Abstract
Aldehyde condensation is a reaction step in the oxidization of a lubricant base stock into high-molecular-weight products, forming sludge and a paint film, which lead to the failure of lubricating oil. Calculations on the basis of the density functional theory (DFT) were employed to investigate the reaction mechanism of the acid-catalyzed aldol condensation of a lubricant base stock. Carbonyl compounds could be converted into their resonant enol structures. However, the activation energy of the process was relatively high, and it was difficult to initiate. The existence of the acid could obviously decrease the activation energy of the reaction from 269.17–287.82 kJ/mol to 177.10–177.63 kJ/mol, and it significantly reduced the difficulty of initiating this reaction. The carbocation formed by the carbonyl compounds and acid could further react with the enol and produce an intermediate reaction product in which the chain of molecules grew longer. This process was not difficult to initiate, with a reaction activation energy of 65.10 kJ/mol. The intermediate product with a larger molecular weight could be converted into carbonyl compounds containing a β-hydroxy by removing a hydrogen proton from it. The energy barrier for this process was 193.15 kJ/mol, and it was not easy to initiate the reaction.
1. Introduction
During the operation of mechanical equipment, the relative movements of friction pairs will cause a large amount of friction and wear, resulting in energy loss and equipment damage. Adopting lubrication technology is a common way to reduce friction and wear, especially in engines. The most commonly used lubrication technology is lubricating oil, which accounts for about 85% of all lubricating materials. The ability of lubricating oil to reduce friction and wear is called lubricating performance. According to the Stribeck curve, the lubricating performance of a lubricant is related to its viscosity, the sliding speed and the normal load of friction pairs [1].
As the service time of a lubricating oil increases, it will gradually oxidize and deteriorate, resulting in an increase in viscosity and even the formation of sludge and other sediments [2,3,4]. The viscosity of a lubricating oil is related to the film thickness and lubricating performance of the lubricating oil [5,6]. Lubricants with high viscosities are beneficial to the formation of an oil film on the surface of a friction pair. However, this will lead to an increase in energy consumption due to the larger internal friction force between the high-viscosity lubricating oils [7,8]. High-viscosity lubricants affect the cold start of engines and increase wear during the low-temperature operation of engines. It was found that the friction will increase when the viscosity of the lubricating oil is higher under certain conditions [9]. Moreover, there will be more heat generated by friction, and the formation of carbon deposits will increase [9]. The heat dissipation capacity of the cylinder will decrease if the formed sludge attaches to the wall of the crankcase, resulting in an increase in the lubricating oil’s temperature and a decline in the engine’s power. The sludge adhering to the internal oil channels of engines will increase the flow resistance of the lubricant, reducing the traffic of lubricating oils. There will be no lubricant between friction pairs if sludge blocks the oil channel, and it will accelerate the damage to friction parts [10,11,12]. This will not only consume more energy due to the increase in friction but also cause engine damage.
Shimonaev et al. [13] studied the oxidation process of lubricating oils in a crankcase via laboratory simulation experiments and analyzed the composition of the lubricating oil sludge obtained after oxidation. It was found that the sludge was mainly composed of non-metallic elements such as carbon, hydrogen, oxygen/sulfur, and small amounts of metallic elements. The most abundant element was carbon, with a mass fraction of 71.8 percent. The hydrogen content was about 7.5 percent, and the oxygen/sulfur content was also high, reaching 15.7 percent. Lillywhite et al. [11] found that a large quantity of carbonyl oxidation products existed in lubricating oil sludge, and they had rich molecular structures and a wide relative molecular weight distribution. It showed that the sludge was mainly composed of hydrocarbons and their oxides and had a certain quantity of polymers or condensates with high relative molecular weights.
At present, many research studies have been carried out on the oxidation of lubricating oils [14,15,16,17,18,19,20,21]. It was found that a variety of oxidation products containing hydroxyl, carboxyl, or carbonyl groups were formed during the oxidation of lubricating oils through the mechanism of a free radical chain reaction [15,16,17]. The molecular weights of some of the oxidation products were obviously larger than those of the molecules in the original lubricating oils. They might be generated by aldol condensation of carbonyl-containing lubricant oxidation products [22]. However, this was only a theoretical speculation, and there was no in-depth study on the specific reaction process. The research about lubricant oxidation mainly focused on the analysis of the reaction products and the mechanisms of the formation of small-molecule oxidation products. Investigations of the formation of oxidation products with larger molecular weights were limited.
Aldol condensation can occur between carbonyl compounds under the catalysis of an acid or base if the α-C of the carbonyl group contains hydrogen atoms. The overall reaction results are as follows. In the aldol condensation reaction, the hydrogen atom of α-C in one carbonyl compound will be transferred to the oxygen atom of the carbonyl group in another carbonyl compound molecule. The rest of the carbonyl compound losing a hydrogen atom will be connected with the carbon atom of the carbonyl group in the carbonyl compound molecule that obtains the hydrogen atom to generate a β-hydroxyketone molecule, which increase the molecular weight. During the oxidation of lubricating oils, organic acids containing carboxylic groups can be formed [15,16,17]. In addition, inorganic acids formed via the combustion of fuels in the engine combustion chamber could enter lubricating oils through the gap between the cylinder wall and the piston [11]. Lubricants will gradually become acidic with the extension of their service time. Aldol condensation due to acid catalyzation is likely to occur. In this paper, the acid-catalyzed aldol condensation process was explored via a calculation made on the basis of the density functional theory (DFT), and the key step affecting the aldol condensation reaction was ascertained, with the aliphatic ketone generated by the oxidation of the lubricant as a reactant. These research results might be helpful in deepening the understanding of the oxidation of lubricating oils. They could also provide theoretical references for inhibiting the oxidation of lubricating oils and slowing down the increase in a lubricant’s viscosity and the formation of sludge and other sediments, which is of great significance to prolonging the service lives of lubricating oils.
2. Materials and Methods
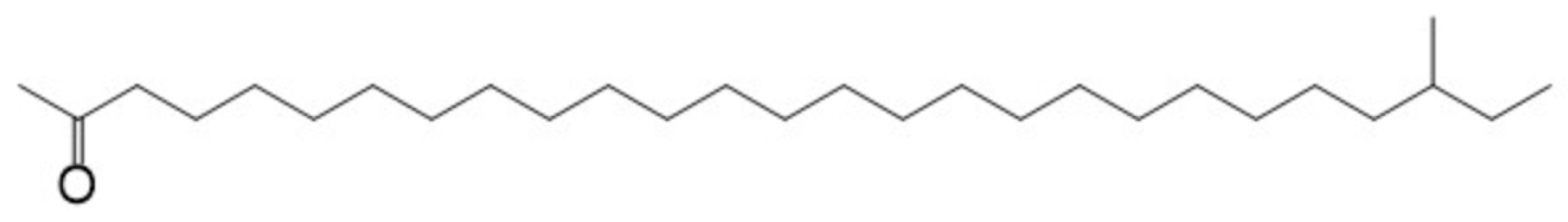
A variety of lubricant oxidation products, such as aliphatic alcohol, aliphatic aldehyde, aliphatic ketone, and aliphatic acid, will be formed during the oxidation of lubricating oils [15,16,17]. There is carbonyl among the aliphatic aldehyde, aliphatic ketone, and aliphatic acid. All of these molecules in which the α-C of carbonyl group contains a hydrogen atom may react to form oxidation products with larger molecular weights through the condensation of the aldol. To simplify the calculation, in this paper, only the aliphatic ketone was selected as the reactant to investigate the mechanism of the acid-catalyzed aldol condensation. The concentration of the base stock, which was a hydrocarbon with 20~40 carbon atoms, is the most commonly found base stock in lubricating oils, and the hydrocarbons in the most-consumed hydrogenated base stock are mainly paraffins [23,24]. Therefore, 2-methyl-2-heptacosanoic ketone was selected as the model aliphatic ketone model, according to the structure of the base stock molecules [23,24]. The structure of aliphatic ketone is shown in Figure 1.
