Evaluation of Aromatic Organic Compounds as Additives on the Lubrication Properties of Castor Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Additives
2.2. Chemical Characterization of Additives
2.3. Lubricants Formulation
2.4. Physical Properties of Lubricants
2.5. Lubricating Regime Estimation
2.6. Tribological Performance Evaluation
2.7. Oxidation Performance Evaluation
3. Results and Discussion
3.1. Chemical Characterization of Additives
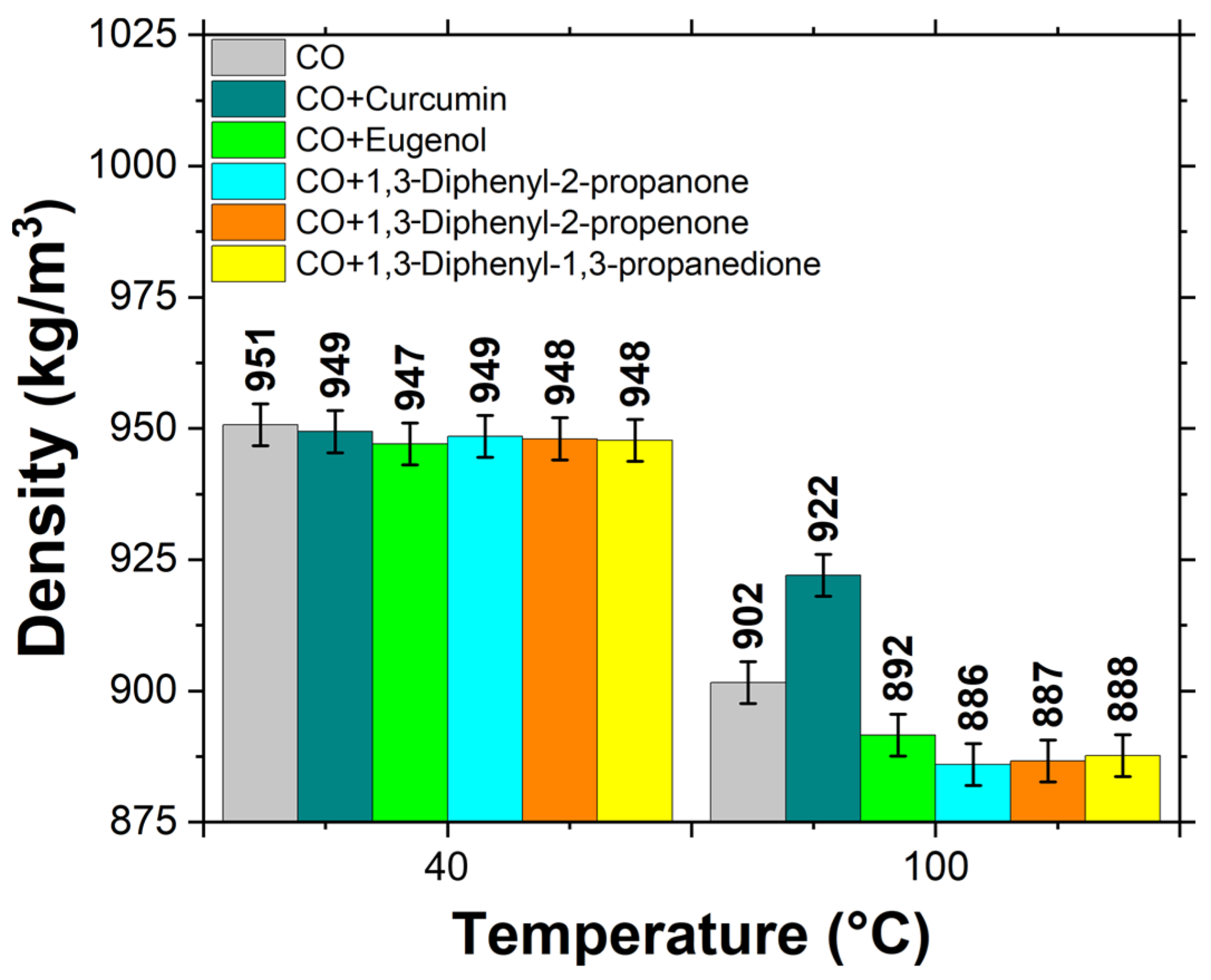
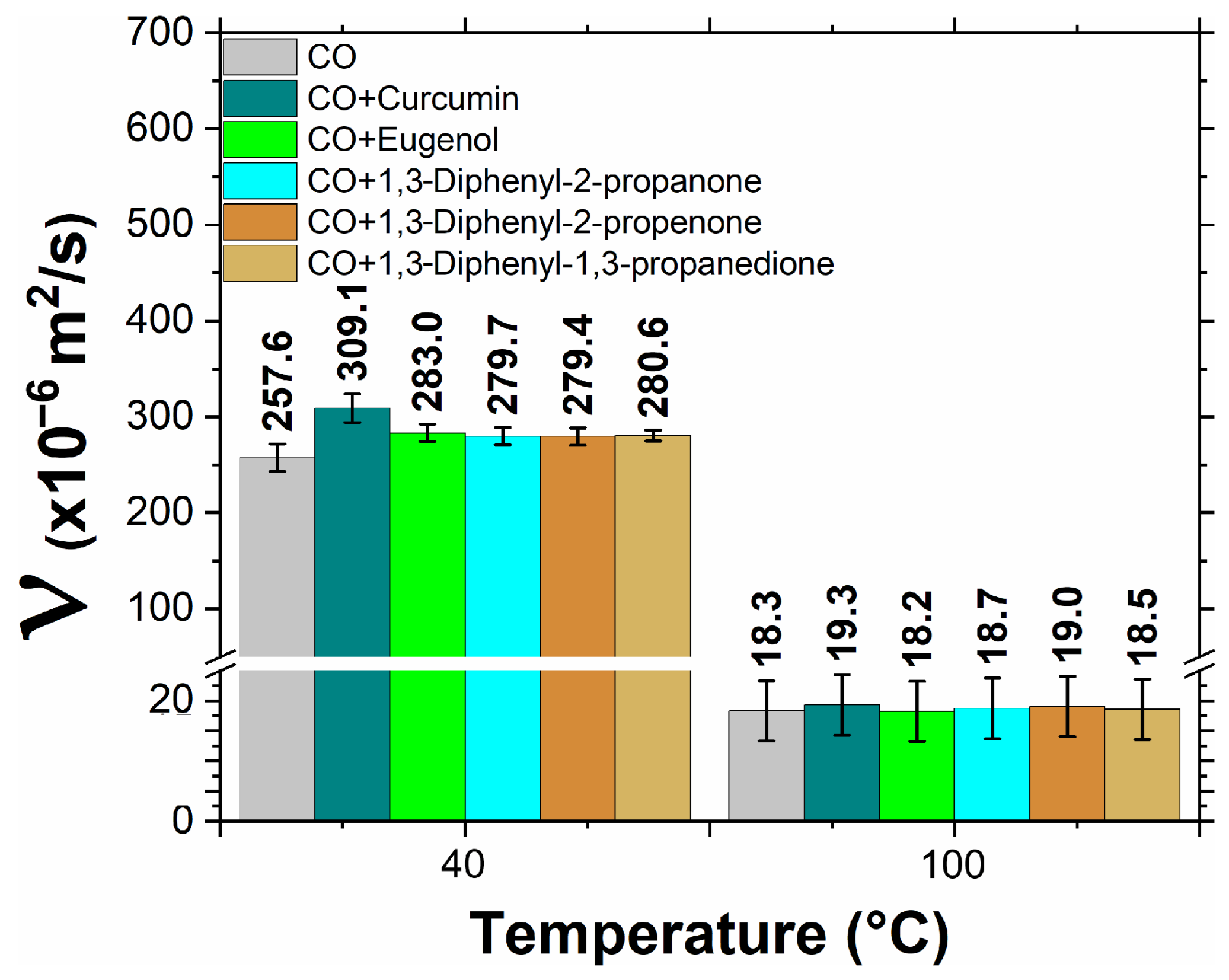
3.2. Physical Properties of Biolubricants
3.3. Lubricating Regime Estimation
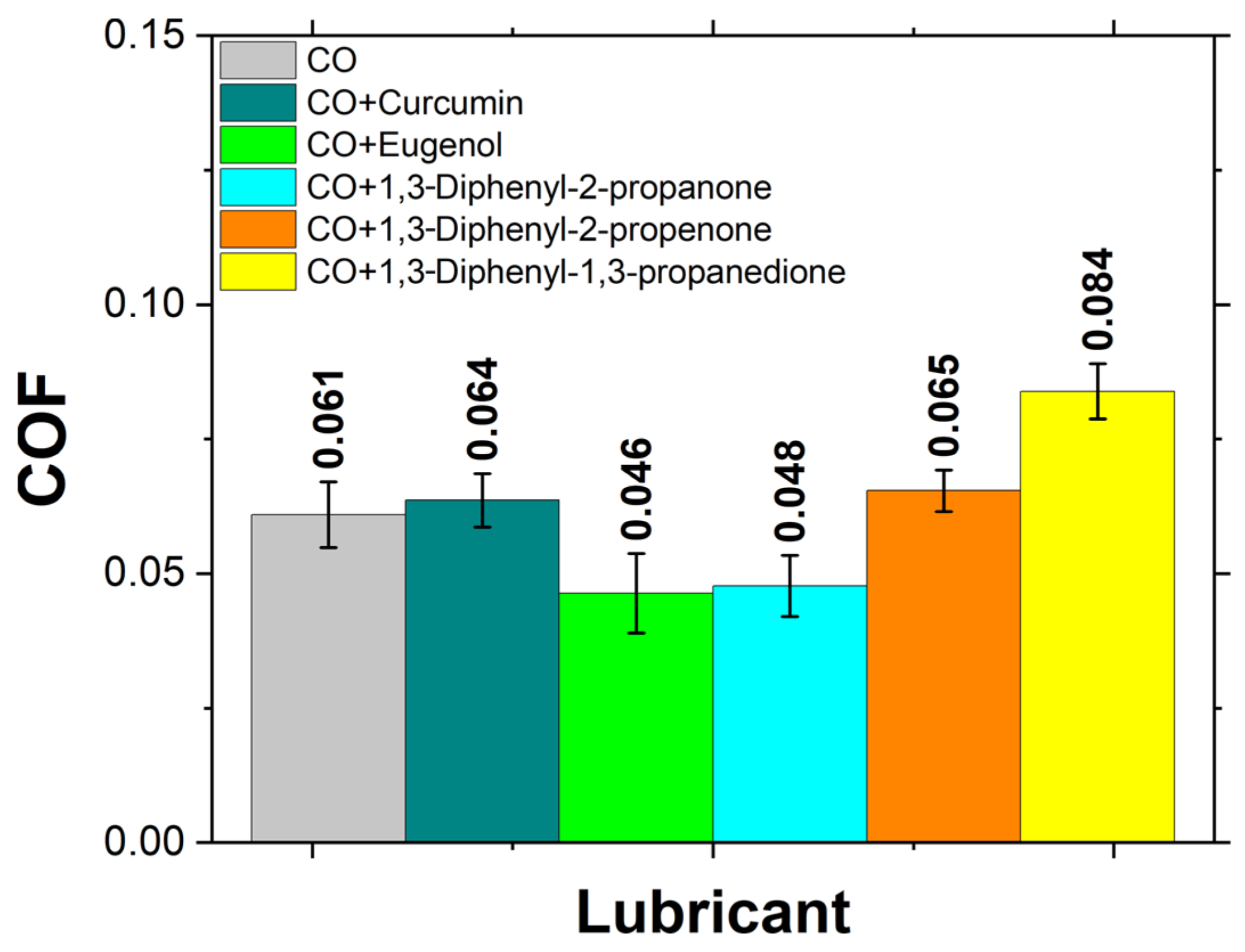
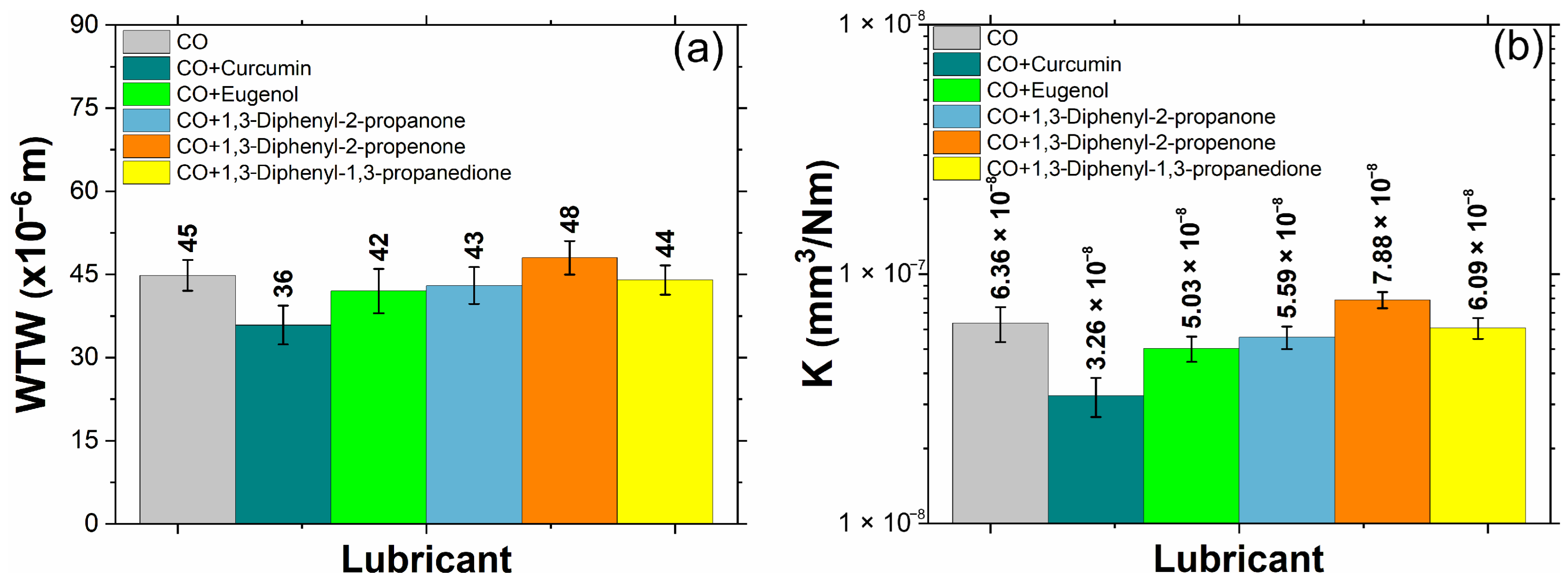
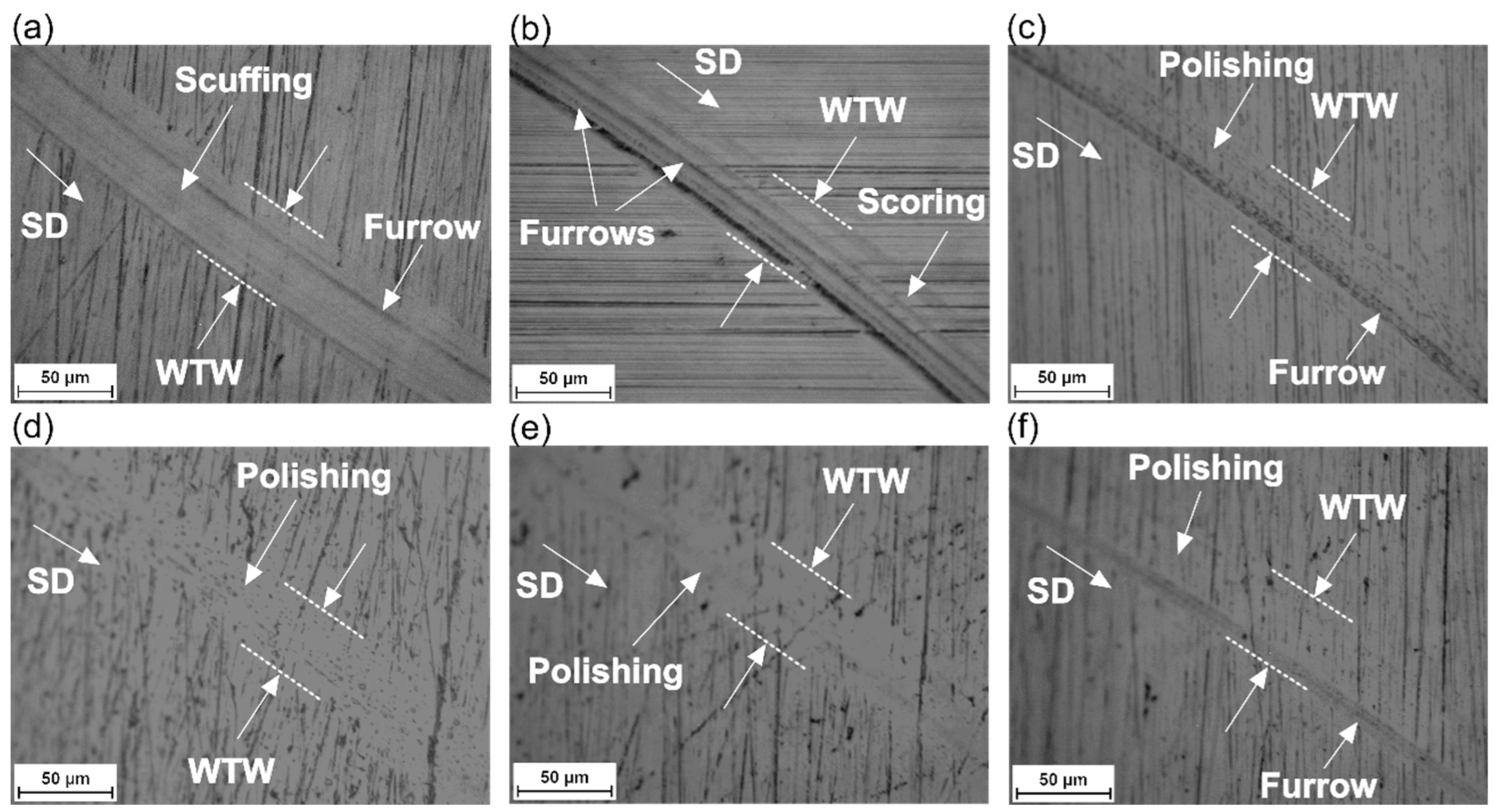
3.4. Tribological Performance
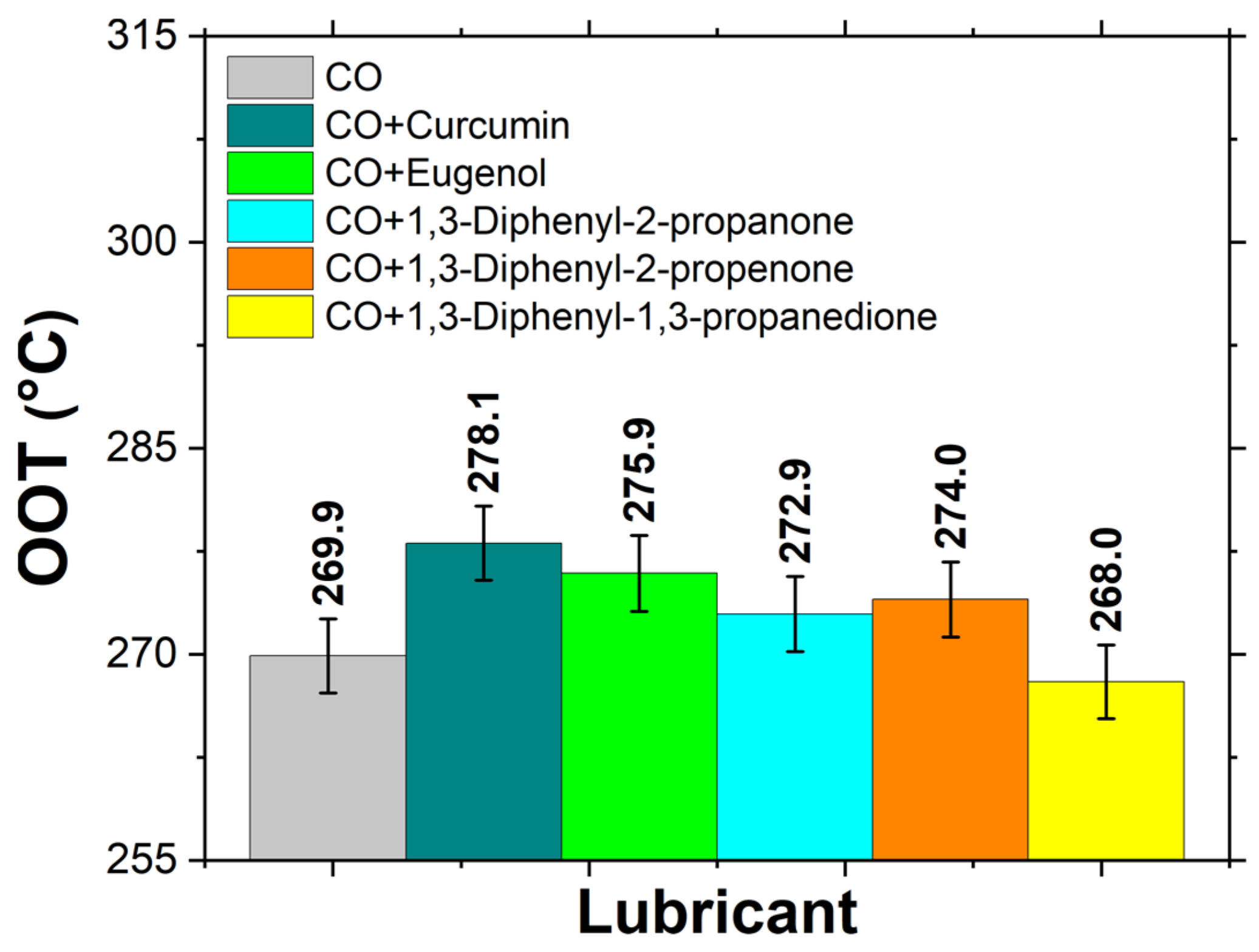
3.5. OOT of Biolubricants
4. Complementary Discussion
- Viscosity: Essential for the formation of the lubricant film and affects the resistance to flow of the lubricant.
