
- Ding, J.C.; Chen, M.; Mei, H.; Jeong, S.; Zheng, J.; Yang, Y.; Wang, Q.; Kim, K.H. Microstructure, Mechanical, and Wettability Properties of Al-Doped Diamond-like Films Deposited Using a Hybrid Deposition Technique: Bias Voltage Effects. Diam. Relat. Mater. 2022, 123, 108861. [Google Scholar] [CrossRef]
- Evaristo, M.; Fernandes, F.; Cavaleiro, A. Room and High Temperature Tribological Behaviour of W-DLC Coatings Produced by DCMS and Hybrid DCMS-HiPIMS Configuration. Coatings 2020, 10, 319. [Google Scholar] [CrossRef]
- Kasiorowski, T.; Lin, J.; Soares, P.; Lepienski, C.M.; Neitzke, C.A.; De Souza, G.B.; Torres, R.D. Microstructural and Tribological Characterization of DLC Coatings Deposited by Plasma Enhanced Techniques on Steel Substrates. Surf. Coat. Technol. 2020, 389, 125615. [Google Scholar] [CrossRef]
- Aijaz, A.; Ferreira, F.; Oliveira, J.; Kubart, T. Mechanical Properties of Hydrogen Free Diamond-like Carbon Thin Films Deposited by High Power Impulse Magnetron Sputtering with Ne. Coatings 2018, 8, 385. [Google Scholar] [CrossRef]
- Yetim, A.F.; Kovacı, H.; Kasapoğlu, A.E.; Bozkurt, Y.B.; Çelik, A. Influences of Ti, Al and V Metal Doping on the Structural, Mechanical and Tribological Properties of DLC Films. Diam. Relat. Mater. 2021, 120, 108639. [Google Scholar] [CrossRef]
- Bai, M.; Yang, L.; Li, J.; Luo, L.; Sun, S.; Inkson, B. Mechanical and Tribological Properties of Si and W Doped Diamond like Carbon (DLC) under Dry Reciprocating Sliding Conditions. Wear 2021, 484, 204046. [Google Scholar] [CrossRef]
- Wang, Q.J.; Chung, Y.-W. Encyclopedia of Tribology: With 3650 Figures and 493 Tables; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 0387928979. [Google Scholar]
- Marinescu, I.D.; Rowe, W.B.; Dimitrov, B.; Inaski, I. Tribology of Abrasive Machining Processes; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 0815519389. [Google Scholar]
- Li, P.; Zhang, F.; Zhang, H.; Wang, T.; Wang, Q.; Qiao, W. Lubrication Performance of Kite-Shaped Microtexture under Hydrodynamic Lubrication. Tribol. Int. 2023, 179, 108144. [Google Scholar] [CrossRef]
- Kalin, M.; Velkavrh, I.; Vižintin, J. The Stribeck Curve and Lubrication Design for Non-Fully Wetted Surfaces. Wear 2009, 267, 1232–1240. [Google Scholar] [CrossRef]
- Linjamaa, A.; Lehtovaara, A.; Kallio, M.; Léger, A. Running-in Effects on Friction of Journal Bearings under Slow Sliding Speeds. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2020, 234, 362–372. [Google Scholar] [CrossRef]
- Xin, Q. Friction and Lubrication in Diesel Engine System Design. Diesel Engine Syst. Des. 2013, 1, 651–758. [Google Scholar]
- Martini, A.; Zhu, D.; Wang, Q. Friction Reduction in Mixed Lubrication. Tribol. Lett. 2007, 28, 139–147. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.J.; Lin, C.; Shi, F. Development of a Set of Stribeck Curves for Conformal Contacts of Rough Surfaces. Tribol. Trans. 2006, 49, 526–535. [Google Scholar] [CrossRef]
- Lu, X.; Khonsari, M.M.; Gelinck, E.R.M. The Stribeck Curve: Experimental Results and Theoretical Prediction. J. Tribol. 2006, 128, 789–794. [Google Scholar] [CrossRef]
- Xiao, H. Ionic Liquid Lubricants: Basics and Applications. Tribol. Trans. 2017, 60, 20–30. [Google Scholar] [CrossRef]
- Kajdas, C. Importance of Anionic Reactive Intermediates for Lubricant Component Reactions with Friction Surfaces. Lubr. Sci. 1994, 6, 203–228. [Google Scholar] [CrossRef]