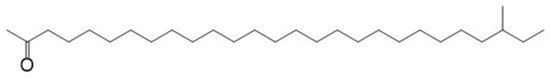
Figure 1.
Structure of aliphatic ketone model molecule.
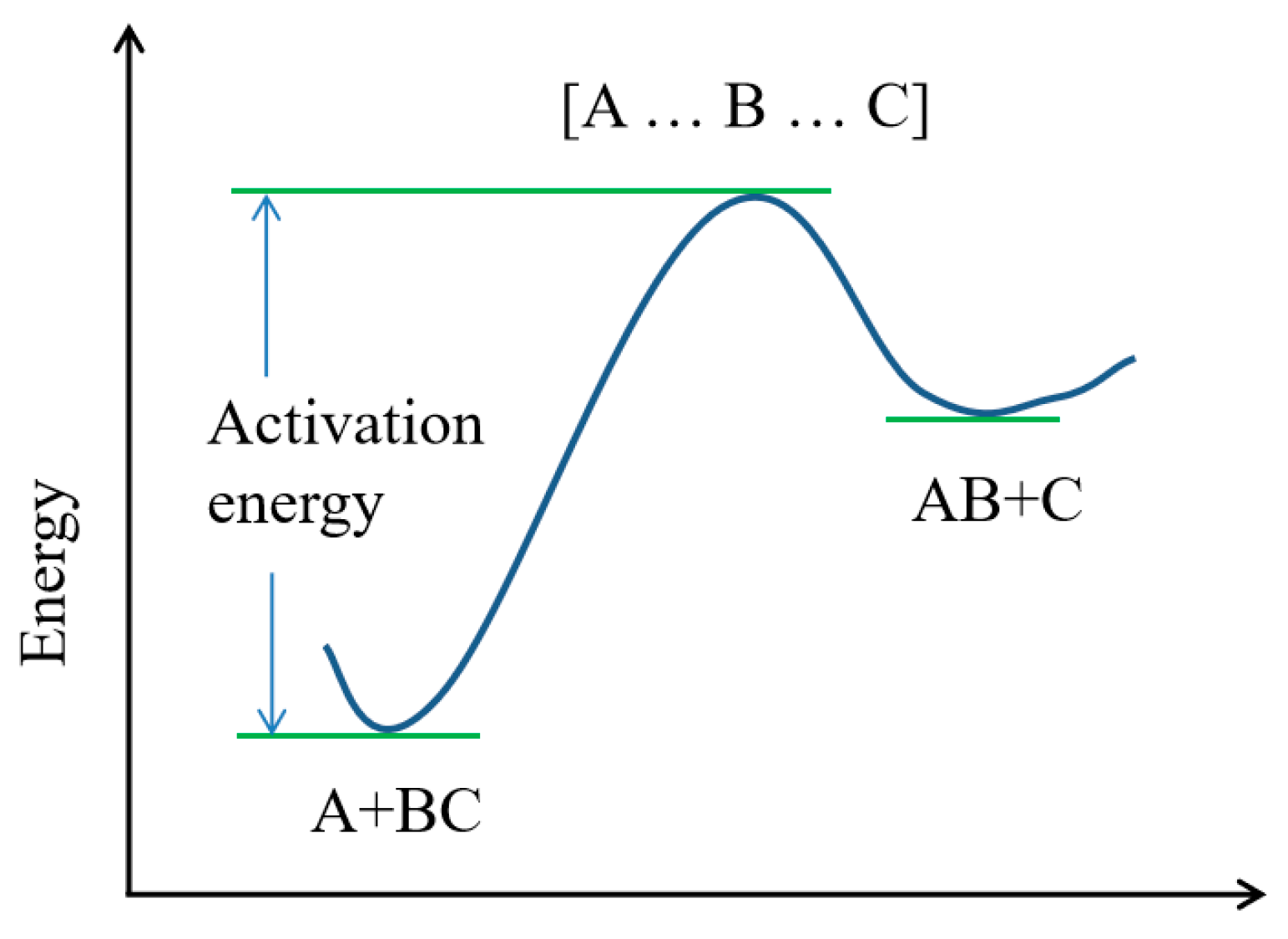
The transition state theory is a theoretical method of studying the mechanism and reaction rate of an elementary reaction. A schematic diagram of the transition state reaction process is shown in Figure 2. For the reaction A + BC→AB + C, transition state theory holds that the actual reaction process is A + BC → [A … B … C] → AB + C. During the reaction, A gradually approaches B in the reactant BC. It has a certain interaction with B and forms a chemical bond A…B, which seems to be weak. At the same time, the original chemical bond between B and C will be gradually weakened and form a seemingly broken chemical bond B … C. The reactants change from two molecules, A and BC, into an unstable [A … B … C], which is the transition state of this reaction. Both reactants and products are in a stable state with low energy, and the transition state is in an unstable state with high energy. The single-point-energy difference between the reactants and the transition state is the activation energy of the reaction.
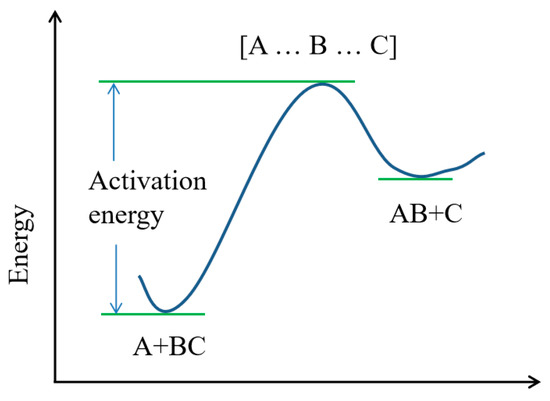
Figure 2.
Schematic diagram of transition state reaction process.
The activation energies of the elementary reactions in aldol condensation were calculated using the transition state theory. In the process of calculating the activation energies of the reactions, the structures of the reactants and products were optimized to help them reach a stable state, and then the transition state was searched. The single point energies of the reactants and the transition state structure were calculated, and the energy difference between them was the activation energy of the reaction. One of the prerequisites for intermolecular reactions is that the distance between molecules is small to a certain extent at a specific angle. The charges of molecules/atoms will directly affect the interaction between them and then affect the reaction. They will repel each other when they have the same charge, and they will attract each other when they have the same charge. The deformation charge density is obtained by subtracting the charge density after bonding from the atomic charge density of the corresponding point. The distribution of the overall charge of the molecules can be obtained from the deformation charge density of the molecules to obtain the regional range of reactive sites. Atomic charge is helpful in accurately comparing the strengths of interactions between different reaction sites. Therefore, the deformation charge densities of molecules and the ESP charges of atoms were calculated.
The initial structure of the molecules was created using Visualizer. All DFT calculations (optimizing the geometric structure and electronic structure of the model molecules, search the transition state of the elementary reaction, and calculate the single point energy and activation energy of the elementary reactions) were performed at DMol3 using Materials Studio 8.0 software. The basis set and local correlation density functional were DNP and the Perdew Wang (PW91), respectively [25]. A zero-point vibration energy (ZPVE) correction was carried out for all the energy calculations, and the threshold of iterative convergence for a self-consistent field (SCF) was set to 1 × 10−5 Ha. The convergence precision of energy was 2 × 10−5 Ha, that of the force was 0.0004 Ha/nm, and that of the displacement was 5 × 10−4 nm. Completely linear synchronous transformations and quadratic synchronous transformations (LST/QST) were used to search the transition state in reactions.
