Phase Structure, Microstructure, Corrosion, and Wear Resistance of Al0.8CrFeCoNiCu0.5 High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. XRD Analysis
3.2. Microstructure
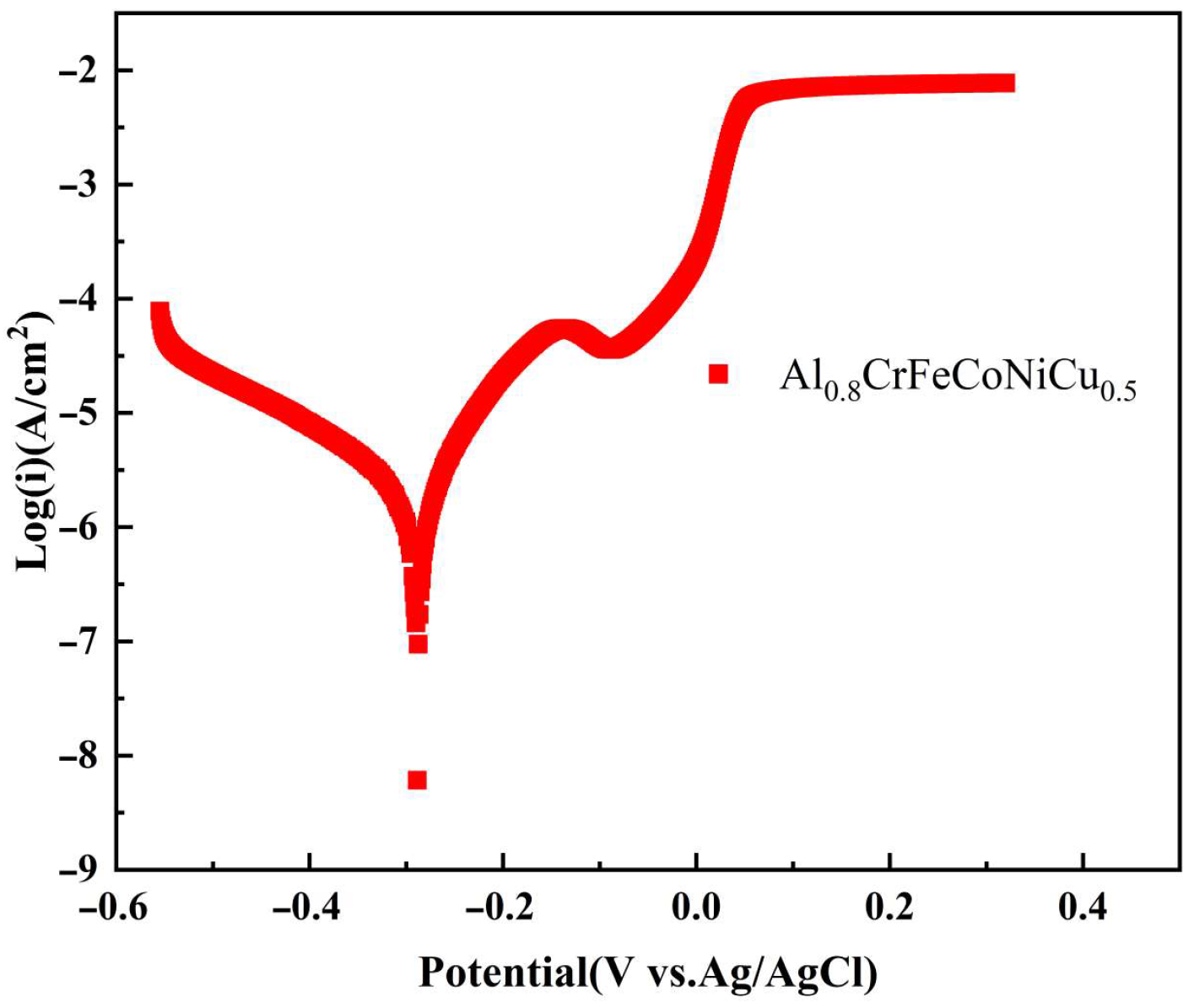
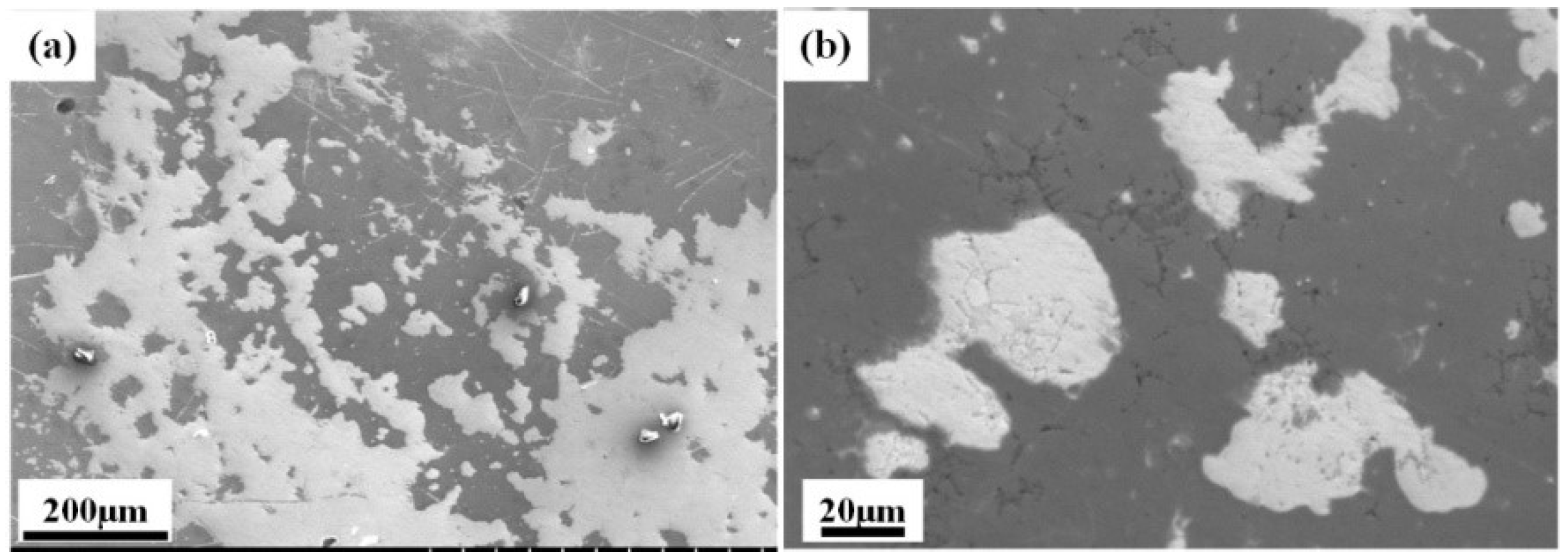
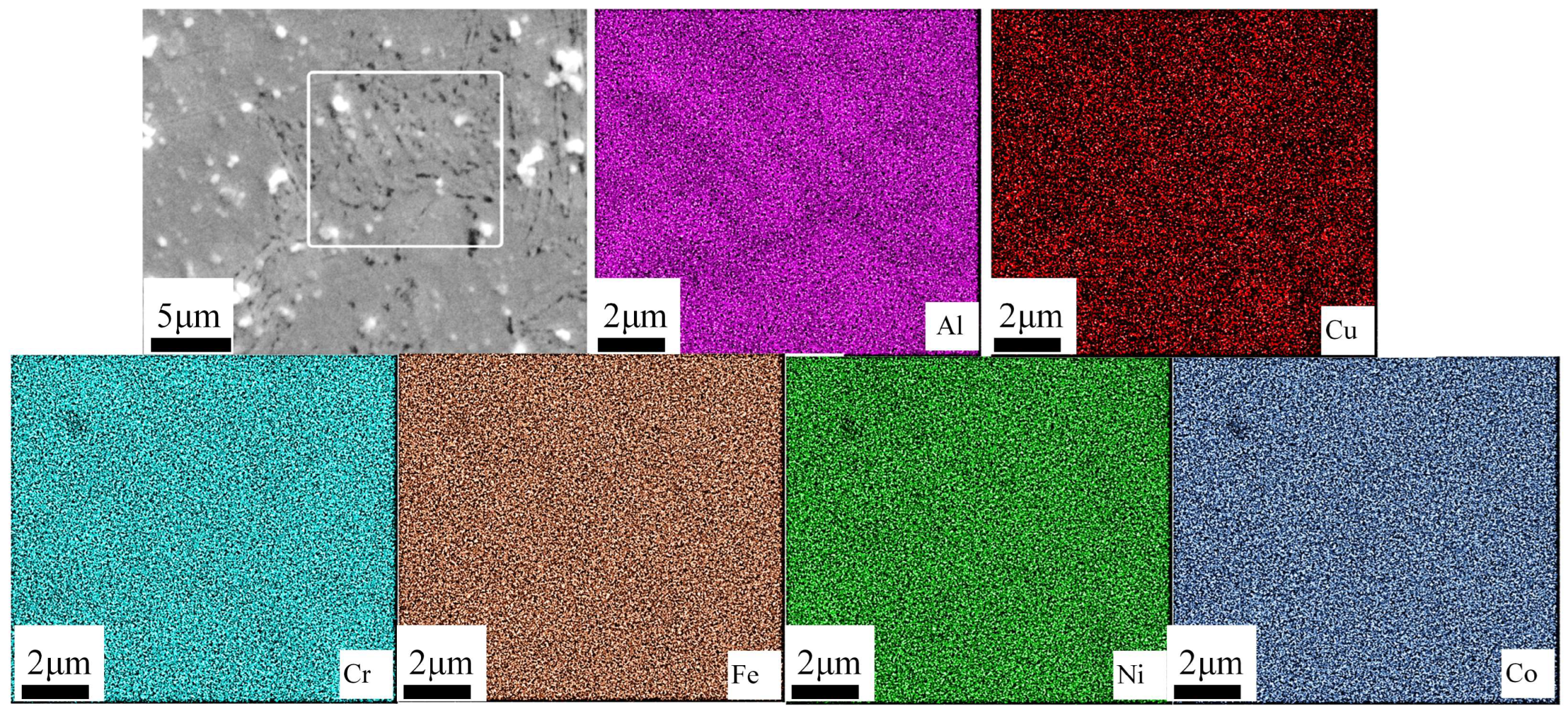
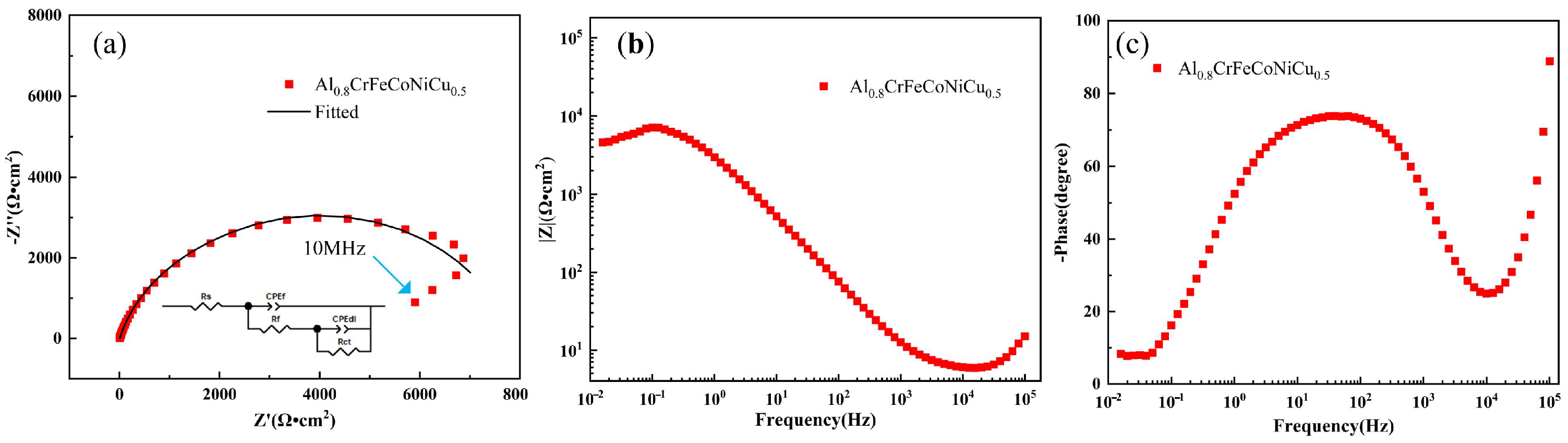