- Friction Coefficient: Indicates the efficiency of the lubricant to reduce friction between surfaces.
- Wear Rate: A key indicator of the durability and useful life of the mechanical system.
- Wear Mechanism: Helps understand how and why wear occurs, which can influence lubricant selection.
- Oxidation Onset Temperature (OOT): Affects the chemical stability and useful life of the lubricant.
- Viscosity = 1
- Friction coefficient = 5
- Wear rate = 3
- Wear mechanism = 4
- Oxidation Onset Temperature (OOT) = 5
5. Conclusions
- All the additives, except for the curcumin at 100 °C, resulted in lower density compared to the castor oil (CO), whereas the additives increased the viscosity of CO, with curcumin showing an up to 20% increase at 40 °C.
- CO+curcumin and CO+eugenol produced the thickest lubricant films and the highest λ compared to the neat CO.
- Eugenol and 1,3-Diphenyl-2-propanone reduced the friction coefficient of CO significantly, while curcumin and other additives slightly increased it.
- Curcumin greatly improved wear track width and wear rate. However, eugenol, 1,3-Diphenyl-2-propanone, 1,3-Diphenyl-2-propenone, and 1,3-Diphenyl-1,3-propanedione notably enhanced the wear mechanism.
- All the studied additives, except for 1,3-Diphenyl-1,3-propanedione, increased the oxidation onset temperature (OOT), improving the oxidative stability of the CO.