3. Results and Discussion
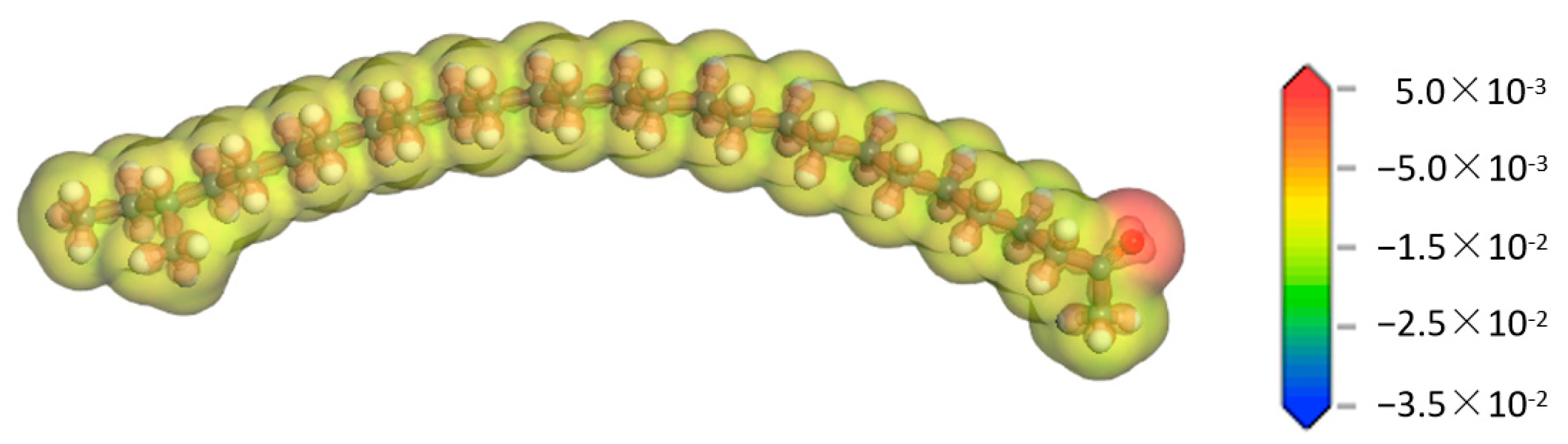
In order to understand the possible reactions and reaction mechanism of aliphatic ketone molecules, it is necessary to have a deep understanding of their properties. Therefore, the deformation charge density of each atom in the aliphatic ketone molecule was calculated, and the result is shown in Figure 3. It can be seen from Figure 3 that the deformation charge density of the alkyl chain in the aliphatic ketone molecule was relatively uniform, while that of the oxygen atom in the carbonyl group was obviously larger, indicating that there was a larger electron density around the oxygen atom. The reason for the heterogeneous distribution of the deformation charge density in the aliphatic ketone molecule lies in the various electronegativities of the atoms in the molecule. The electronegativity of oxygen atoms is distinctly stronger than that of carbon atoms [26]. They have a stronger ability to attract electrons in the C=O bond, which would make the electron density around the oxygen atom higher.
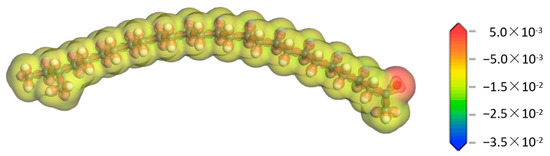
Figure 3.
Deformation charge density of the aliphatic ketone molecule.  —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
 —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
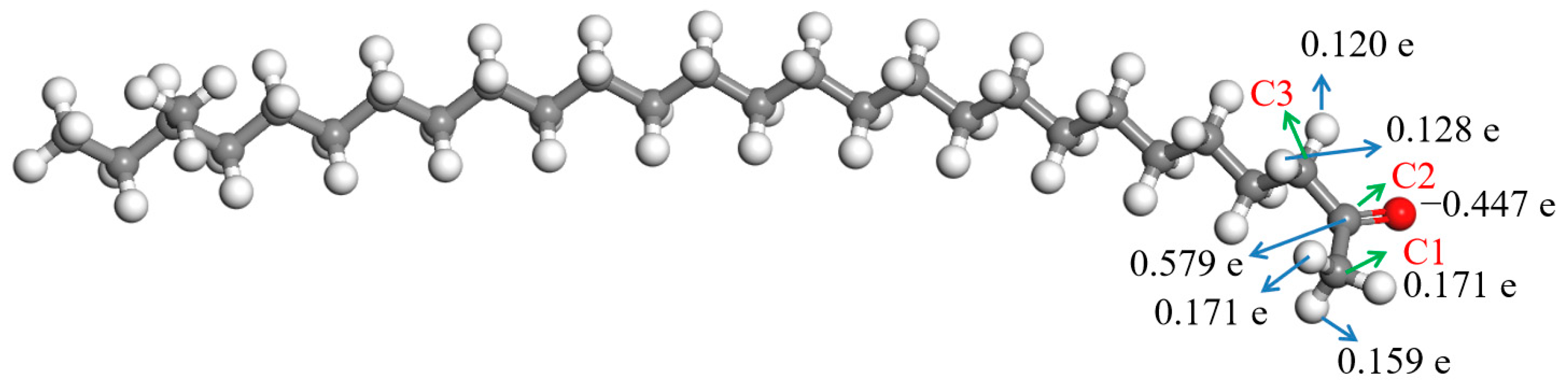
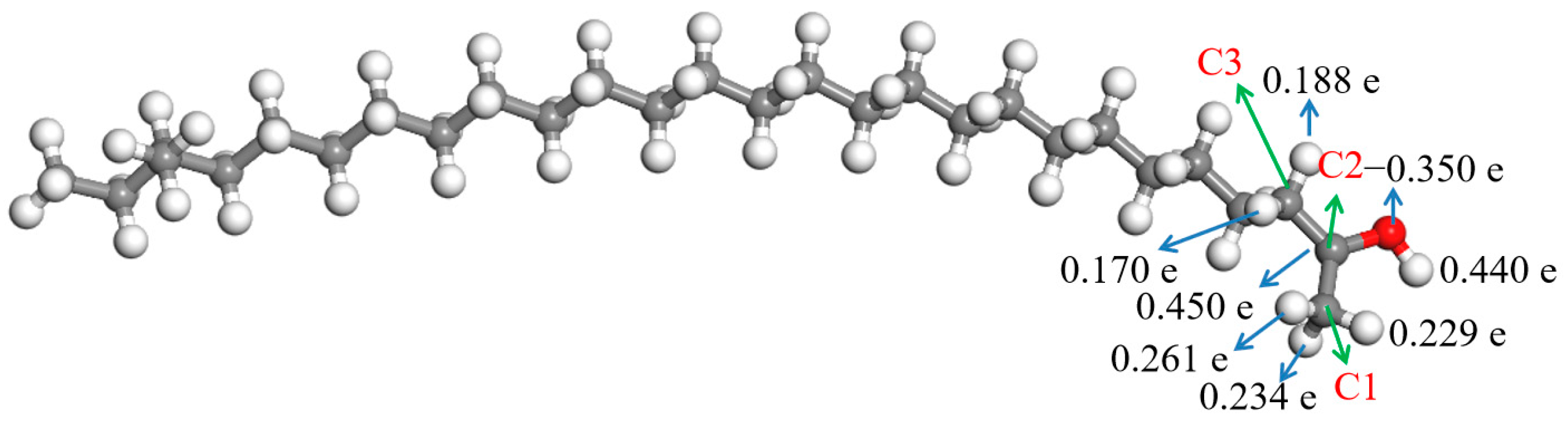
The ESP charges of atoms in the aliphatic ketone molecule were calculated to understand the charges on the atoms more precisely, and the result is shown in Figure 4. Herein, only the atoms in and near the carbonyl group are discussed about due to the uneven distribution of the deformation charge density, which was mainly located in them. Figure 4 shows that there were some negative charges on the oxygen atom in the carbonyl group, while the charge of the carbon atom in the carbonyl group (C2) was positive; they were −0.447 e and 0.579 e, respectively. There were some positive charges on the hydrogen atoms bonded with the neighboring carbon atoms (C1 and C3) of the carbonyl group. The charges of hydrogen atoms bonded with C1 were 0.159–0.171 e, and those of hydrogen atoms bonded with C3 were 0.120–0.128 e.