3.3. Corroison Resistance
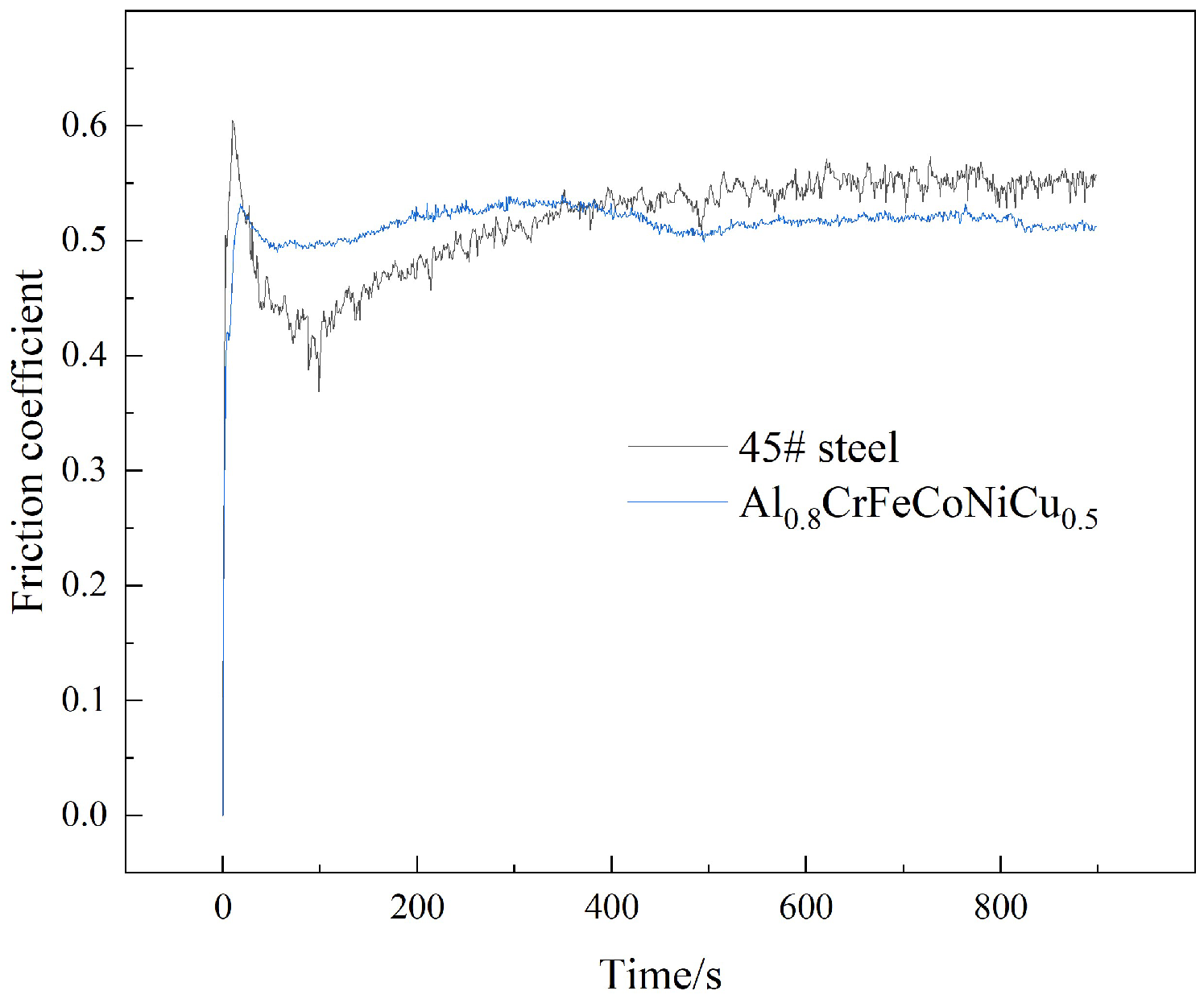
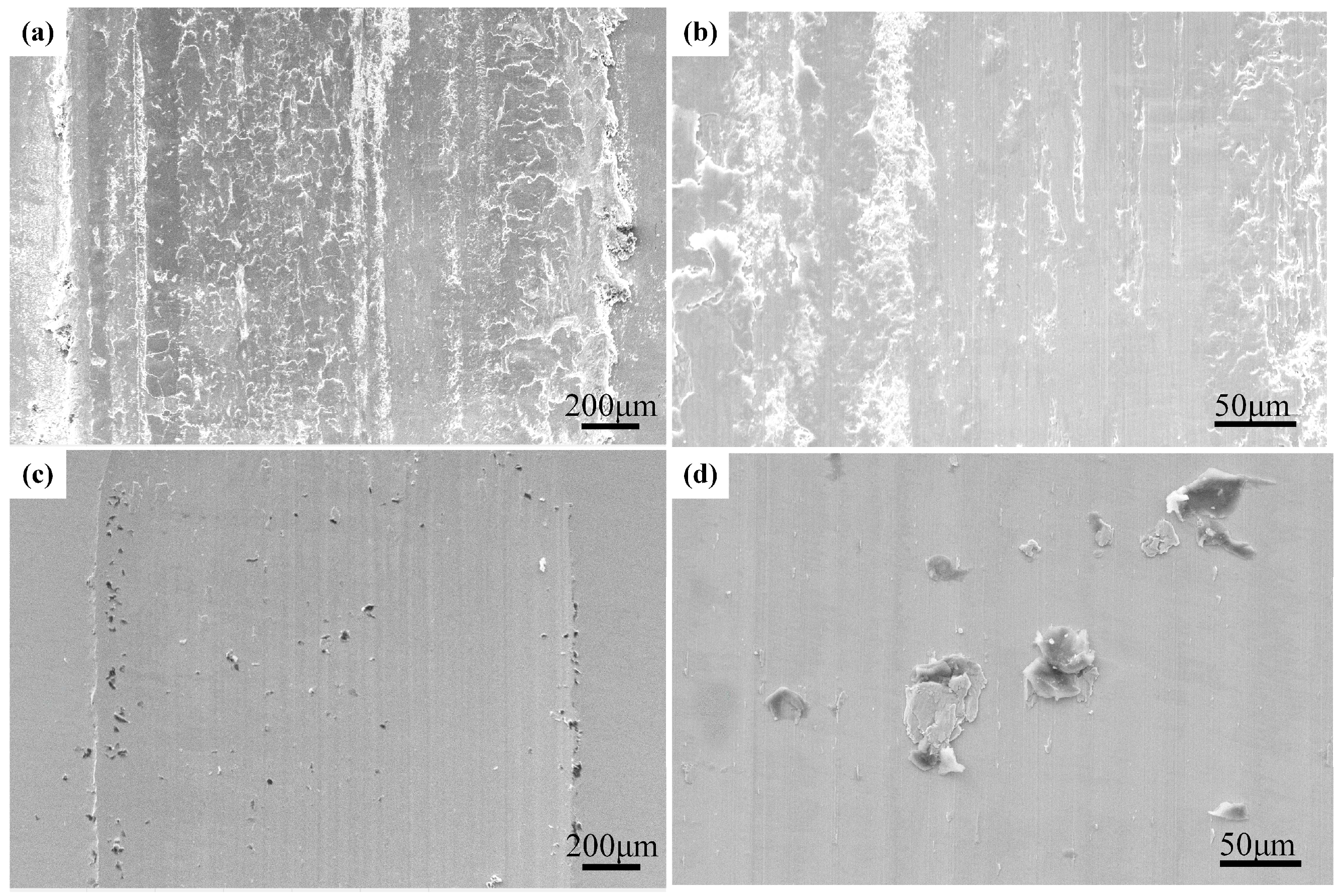
3.4. Wear Resistance
4. Conclusions
- (1)
- The Al0.8CrFeCoNiCu0.5 high-entropy alloy exhibits the presence of BCC1 and BCC2 phases, corresponding to (Fe-Cr) and Al-Ni, respectively. The identified FCC phase aligns with the Cu element.
- (2)
- Te Al0.8CrFeCoNiCu0.5 high-entropy alloy exhibits an equiaxed grain structure with a fishbone-like morphology at the grain boundaries and the presence of modulated structures within the grains. The intragranular regions predominantly comprise the BCC, while the intergranular areas primarily comprise the FCC. The alloy demonstrates minimal residual stress and strain within its internal structure. The alloy grains tend to grow along the <001> direction.
- (3)
- The Al0.8CrFeCoNiCu0.5 high-entropy alloy showed a corrosion potential of −0.332 V and a corrosion current density of 2.61 × 10−6 A/cm2. The corrosion surface of the alloy remained relatively intact, with some areas exhibiting intergranular corrosion. The intergranular corrosion regions exhibited significant depletion of Al and Cu elements, while the corrosion products primarily consisted of Al and Cu elements. Al and Cu phases contribute to the alloy’s susceptibility to corrosion.