- Through a decision matrix, it was observed that in situations where it is essential to have reduced friction, strong oxidation resistance, and minimal wear, the most effective additive for CO is eugenol, followed by curcumin, and then 1,3-Diphenyl-2-propanone.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, R.; Woydt, M.; Zhang, S. The Economic and Environmental Significance of Sustainable Lubricants. Lubricants 2021, 9, 21. [Google Scholar] [CrossRef]
- Granja, V.; Jogesh, K.; Taha-Tijerina, J.; Higgs, C.F., III. Tribological Properties of h-BN, Ag and MgO Nanostructures as Lubricant Additives in Vegetable Oils. Lubricants 2024, 12, 66. [Google Scholar] [CrossRef]
- Freschi, M.; Paniz, A.; Cerqueni, E.; Colella, G.; Dotelli, G. The Twelve Principles of Green Tribology: Studies, Research, and Case Studies—A Brief Anthology. Lubricants 2022, 10, 129. [Google Scholar] [CrossRef]
- Moreno, K.J.; Hernández-Sierra, M.T.; Báez, J.E.; Rodríguez-deLeón, E.; Aguilera-Camacho, L.D.; García-Miranda, J.S. On the Tribological and Oxidation Study of Xanthophylls as Natural Additives in Castor Oil for Green Lubrication. Materials 2021, 14, 5431. [Google Scholar] [CrossRef]
- Hernández-Sierra, M.T.; Báez, J.E.; Aguilera-Camacho, L.D.; García-Miranda, J.S.; Moreno, K.J. Friction and wear improvement by using Curcumin as a natural additive in green lubricants. Proc. Inst. Mech. Eng. Part J 2023, 237, 578–588. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Z.; Li, K.; Huang, T.; Ma, J.; Wen, G. Degradation of Micropollutants and Formation of Oxidation By-Products during the Ozone/Peroxymonosulfate System: A Critical Review. Water 2021, 13, 3126. [Google Scholar] [CrossRef]
- Wu, T. Byproduct formation in heterogeneous catalytic ozonation processes. Environ. Sci. Adv. 2023, 2, 558–569. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Hinestroza, J.P.; Martín-Alfonso, J.E.; Franco, J.M. Environmentally friendly tailor-made oleo-dispersions of electrospun cellulose acetate propionate nanostructures in castor oil for lubricant applications. Nano Mater. Sci. 2024. [Google Scholar] [CrossRef]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Dubyne, D. Castor Bean. Available online: https://www.oilseedcrops.org/castor-bean/ (accessed on 1 July 2024).
- Gupta, R.N.; Harsha, A. Antiwear and extreme pressure performance of castor oil with nano-additives. Proc. Inst. Mech. Eng. Part J 2018, 232, 1055–1067. [Google Scholar] [CrossRef]
- Qian, S.; Wang, H.; Huang, C.; Zhao, Y. Experimental investigation on the tribological properties of modified carbon nanotubes as the additive in castor oil. Ind. Lubr. Tribol. 2018, 70, 499–505. [Google Scholar] [CrossRef]
- Singh, Y.; Chaudhary, V.; Pal, V. Friction and wear characteristics of the castor oil with TiO2 as an additive. Mater. Today Proc. 2020, 26, 2972–2976. [Google Scholar] [CrossRef]
- Ouyang, T.; Lei, W.; Tang, W.; Shen, Y.; Mo, C. Experimental investigation of the effect of IF-WS2 as an additive in castor oil on tribological property. Wear 2021, 486, 204070. [Google Scholar] [CrossRef]
- Adithyan, K.S.; Edla, S.; Thampi, A.D.; Kumar, S.A.; Sasidharan, B.; Arif, M.M.; Rani, S. Experimental investigation on the effects of clove oil as an anti-oxidant additive on the lubricant properties of transesterified rice bran oil. Mater. Today Proc. 2023, 72, 2892–2896. [Google Scholar] [CrossRef]
- Al-Shibli, F.S.; Al-Tamemi, R.K.; Al-Assdi, K.A. Synthesis of the Antioxidant Compounds from the Eugenol to the Lubricating Oils. J. Kufa Chem. Sci. 2022, 2, 501–518. [Google Scholar] [CrossRef]
- Sneha, E.; Karthik, G.V.S.; Thampi, A.D.; Krishna, A.; Revikumar, A.; Rani, S. Experimental and quantum chemical investigation of bio-fuels/lubricants for its oxidative stability. J. Mol. Liq. 2021, 340, 117292. [Google Scholar] [CrossRef]
- Edla, S.; Thampi, A.D.; Pillai, A.B.; Sivan, V.V.; Arif, M.M.; Sasidharan, B. Formulation of rice bran oil-based green cutting fluid with holy basil oil and clove oil as bio-additives. Biomass Convers. Biorefin. 2022, 13, 16877–16886. [Google Scholar] [CrossRef]
- Gamayel, A.; Mohammed, M.; Al-Zubaidi, S.; Yusuf, E. Characterization and physicochemical properties of biofuel from mixed crude jatropha oil and clove oil. Sys. Rev. Pharm. 2020, 11, 910–916. [Google Scholar]
- ASTM G99; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus, Annual Book of Standards. ASTM International: West Conshohocken, PA, USA, 2017.