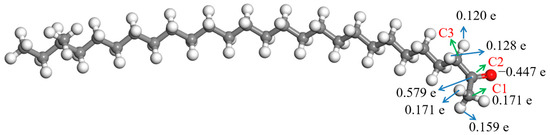
Figure 4.
ESP charges of the atoms in the aliphatic ketone molecule.  —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
 —Oxygen atom,
—Oxygen atom, 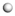 —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
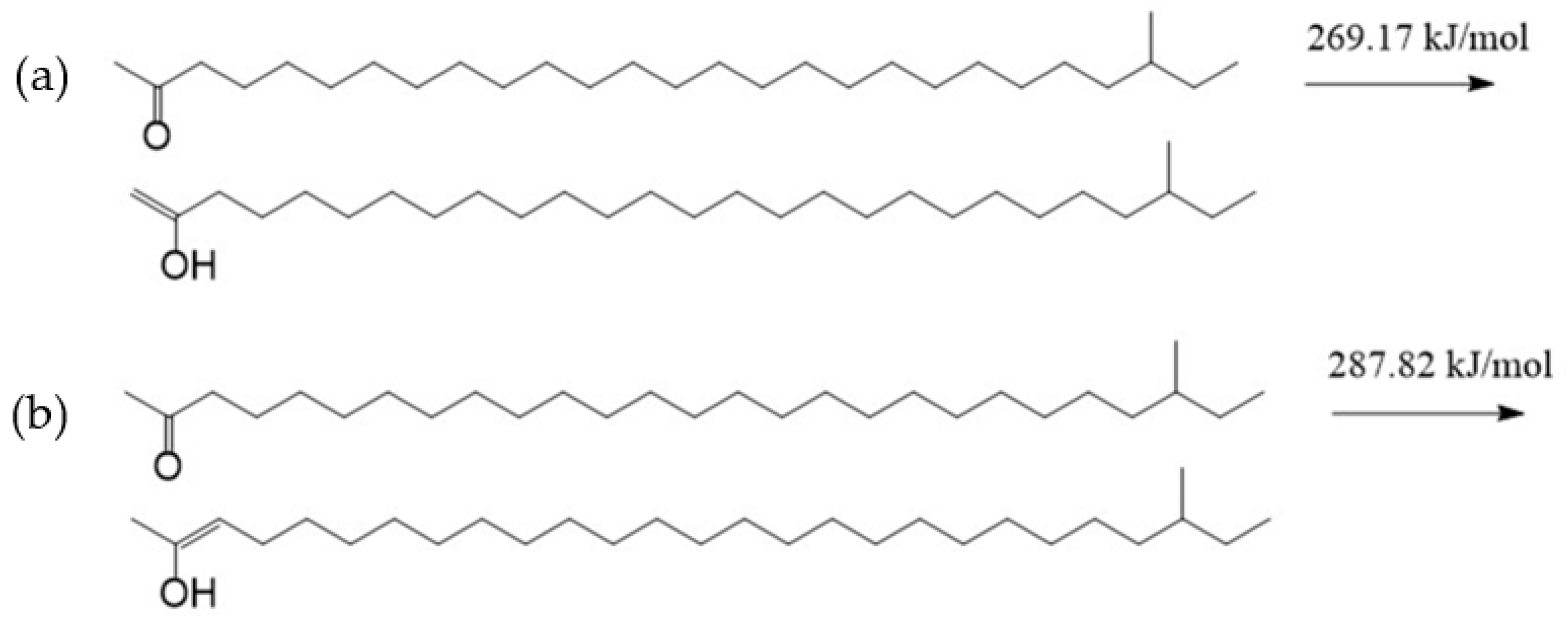
The carbonyl group in the aliphatic ketone contains a C-O π bond, and the electron cloud density in the π bond is low because of the low overlap of the electron orbits forming the π bond. The electron cloud in the C-H bond adjacent to the carbonyl group will partially delocalize to the π bond, which will produce a p–π hyperconjugate effect, thus making the molecule more stable. However, this process will make the stability of the C-H bonds involved in the hyperconjugate effect worse. Because the oxygen atom on the carbonyl group has more negative charges and the hydrogen atom in the C-H bond adjacent to the carbonyl group has some positive charges, there will be a certain electrostatic attraction between them. The ortho-C-H bond of the carbonyl group and the C-O π bond in the carbonyl group will be broken when the molecule absorbs a certain amount of energy. The hydrogen atom in the C-H bond will form an H-O σ bond with the oxygen atom in the carbonyl group, and the carbon atom in the C-H bond will form a C-C π bond with the carbon atom in the carbonyl group. The above process is the conversion of an aliphatic ketone into enol. An enol is the resonance structure of an aliphatic ketone [27]. The hydrogen atom that was captured by the oxygen atom may be bonded with C1 or C3. The activation energies of the above two reaction processes were calculated, and the results are shown in Figure 5. The activation energy of the reaction in which the oxygen atom captured the hydrogen atom bonded with C1 was 269.17 kJ/mol, while that of the reaction in which the oxygen atom captured the hydrogen atom bonded with C3 was 287.82 kJ/mol. This indicates that the oxygen atom in the carbonyl group was captured the hydrogen atom in the shorter alkyl more easily compared with the hydrogen atom in the longer alkyl. The reason for this may be that there are more positive charges in the hydrogen atom bonded with C1 compared with the hydrogen atom bonded with C3 (Figure 4). The attraction between the oxygen atom and the hydrogen atom bonded with C1 is relatively large. However, the activation energies of the above two reactions were both relatively high, which manifested in the conversion of the aliphatic ketone into enol being quite difficult.
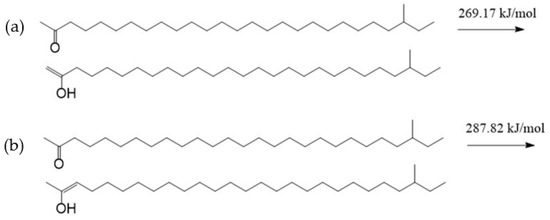
Figure 5.
The conversion of an aliphatic ketone into enol (a) the oxygen atom is bonded with C1; (b) the oxygen atom is bonded with C3.