- (4)
- The wear scar width of Al0.8CrFeCoNiCu0.5 high-entropy alloy is 1.65 mm, which is less than 45# steel, and high-entropy alloy has more excellent wear resistance. Compared with 45# steel, the wear surface of Al0.8CrFeCoNiCu0.5 high-entropy alloy is more intact, and the wear form is mainly adhesive wear.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zhang, M.; Peng, P. First-principles calculations on brazed diamond with FeCoCrNi high entropy alloys doped with strong carbide-forming elements. Solid State Commun. 2022, 357, 114980. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, Y. Simulations of irradiation resistance and mechanical properties under irradiation of high-entropy alloy NiCoCrFe. Mater. Today Commun. 2022, 33, 104308. [Google Scholar] [CrossRef]
- Ali, M.M.; Abd Elmoamen, S.S.; Gepreel, M.A.H.; Ahmed, H.A.; Elrefaie, F.A. High temperature oxidation of non equi-atomic Al5Cr12Fe35Mn28Ni20 high entropy alloy. Mater. Res. Express 2021, 8, 036508. [Google Scholar] [CrossRef]
- Duan, X.T.; Han, T.Z.; Guan, X.; Wang, Y.N.; Su, H.H.; Ming, K.S.; Wang, J.; Zheng, S.J. Cooperative effect of Cr and Al elements on passivation enhancement of eutectic high-entropy alloy AlCoCrFeNi 2.1 with precipitates. J. Mater. Sci. Technol. 2023, 136, 97–108. [Google Scholar] [CrossRef]
- Li, X.F.; Yang, X.H.; Yi, D.H.; Liu, B.S.; Zhu, J.C.; Li, J.X.; Gao, C.; Wang, L.Z. Effects of NbC content on microstructural evolution and mechanical properties of laser cladded Fe50Mn30Co10Cr10-xNbC composite coatings. Intermetallics 2021, 138, 107309. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Zhou, X.; Fu, R. A Driving Fatigue Feature Detection Method Based on Multifractal Theory. IEEE Sens. J. 2022, 22, 19046–19059. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Wu, X.L. Heterostructured materials. Prog. Mater. Sci. 2023, 131, 101019. [Google Scholar] [CrossRef]
- Sathiyamoorthi, P.; Kim, H.S. High-entropy alloys with heterogeneous microstructure: Processing and mechanical properties. Prog. Mater. Sci. 2022, 123, 100709. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent high-entropy Cantor alloys. Prog. Mater. Sci. 2021, 120, 100754. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Liu, X.B.; Liu, Y.F.; Zhang, S.Y.; Meng, Y.; Zhou, H.B.; Zhang, S.H. Effects of Cu/Si on the microstructure and tribological properties of FeCoCrNi high entropy alloy coating by laser cladding. Wear 2023, 512, 204533. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, H.; Liu, Y.; Niu, T.; Li, H. Effect of Ti on the corrosion behavior of (FeCrCoNi)100−xTix alloy. Corros. Sci. 2022, 209, 110807. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Yao, K.D.; Duan, K.; Wu, F.F.; Zhao, R.D.; Zhang, Y.; Shang, J.; Chen, M.H. The evolution of eutectic microstructure and mechanical properties of AlxCoCrFeNi2.1 high-entropy alloys. J. Mater. Res. 2022, 37, 2082–2092. [Google Scholar] [CrossRef]