- Perkin, S.; Albrecht, T.; Klein, J. Layering and Shear Properties of an Ionic Liquid, 1-Ethyl-3-Methylimidazolium Ethylsulfate, Confined to Nano-Films between Mica Surfaces. Phys. Chem. Chem. Phys. 2010, 12, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Atkin, R.; El Abedin, S.Z.; Hayes, R.; Gasparotto, L.H.S.; Borisenko, N.; Endres, F. AFM and STM Studies on the Surface Interaction of [BMP] TFSA and [EMIm] TFSA Ionic Liquids with Au (111). J. Phys. Chem. C 2009, 113, 13266–13272. [Google Scholar] [CrossRef]










| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Conditions | Potentiometer | 1.6 | 4.9 | 11.2 | 24.7 | 45.5 |
| Sliding Speed (m/s) | 0.02 | 0.07 | 0.18 | 0.40 | 0.74 | |
| Rotational speed (rpm) | 4 | 13 | 32 | 64 | 127 | |
| Rotational speed (rps) | 0.07 | 0.21 | 0.53 | 1.06 | 2.12 | |
| Number of turns | 100 | 250 | 500 | 1000 | 1000 | |
| Sliding time (s) | 1500 | 1181 | 943 | 942 | 471 |
| T = 60 °C | ||||||
|---|---|---|---|---|---|---|
| Sliding speed—u (m/s) | 0.0241 | 0.0765 | 0.1916 | 0.3835 | 0.7671 | |
| PAO 8 + 1 wt.% {[P66614][DEHP]} | Film Thickness—h0 (m) | 1.97 × 10−8 | 4.32 × 10−8 | 8.06 × 10−8 | 1.29 × 10−7 | 2.07 × 10−7 |
| Tallian Parameter—λ | 0.3226 | 0.7077 | 1.3213 | 2.1184 | 3.3939 | |
| PAO 8 + 1 wt.% {[EMIM][DEP]} | Film Thickness—h0 (m) | 1.87 × 10−8 | 4.1 × 10−8 | 7.65 × 10−8 | 1.23 × 10−7 | 1.96 × 10−7 |
| Tallian Parameter—λ | 0.3058 | 0.6710 | 1.2528 | 2.0086 | 3.2180 | |
| T = 80 °C | ||||||
|---|---|---|---|---|---|---|
| Sliding speed—u (m/s) | 0.0241 | 0.0765 | 0.1916 | 0.3835 | 0.7671 | |
| PAO 8 + 1 wt.% {[P66614][DEHP]} | Film Thickness—h0 (m) | 1.22 × 10−8 | 2.68 × 10−8 | 5.01 × 10−8 | 8.03 × 10−8 | 1.29 × 10−7 |
| Tallian Parameter—λ | 0.2002 | 0.4393 | 0.8202 | 1.3151 | 2.1070 | |
| PAO 8 + 1 wt.% {[EMIM][DEP]} | Film Thickness—h0 (m) | 1.18 × 10−8 | 2.59 × 10−8 | 4.83 × 10−8 | 7.74 × 10−8 | 1.24 × 10−7 |
| Tallian Parameter—λ | 0.1931 | 0.4237 | 0.7910 | 1.2683 | 2.0319 | |
| T = 100 °C | ||||||
|---|---|---|---|---|---|---|
| Sliding speed—u (m/s) | 0.0241 | 0.0765 | 0.1916 | 0.3835 | 0.7671 | |
| PAO 8 + 1 wt.% {[P66614][DEHP]} | Film Thickness—h0 (m) | 8.1 × 10−9 | 1.78 × 10−8 | 3.32 × 10−8 | 5.32 × 10−8 | 8.52 × 10−8 |
| Tallian Parameter—λ | 0.1327 | 0.2911 | 0.5435 | 0.8714 | 1.3961 | |
| PAO 8 + 1 wt.% {[EMIM][DEP]} | Film Thickness—h0 (m) | 7.92 × 10−9 | 1.74 × 10−8 | 3.25 × 10−8 | 5.2 × 10−8 | 8.34 × 10−8 |
| Tallian Parameter—λ | 0.1298 | 0.2848 | 0.5317 | 0.8525 | 1.3658 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, S.; Omiya, T.; Cavaleiro, A.; Vilhena, L.; Ramalho, A.; Ferreira, F. Impact of Temperature Variation on Friction Behaviour of Rare Earth-Doped Diamond-like Carbon Coatings with Ionic Liquid Lubricants. Lubricants 2023, 11, 302. https://doi.org/10.3390/lubricants11070302
Shaikh S, Omiya T, Cavaleiro A, Vilhena L, Ramalho A, Ferreira F. Impact of Temperature Variation on Friction Behaviour of Rare Earth-Doped Diamond-like Carbon Coatings with Ionic Liquid Lubricants. Lubricants. 2023; 11(7):302. https://doi.org/10.3390/lubricants11070302
Chicago/Turabian StyleShaikh, Shahsharif, Takeru Omiya, Albano Cavaleiro, Luis Vilhena, Amilcar Ramalho, and Fábio Ferreira. 2023. "Impact of Temperature Variation on Friction Behaviour of Rare Earth-Doped Diamond-like Carbon Coatings with Ionic Liquid Lubricants" Lubricants 11, no. 7: 302. https://doi.org/10.3390/lubricants11070302
APA StyleShaikh, S., Omiya, T., Cavaleiro, A., Vilhena, L., Ramalho, A., & Ferreira, F. (2023). Impact of Temperature Variation on Friction Behaviour of Rare Earth-Doped Diamond-like Carbon Coatings with Ionic Liquid Lubricants. Lubricants, 11(7), 302. https://doi.org/10.3390/lubricants11070302