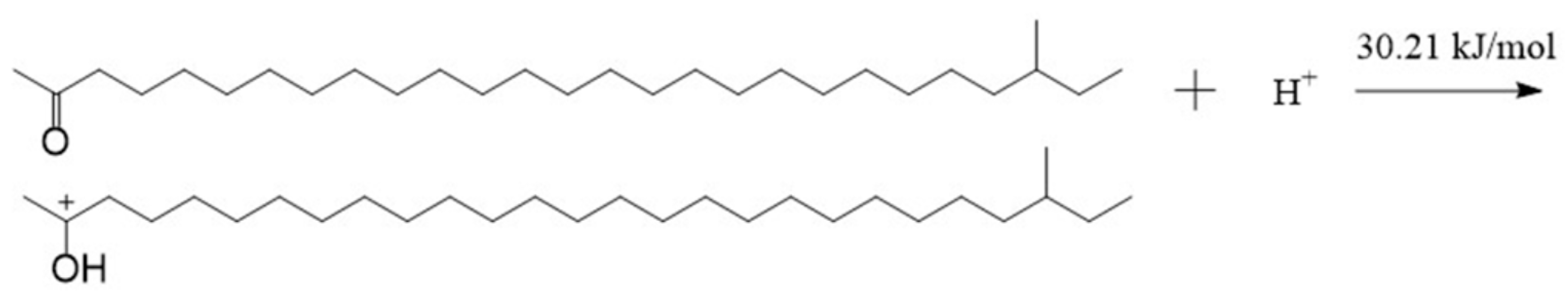
If there is acid in the lubricant, an oxygen atom can also produce a strong electrostatic attraction to hydrogen protons owing to the negative charge on it. There are lone pairs of electrons on the oxygen atom, and the hydrogen atom in the hydrogen proton has vacant orbitals [28,29]. A coordinate bond may be formed between them when they get close enough. And then, an intramolecular electron rearrangement may occur in the structure formed by the aliphatic ketone and the hydrogen proton. Thus, the oxygen atom in the aliphatic ketone and the hydrogen atom in the hydrogen proton will form a hydroxyl, and the carbon atom bonded with the hydroxyl becomes a carbocation. The formed structure is called TTZ in this paper. The activation energy of the process was calculated, as shown in Figure 6. As can be seen in the result, the activation energy of the reaction between the aliphatic ketone and the hydrogen proton was only 30.21 kJ/mol. This is so low that the reaction can be carried out very easily. In order to study the properties and possible reactions of TTZ, the ESP charges of its atoms were calculated, and the result is shown in Figure 7.
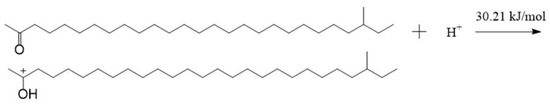
Figure 6.
The reaction between the aliphatic ketone and the hydrogen proton.
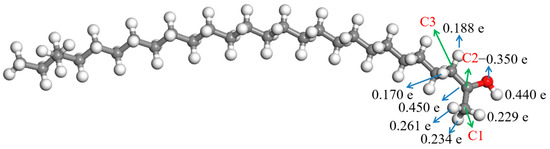
Figure 7.
ESP charges of atoms in TTZ.  —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
 —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
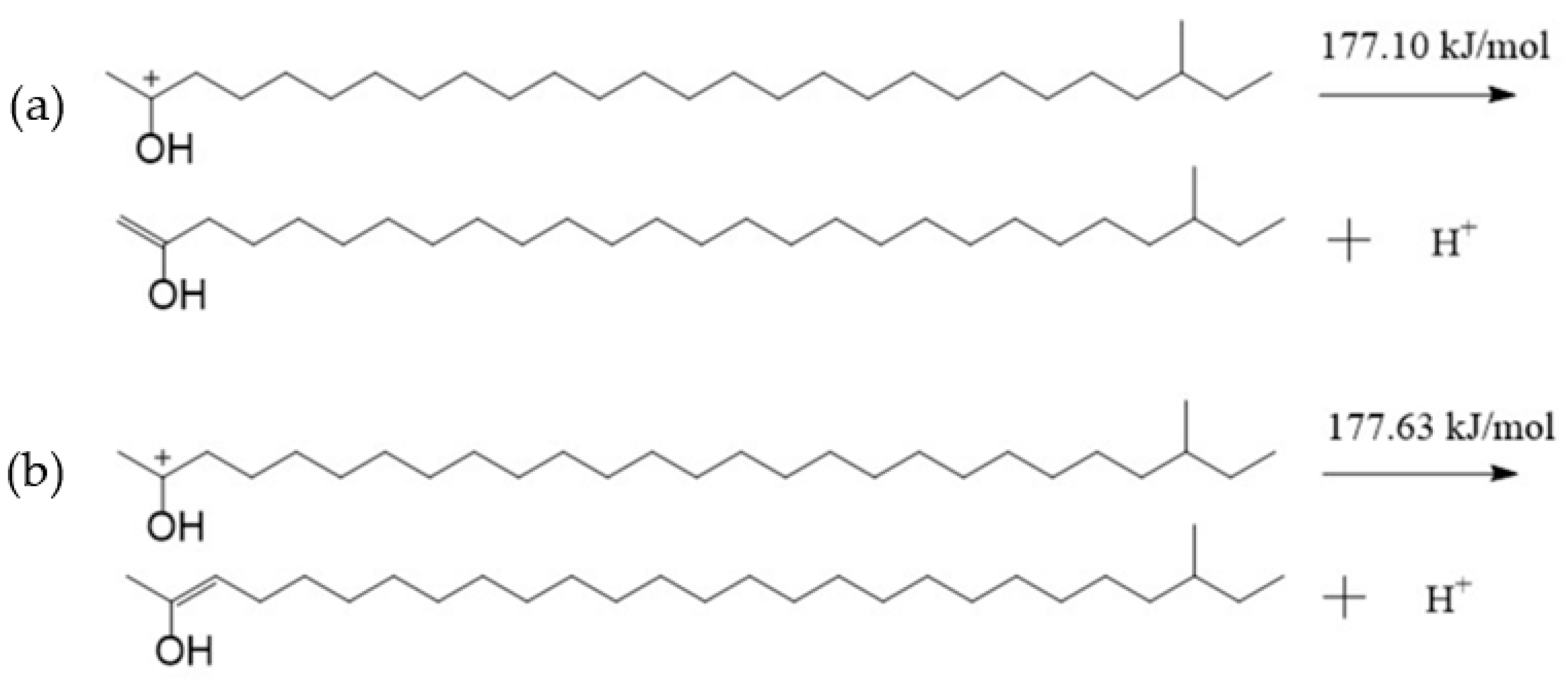
Figure 7 showed that the negative charge of the oxygen atom in TTZ was −0.350 e, which was less than that of the oxygen atom in the aliphatic ketone (Figure 4). However, there were more positive charges in the hydrogen atoms bonded with C1 and C3, which were 0.170–0.261 e. A hydrogen atom bonded with C1 or C3 might break away from TTZ in the form of a hydrogen proton, and the bond between C2 and C1 or C3 might be changed from a C—C bond to C=C bond if the TTZ absorbed enough energy. Then, an enol would be formed. The activation energies of the above reaction processes were calculated, and results are shown in Figure 8. The activation energy of the reaction in which the hydrogen atom bonded with the C1 separated from TTZ was 177.10 kJ/mol, while that of the reaction in which the hydrogen atom bonded with the C3 separated from TTZ was 177.63 kJ/mol. The activation energies of the two reactions in Figure 8 are almost the same, indicating that the degree of difficulty and possibility of these two reactions are similar. Compared with the results calculated in Figure 5, Figure 6 and Figure 8, the energy barrier required for the conversion of an aliphatic ketone into an enol structure was greatly reduced with the catalysis of acid. In order to study the property and possible reactions of the enol, the ESP charges of the atoms in the enol were calculated, and the result is shown in Figure 9. To simplify the calculation, only the enol generated by the reaction in Figure 8a was analyzed.