- Pirro, D.M.; Webster, M.; Daschner, E. Lubrication Fundamentals, 3rd ed.; Taylor & Francis Group: New York, NY, USA, 2016; pp. 35–67. [Google Scholar]
- ASTM D 891; Standard Test Methods for Specific Gravity, Apparent, of Liquid Industrial Chemicals. ASTM International: West Conshohocken, PA, USA, 1993.
- ASTM D 445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (the Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2024.
- Hamrock, B.J.; Dowson, D. Isothermal elastohydrodynamic lubrication of point contacts: Part III—Fully flooded results. J Lubr. Technol. 1977, 99, 264–275. [Google Scholar] [CrossRef]
- Hernández-Sierra, M.T.; Bravo-Sánchez, M.G.; Báez, J.E.; Aguilera-Camacho, L.D.; García-Miranda, J.S.; Moreno, K.J. Improvement effect of green lubricants on the tribological and mechanical performance of 4140 steel. Appl. Sci. 2019, 9, 4896. [Google Scholar] [CrossRef]
- ASTM E 2009; Standard Test Method for Oxidation Onset Temperature of Hydrocarbons by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2008.
- Fugita, R.A.; Gálico, D.A.; Guerra, R.B.; Perpétuo, G.L.; Treu-Filho, O.; Galhiane, M.S.; Mendes, R.A.; Bannach, G. Thermal behaviour of curcumin. Braz. J. Therm. Anal. 2012, 1, 19–23. [Google Scholar]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef]
- de Sousa, A.S.; Silva, R.; Tay, F.H.; Simplício, A.L.; Kazarian, S.G.; Duarte, C.M. Solubility enhancement of trans-chalcone using lipid carriers and supercritical CO2 processing. J. Supercrit. Fluids. 2009, 48, 120–125. [Google Scholar] [CrossRef]
- Horozić, E.; Suljagić, J.; Husejnagić, D.; Hasić, N.; Bratovčić, A. Synthesis, characterization and in vitro biological evaluation of the Schiff base derived from benzidine and 1,3-diphenyl-1,3-propanedione. J. Eng. Process. Manag. 2019, 11, 112–116. [Google Scholar] [CrossRef]
- Krzyzak, Z.; Pawlus, P. ‘Zero-wear’ of piston skirt surface topography. Wear 2006, 260, 554–561. [Google Scholar] [CrossRef]
- Kim, I.J. (Ed.) Wear Behaviours and Mechanisms. In Engineering Metrology for Pedestrian Falls Prevention and Protection; Springer Nature: Cham, Switzerland, 2022; pp. 153–191. [Google Scholar] [CrossRef]
- Jabbarzadeh, A. Tribological Properties of Interfacial Molecular Films. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 864–874. [Google Scholar]
- Cyriac, F.; Akchurin, A. Thin Film Lubrication, Lubricants and Additives. In Tribology in Materials and Applications; Katiyar, J.K., Ramkumar, P., Rao, T.V.V.L.N., Davim, J.P., Eds.; Springer Nature: Geneva, Switzerland, 2020; pp. 33–76. [Google Scholar]
- Budinski, K.G.; Budinski, S.T. Interpretation of galling tests. Wear 2015, 332, 1185–1192. [Google Scholar] [CrossRef]
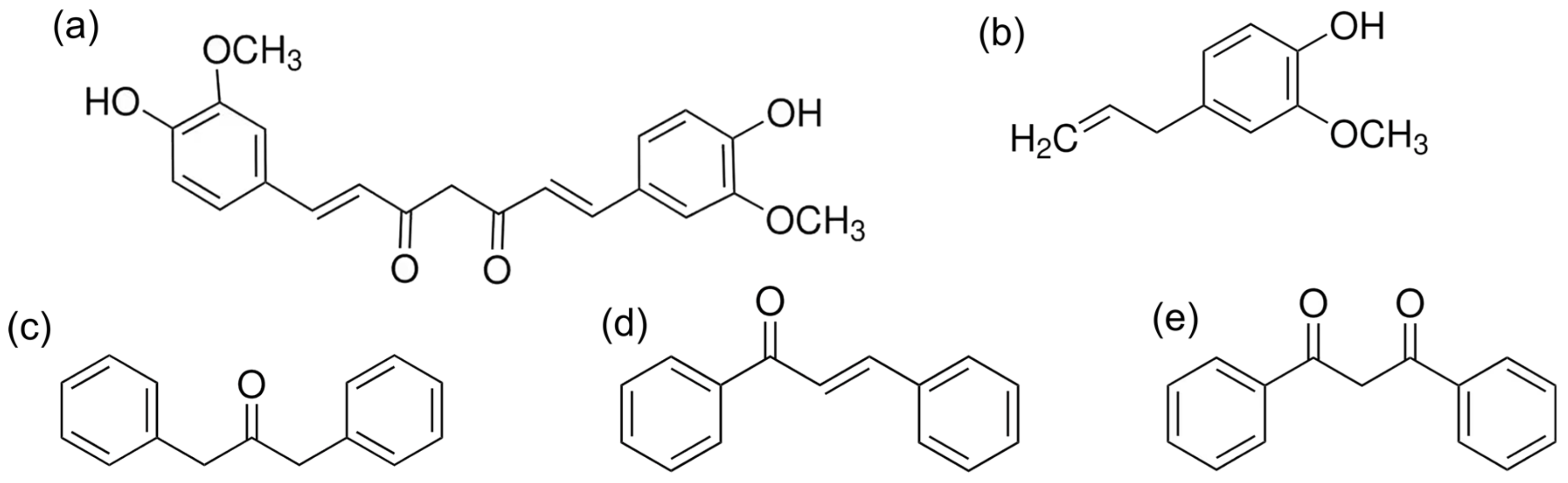


| Compound | Other Names | Molecular Formula | Molecular Weight (g/mol) | Melting Point (°C) | Density (g/cm3) |