- Wan, Q.; Hua, K.; Zhou, Z.; Zhang, F.; Wu, H.; Zhou, Q.; Wang, H. Revealing the B addition on tribology performance in TiZrHfTa0.5 refractory high-entropy alloy at ambient and elevated temperature. J. Alloys Compd. 2023, 931, 167521. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y.; Wang, H.X.; Zhou, B.J.; Li, D.F.; Lin, H.; Wang, J.Q. Effect of Y on Microstructure and Properties of Al0.8FeCrCoNiCu0.5 High Entropy Alloy Coating on 5083 Aluminum by Laser Cladding. Lubricants 2023, 11, 50. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chen, X.Z.; Jayalakshmi, S.; Singh, R.A.; Deev, V.B.; Prusov, E.S. Factors determining solid solution phase formation and stability in CoCrFeNiX0.4 (X=Al, Nb, Ta) high entropy alloys fabricated by powder plasma arc additive manufacturing. J. Alloys Compd. 2021, 857, 157625. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, P.; Bai, S.; Qin, B.; Zhang, D. Influence of Laser Power on Microstructure and Properties of Al-Si+Y2O3 Coating. Coatings 2023, 13, 1289. [Google Scholar] [CrossRef]
- Pandey, V.K.; Shadangi, Y.; Shivam, V.; Sarma, B.N.; Mukhopadhyay, N.K. Theoretical and experimental study on phase stability of TiVZrMoW refractory high entropy alloy. Philos. Mag. 2022, 102, 480–503. [Google Scholar] [CrossRef]
- Huang, C.; Yao, Y.; Yang, B.; Chen, S.H. Hierarchical toughening mechanisms in face centered cubic CoCrFeMnNi high-entropy alloy at room temperature and cryogenic temperatures. Phys. Rev. B 2023, 107, 174103. [Google Scholar] [CrossRef]
- Zhang, P.L.; Qi, Y.F.; Cheng, Q.Q.; Sun, X.M. Welding Dissimilar Alloys of CoCrFeMnNi High-Entropy Alloy and 304 Stainless Steel Using Gas Tungsten Arc Welding. J. Mater. Eng. Perform. 2023, 212, 348–365. [Google Scholar] [CrossRef]
- Lee, U.H. Precipitation growth of a-phase along the interface in CoCrFeMnNi high-entropy alloy. Vacuum 2023, 213, 112103. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Q.Y.; Li, W.; Li, D.Y. Microstructure, mechanical properties, and wear behavior of AlCoCrFeNi high-entropy alloy and AlCrFeNi medium-entropy alloy with WC addition. Wear 2023, 522, 204701. [Google Scholar] [CrossRef]
- Li, Y.T.; Fu, H.G.; Wang, K.M.; Yang, X.J.; Zong, B.; Lin, J. Effect of Mo addition on microstructure and wear resistance of laser clad AlCoCrFeNi-TiC composite coatings. Appl. Surf. Sci. 2023, 623, 157071. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Lee, T.-D.; Chen, S.-K.; Chang, Y.-S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Geant, V.; Voiculescu, I.; Cotrut, M.C.; Vranceanu, M.; Vasile, I.M.; Rosca, J. Effect of Al on Corrosion Behavior in 3.5%NaCl Solution of Al_xCoCrFeNi High Entropy Alloys. Int. J. Eng. Res. Afr. 2021, 53, 20–30. [Google Scholar] [CrossRef]
- Yan, X.; Guo, H.; Yang, W.; Pang, S.; Wang, Q.; Liu, Y.; Liaw, P.K.; Zhang, T. Al0.3CrxFeCoNi high-entropy alloys with high corrosion resistance and good mechanical properties. J. Alloys Compd. 2021, 860, 158436. [Google Scholar] [CrossRef]
- Shi, Y.; Collins, L.; Feng, R.; Zhang, C.; Balke, N.; Liaw, P.K.; Yang, B. Homogenization of AlxCoCrFeNi high-entropy alloys with improved corrosion resistance. Corros. Sci. 2018, 133, 120–131. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y.; Olugbade, E. Microstructure, mechanical, and corrosion resistance properties of Al0.8CrFeCoNiCux high-entropy alloy coatings on aluminum by laser cladding. Mater. Res. Express 2020, 7, 026504. [Google Scholar] [CrossRef]