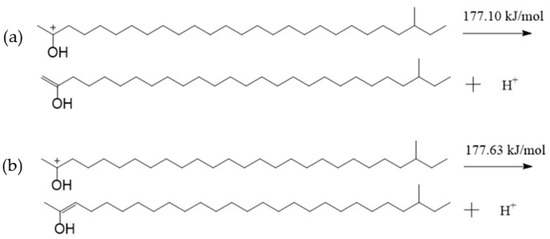
Figure 8.
The reaction of a hydrogen proton bonded with C1 (a) or C3 (b) breaking away from TTZ.
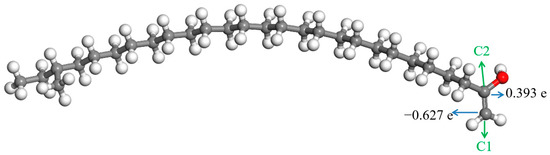
Figure 9.
ESP charges of the atoms in the enol molecule.  —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
 —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
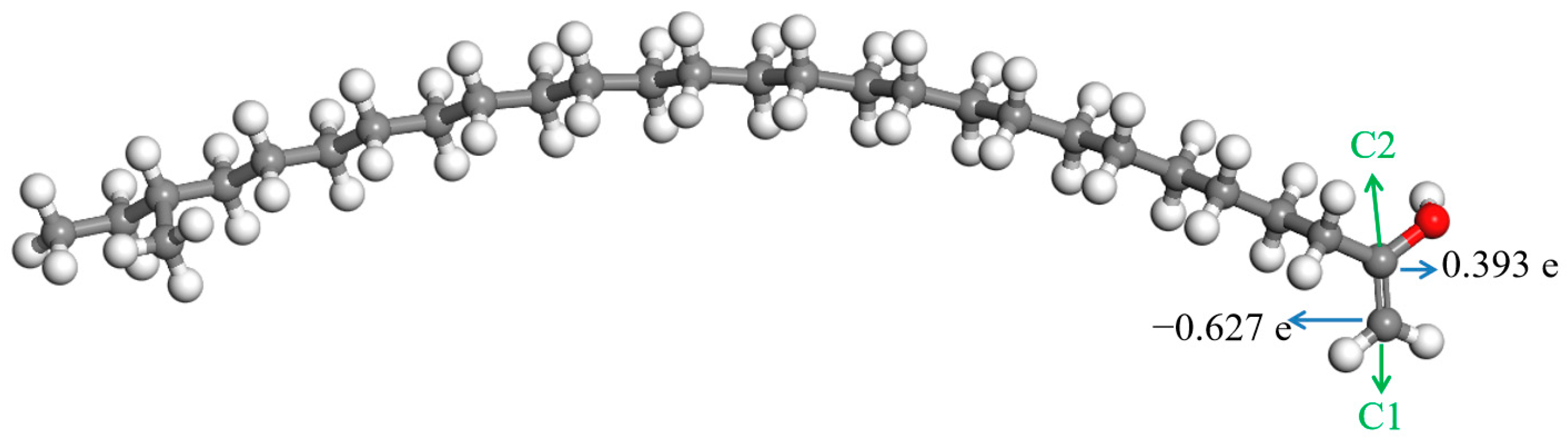
It can be seen from Figure 9 that the charges on the two carbon atoms in the C=C bond of the enol molecule were quite different. There were some positive charges on the carbon atom bonded with the hydroxyl, while the charge on the other carbon atom in the C=C bond was negative; they were 0.393 e and −0.627 e, respectively. The electronegativity of the oxygen atom in the hydroxyl is distinctly stronger than that of the carbon atom bonded with it. It has a stronger attraction to the electrons in the C—O bond. Therefore, the electron density around the carbon atom is low, and the charge on it is positive. The electronegativity of the other carbon atom in the C=C bond is stronger than that of hydrogen atoms bonded with it, leading to the negative charges on it.
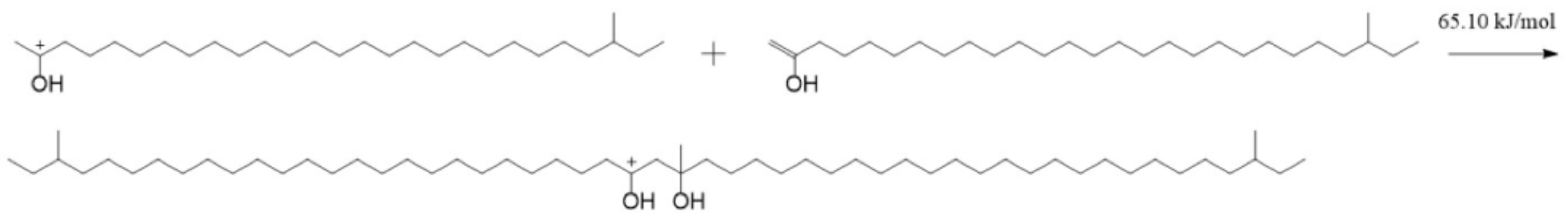
The aliphatic ketone reacts easily with the hydrogen proton and forms TTZ, according to Figure 6. Therefore, a large portion of the aliphatic ketone will react to TTZ when there are enough acids in lubricants. Some TTZ may further change into enols by the way of Figure 8. Therefore, TTZ and enols coexist in lubricating oils. There are electrostatic attractions between the positive C2 in TTZ and the negative C1 in the enol. A new C—C bond may be formed between them if they absorb enough energy. At the same time, the C=C bond in the enol will change into a C—C bond, and the intramolecular electron rearrangement will occur in the structure formed by TTZ and enols, which is called SCTZ in this paper. The activation energy of the above reaction was calculated, and it is shown in Figure 10. It can be seen from the result that the activation energy of the reaction between the TTZ and enols was only 65.10 kJ/mol, indicating that the reaction occurred very easily. In order to study the property of SCTZ, the ESP charges of its atoms were calculated, and the result is shown in Figure 11.
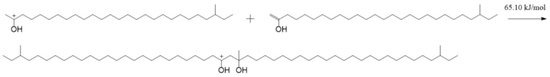
Figure 10.
The reaction between the aliphatic ketone and TTZ.
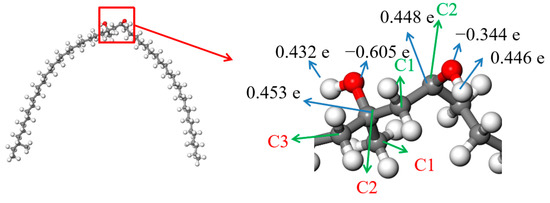
Figure 11.
ESP charges of the atoms in SCTZ.  —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
 —Oxygen atom,
—Oxygen atom,  —Hydrogen atom, and
—Hydrogen atom, and  —Carbon atom.
—Carbon atom.