|---|---|---|---|---|---|
| Curcumin | Diferuloylmethane, Natural Yellow 3 | C21H20O6 | 368.38 | 183 | 1.3 |
| Eugenol | 4-Allyl-2-methoxyphenol, 2-Methoxy-4-(2-propenyl)phenol | C10H12O2 | 164.20 | (−12)–(−10) | 1.067 |
| 1,3-Diphenyl-2-propanone | 1,3-Diphenylacetone, Dibenzylketone | C15H14O | 210.27 | 32–34 | 1.070 |
| 1,3-Diphenyl-2-propenone | Trans-chalcone, Benzylideneacetophenone | C15H12O | 208.26 | 55–57 | 1.071 |
| 1,3-Diphenyl-1,3-propanedione | Dibenzoylmethane | C15H12O2 | 224.25 | 77–79 | 0.800 |
| Lubricant | hc (nm) | λc | Lubricating Regime | hmin (nm) | λmin | Lubricating Regime |
|---|---|---|---|---|---|---|
| 280.6 | 4 | 0.09 | Boundary | 2 | 0.05 | Boundary |
| CO+curcumin | 6 | 0.13 | Boundary | 3 | 0.07 | Boundary |
| CO+eugenol | 6 | 0.13 | Boundary | 3 | 0.07 | Boundary |
| CO+1,3-Diphenyl-2-propanone | 4 | 0.09 | Boundary | 2 | 0.05 | Boundary |
| CO+1,3-Diphenyl-2-propenone | 4 | 0.09 | Boundary | 2 | 0.05 | Boundary |
| CO+1,3-Diphenyl-1,3-propanedione | 3 | 0.08 | Boundary | 2 | 0.04 | Boundary |
| Score | Viscosity at 40 °C (×10−6 m2/s) | Friction Coefficient | Wear Rate, (×10−8 mm3/Nm) | Wear Mechanism | OOT (°C) |
|---|---|---|---|---|---|
| 1 | 245–260 | 0.08–0.09 | 7–8 | Incipient galling | 268–270 |
| 2 | 260–275 | 0.07–0.08 | 6–7 | Scuffing | 270–272 |
| 3 | 275–290 | 0.06–0.07 | 5–6 | Scoring | 272–274 |
| 4 | 290–305 | 0.05–0.06 | 4–5 | Polishing and some furrows | 274–276 |
| 5 | 305–320 | 0.04–0.05 | 3–4 | No damage or polishing | 276–278 |
| Viscosity | Friction Coefficient | Wear Rate | Wear Mechanism | OOT | Total | |
|---|---|---|---|---|---|---|
| None | 1 | 15 | 6 | 8 | 5 | 35 |
| Curcumin | 5 | 15 | 15 | 12 | 25 | 72 |
| Eugenol | 3 | 25 | 9 | 16 | 20 | 73 |
| 1,3-Diphenyl-2-propanone | 3 | 25 | 9 | 20 | 15 | 72 |
| 1,3-Diphenyl-2-propenone | 3 | 15 | 3 | 20 | 15 | 56 |
| 1,3-Diphenyl-1,3-propanedione | 3 | 5 | 6 | 16 | 5 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Sierra, M.T.; Báez, J.E.; Aguilera-Camacho, L.D.; García-Miranda, J.S.; Moreno, K.J. Evaluation of Aromatic Organic Compounds as Additives on the Lubrication Properties of Castor Oil. Lubricants 2024, 12, 244. https://doi.org/10.3390/lubricants12070244
Hernández-Sierra MT, Báez JE, Aguilera-Camacho LD, García-Miranda JS, Moreno KJ. Evaluation of Aromatic Organic Compounds as Additives on the Lubrication Properties of Castor Oil. Lubricants. 2024; 12(7):244. https://doi.org/10.3390/lubricants12070244
Chicago/Turabian StyleHernández-Sierra, María Teresa, José E. Báez, Luis Daniel Aguilera-Camacho, J. Santos García-Miranda, and Karla J. Moreno. 2024. "Evaluation of Aromatic Organic Compounds as Additives on the Lubrication Properties of Castor Oil" Lubricants 12, no. 7: 244. https://doi.org/10.3390/lubricants12070244