- Li, H.; Zeng, S.; Zhou, Y.K.; Li, H.L.; Zhang, H.W.; Zhang, H.F.; Zhu, Z.W. High Tensile Strength and Superelasticity of Directionally Solidified Ti30Ni30Fe10Hf10Nb20 Eutectic High Entropy Alloy. Acta Metall. Sin.-Engl. Lett. 2022, 35, 1583–1590. [Google Scholar] [CrossRef]
- Thirugnanasambantham, K.G.; Singh, A.; Jegannathan, P.; Barathwaj, A.; Vignesh, P.; Vinayak, S.; Vikram, S.V.; Cheepurupalli, M.V. A novel alloy design for non-equiatomic high-entropy alloy (Cr-Fe-Ni-Ti-Nb): Predicting entropy mix and enthalpy mix. Int. J. Interact. Des. Manuf. IJIDeM 2023, 6, 467–489. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Juan, Y.F.; Lu, Z.J.; Jia, W.L. Evolution in microstructure and corrosion behavior of AlCoCrxFeNi high-entropy alloy coatings fabricated by laser cladding. J Alloy. Compd. 2019, 775, 1–14. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Cao, D.D.; Zhang, J.Y.; Zhang, P.; Tao, Q.; Yang, X.Q. Concurrence of spinodal decomposition and nano-phase precipitation in a multi-component AlCoCrCuFeNi high-entropy alloy. J. Mater. Res. Technol. 2019, 8, 726–736. [Google Scholar] [CrossRef]
- Li, L.L.; Kamachali, R.D.; Li, Z.M.; Zhang, Z.F. Grain boundary energy effect on grain boundary segregation in an equiatomic high-entropy alloy. Phys. Rev. Mater. 2020, 4, 053603. [Google Scholar] [CrossRef]
- Della Rovere, C.A.; Alano, J.H.; Silva, R.; Nascente, P.A.P.; Otubo, J.; Kuri, S.E. Influence of alloying elements on the corrosion properties of shape memory stainless steels. Mater. Chem. Phys. 2012, 133, 668–673. [Google Scholar] [CrossRef]
- Della Rovere, C.A.; Alano, J.H.; Silva, R.; Nascente, P.A.P.; Otubo, J.; Kuri, S.E. Characterization of passive films on shape memory stainless steels. Corros. Sci. 2012, 57, 154–161. [Google Scholar] [CrossRef]

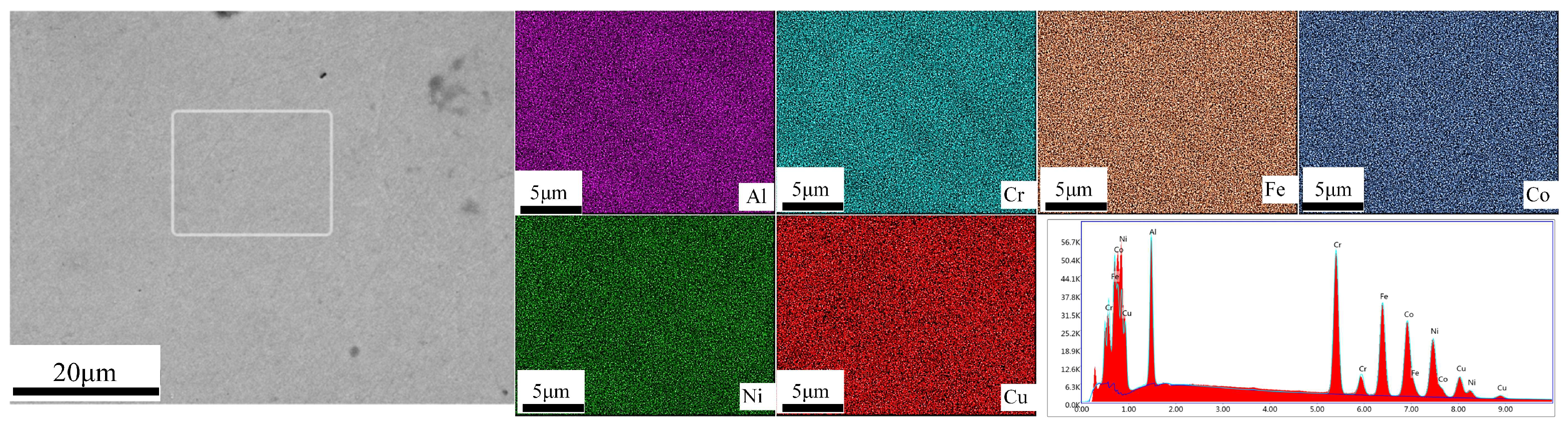


| Element | Al | Cr | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| Atomic % | 15.09 | 18.86 | 18.86 | 18.86 | 18.86 | 9.43 |
| Element | Al | Cr | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| Atomic % | 13.25 | 20.34 | 19.25 | 19.33 | 19.00 | 8.83 |
| Rs (Ω·cm2) | Rf (Ω·cm2) | CPEdl (μF/cm−2·S(α−1)) | Rct (Ω·cm2) | (χ2) |
|---|---|---|---|---|
| 5.155 | 7339 | 1.162 | 487.6 | 4.1E3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; Shi, Z.; Liang, J. Phase Structure, Microstructure, Corrosion, and Wear Resistance of Al0.8CrFeCoNiCu0.5 High-Entropy Alloy. Lubricants 2023, 11, 358. https://doi.org/10.3390/lubricants11090358
Li Y, Wang X, Shi Z, Liang J. Phase Structure, Microstructure, Corrosion, and Wear Resistance of Al0.8CrFeCoNiCu0.5 High-Entropy Alloy. Lubricants. 2023; 11(9):358. https://doi.org/10.3390/lubricants11090358
Chicago/Turabian StyleLi, Yanzhou, Xingfu Wang, Zimu Shi, and Juhua Liang. 2023. "Phase Structure, Microstructure, Corrosion, and Wear Resistance of Al0.8CrFeCoNiCu0.5 High-Entropy Alloy" Lubricants 11, no. 9: 358. https://doi.org/10.3390/lubricants11090358