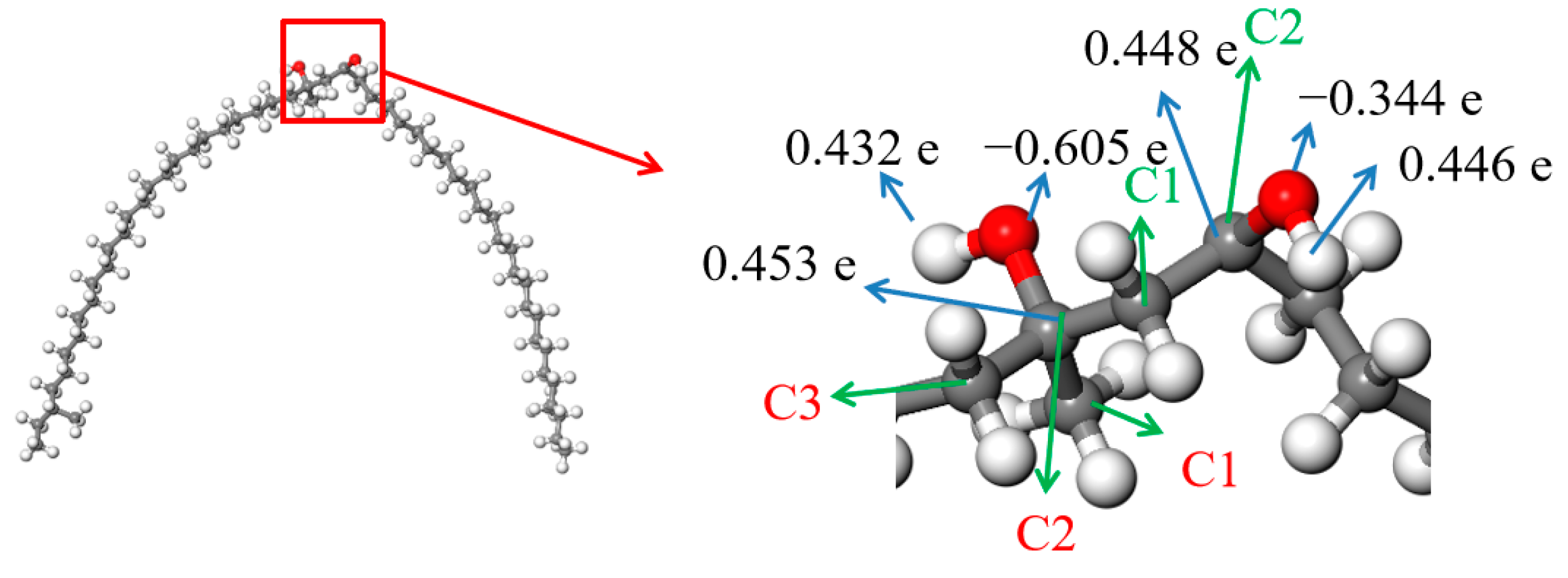
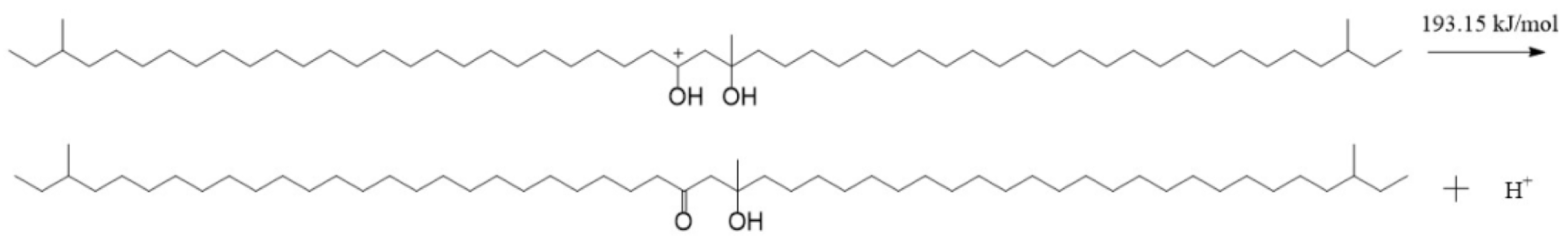
The result in Figure 11 shows that both of the carbon atoms bonded with hydroxyls were positive, and the charges on them were 0.453 e and 0.448 e, respectively. There were some negative charges on the oxygen atoms in the SCTZ hydroxyls, while the hydrogen atoms in the hydroxyls were positive. The charges on the oxygen atoms were −0.605 e and −0.344 e, respectively. There were charges of 0.432 e and 0.446 e on the hydrogen atoms. The whole molecule was positive. The hydrogen atom with more positive charge may break away from SCTZ and form an electrically neutral, stable structure, which is the β-hydroxy aliphatic ketone. The activation energy of the process was calculated and is shown in Figure 12. The result shows that the activation energy of the reaction was 193.15 kJ/mol, which is not low. It indicates that the reaction did not occur easily.
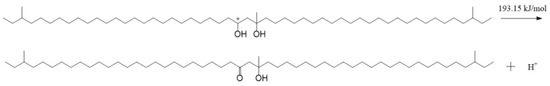
Figure 12.
The reaction of a hydrogen proton breaking away from SCTZ.
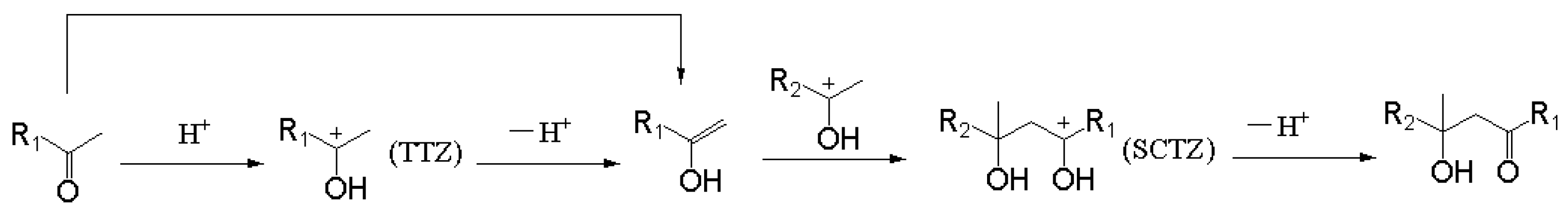
The reaction mechanism of an acid-catalyzed aldol condensation was obtained through the above studies, as shown in Figure 13. The aliphatic ketone can be converted into its resonance structure (enol) under certain conditions. This reaction will be obviously easier under the catalysis of acid when compared with a direct transformation. The formation of the enol may be mainly catalyzed by an acid. The intermediate product of this process (TTZ) can react with the enol through the addition reaction and form a carbocation intermediate (SCTZ) with a larger molecular weight. A hydrogen atom in a hydroxyl may be removed from SCTZ in the form of a hydrogen proton to form the β-hydroxy aliphatic ketone. The last reaction may be the rate-controlling step in the whole acid-catalyzed aldol condensation process, compared with the activation energies of each elementary reaction.
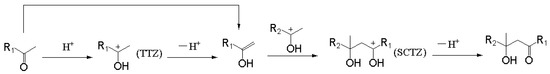
Figure 13.
Reaction mechanism of acid-catalyzed aldol condensation.
4. Conclusions
Aliphatic ketone, a product of lubricant oxidation, was selected as a reactant to investigate the mechanism of acid-catalyzed aldol condensation. The elementary reactions of acid-catalyzed aldol condensation were investigated via a DFT calculation. The results can be summarized as follows:
(1) Carbonyl compounds are difficult to convert into enols directly, which represent a significant reactant in the aldol condensation. However, acids can react with carbonyl compounds and form a carbocation. The intermediate product can be changed into enols more easily. Therefore, this process will be easier with the catalysis of acids.
(2) The enol can be easily reacted with the carbocation formed by the carbonyl compounds and acids; then, a new carbocation with a larger molecular weight will be formed. A hydrogen proton can be removed from it, and it can be further converted into carbonyl compounds with a β-hydroxy. The activation energy of this process is relatively high, and it may be the rate-controlling step in the whole acid-catalyzed aldol-condensation process.
Author Contributions
Conceptualization, L.X. and Y.L.; methodology, L.X.; validation, R.L.; formal analysis, Y.L.; investigation, L.X.; resources, L.X.; data curation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, L.X.; visualization, L.X.; supervision, R.L.; project administration, R.L.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Science and Technology Liaoning TalentProject Grants (No. 601010314), and the Natural Science Foundation of China (NSFC; No.52274338).
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the calculation support of the Key Laboratory of Molecular Oil Refining of Research Institute of Petroleum Processing in SINOPEC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials, 2nd ed.; Arnold: London, UK, 2017. [Google Scholar]
- Kudish, I. Effect of lubricant degradation on contact fatigue. ASLE Trans. 2005, 48, 100–107. [Google Scholar] [CrossRef]
- Thompson, B.A.W.; Davies, N.W.; Goldsworthy, P.M.; Riddle, M.J.; Snape, I.; Stark, J.S. In situ lubricant degradation in Antarctic marine sediments. 1. Short-term changes. Environ. Toxicol. Chem. 2010, 25, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.O.; Santos, I.M.G.D.; Souza, A.G.; Sobrinho, E.V.; Fernandes, V.J., Jr.; Silva, A.J.N. Thermoanalytical and rheological characterization of automotive mineral lubricants after thermal degradation. Fuel 2004, 83, 2393–2399. [Google Scholar] [CrossRef]
- Fryza, J.; Sperka, P.; Kaneta, M.; Krupka, I.; Hartl, M. Effects of lubricant rheology and impact speed on EHL film thickness at pure squeeze action. Tribol. Int. 2017, 106, 1–9. [Google Scholar] [CrossRef]
- Guo, L.; Wong, P.L.; Guo, F. Effect of viscosity and sliding speed on boundary slippage in thin film hydrodynamic lubrication. Tribol. Int. 2017, 107, 85–93. [Google Scholar] [CrossRef]
- Carvalho, M.J.S.; Seidl, P.R.; Belchior, C.R.P.; Sodre, J.R. Lubricant viscosity and viscosity improver additive effects on diesel fuel economy. Tribol. Int. 2010, 43, 2298–2302. [Google Scholar] [CrossRef]
- Macián, V.; Tormos, B.; Ruiz, S.; Miro, G. Low viscosity engine oils: Study of wear effects and oil key parameters in a heavy duty engine fleet test. Tribol. Int. 2016, 94, 240–248. [Google Scholar] [CrossRef]
- Kim, H.G.; Jeon, S.I. Effect on friction of engine oil seal with engine oil viscosity. Int. J. Auto. Technol.-Kor. 2008, 9, 601–606. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Pang, C. Composition and degradation of turbine oil sludge. J. Therm. Anal. Calorim. 2016, 125, 155–162. [Google Scholar] [CrossRef]
- Lillywhite, J.R.F.; Sant, P.; Saville, S.B. Sludge formation: Investigation of sludge formation in gasoline engines. Ind. Lubr. Tribol. 1990, 42, 4–10. [Google Scholar] [CrossRef]
- Spilners, I.J.; Hedenburg, J.F. Chemistry of Engine Combustion Deposits-Effect of Fuel and Lubricant Composition on Engine Deposit Formation; Springer: Boston, MA, USA, 1985. [Google Scholar]
- Shimonaev, G.S.; Shchegolev, N.V.; Filatov, P.G.; Penchul, A.F.; Stepanova, L.S.; Boiko, L.V.; Voinova, L.A.; Mikulin, Y.V. Composition of sludge formed in motor oil during engine operation. Chem. Technol. Fuels Oil+ 1976, 12, 538–541. [Google Scholar] [CrossRef]
- Battin-Leclerc, F.; Herbinet, O.; Glaude, P.A.; Fournet, R.; Qi, F. Experimental confirmation of the low-temperature oxidation scheme of alkanes. Angew. Chem. Int. Edit. 2010, 49, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Blaine, S.; Savage, P.E. Reaction pathways in lubricant degradation. 1. Analytical characterization of n-hexadecane autoxidation products. Ind. Eng. Chem. Res. 1991, 30, 792–798. [Google Scholar] [CrossRef]
- Blaine, S.; Savage, P.E. Reaction pathways in lubricant degradation. 2. n-Hexadecane autoxidation. Ind. Eng. Chem. Res. 1991, 30, 2185–2191. [Google Scholar] [CrossRef]
- Blaine, S.; Savage, P.E. Reaction pathways in lubricant degradation. 3. Reaction model for n-hexadecane autoxidation. Ind. Eng. Chem. Res. 1992, 31, 69–75. [Google Scholar] [CrossRef]
- Gracia, N.; Thomas, S.; Bazin, P.; Duponchel, L.; Thibault-Starzyk, F.; Lerasle, O. Combination of mid-infrared spectroscopy and chemometric factorization tools to study the oxidation of lubricating base oils. Catal. Today 2010, 155, 255–260. [Google Scholar] [CrossRef]
- Diaby, M.; Sablier, M.; Lenegrate, A.; Fassi, M.E.; Bocquet, J. Understanding carbonaceous deposit formation resulting from engine oil degradation. Carbon 2009, 47, 355–366. [Google Scholar] [CrossRef]
- Pfaendtner, J.; Broadbelt, L.G. Mechanistic Modeling of Lubricant Degradation.1. Structure -Reactivity Relationships for Free-Radical Oxidation. Ind. Eng. Chem. Res. 2008, 47, 2886–2896. [Google Scholar] [CrossRef]
- Pfaendtner, J.; Broadbelt, L.G. Mechanistic Modeling of Lubricant Degradation.2.The autoxidation of decane and octane. Ind. Eng. Chem. Res. 2008, 47, 2897–2904. [Google Scholar] [CrossRef]
- Mortier, R.M.; Fox, M.F.; Orszulik, S.T. Chemistry and Technology of Lubricants; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Wang, X.; Zhang, Q.; Zhu, X.; Song, C.; Wang, L. Study on compositions and viscosity-temperature performance of oils in producing base oil from hydrogenation of VGO. Petrol. Process. Petrochem. 2019, 50, 28–32. [Google Scholar] [CrossRef]
- Hourani, N.; Muller, H.; Adam, F.M.; Panda, S.K.; Sarathy, S.M. Structural Level Characterization of Base Oils Using Advanced Analytical Techniques. Energy Fuels 2015, 29, 2962–2970. [Google Scholar] [CrossRef]
- Kurita, N.; Inoue, H.; Sekino, H. Adjustment of Perdew-Wang exchange functional for describing van der Waals and DNA base-stacking interactions. Chem. Phys. Lett. 2003, 370, 161–169. [Google Scholar] [CrossRef]
- Boyd, R.J.; Edgecombe, K.E. Atomic and group electronegativities from the electron-density distributions of molecules. J. Am. Chem. Soc. 1988, 110, 4182–4186. [Google Scholar] [CrossRef]
- Moradi, R.; Jameh-Bozorghi, S.; Kadivar, R.; Mahdiani, A.; Soleymanabadi, H. Study of Mechanism Keto-Enol Tautomerism (isomeric reaction) Structure Cyclohexanone by Using Ab initio Molecular Orbital and Density Functional Theory (DFT) Method with NBO Analysis. APCBEE Procedia 2012, 3, 70–74. [Google Scholar] [CrossRef][Green Version]
- Tang, K.; Tao, H.; Jiang, X. Possible Reaction between Titanium(IV) and Hydrogen Peroxide in Acidic Condition: An ab-initio Study on Its Mechanism. Acta Chim. Sinica 2012, 70, 2091–2096. [Google Scholar] [CrossRef]
- Kaviani, S.; Izadyar, M.; Housaindokht, M.R. Solvent and spin state effects on molecular structure, IR spectra, binding energies and quantum chemical reactivity indices of deferiproneCferric complex: DFT study. Polyhedron 2016, 117, 623–627. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).