The Preparation of MoS2/Metal Nanocomposites Functionalized with N-Oleoylethanolamine: Application as Lubricant Additives
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Synthesis
2.2. Characterization
3. Results and Discussion
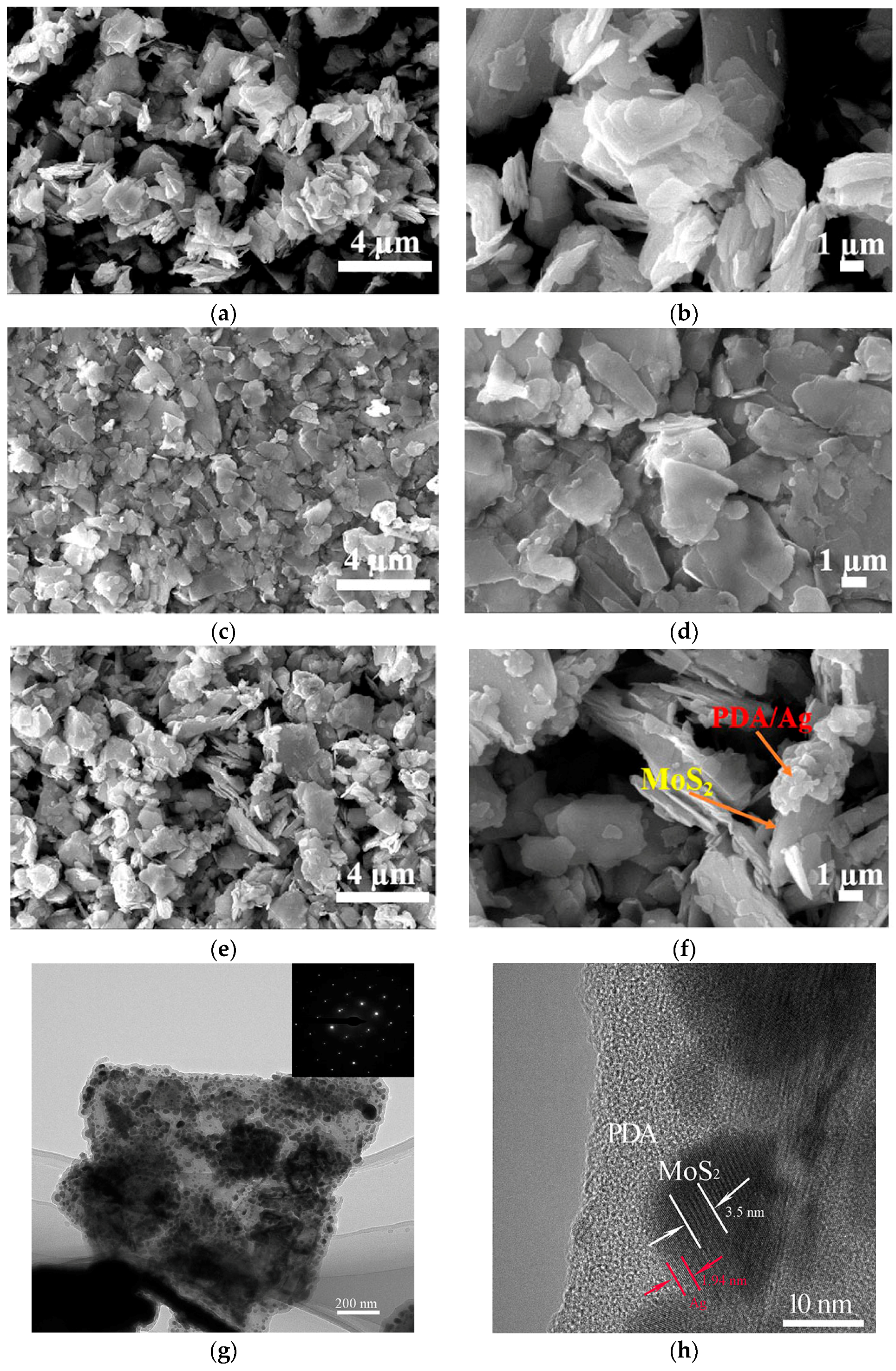
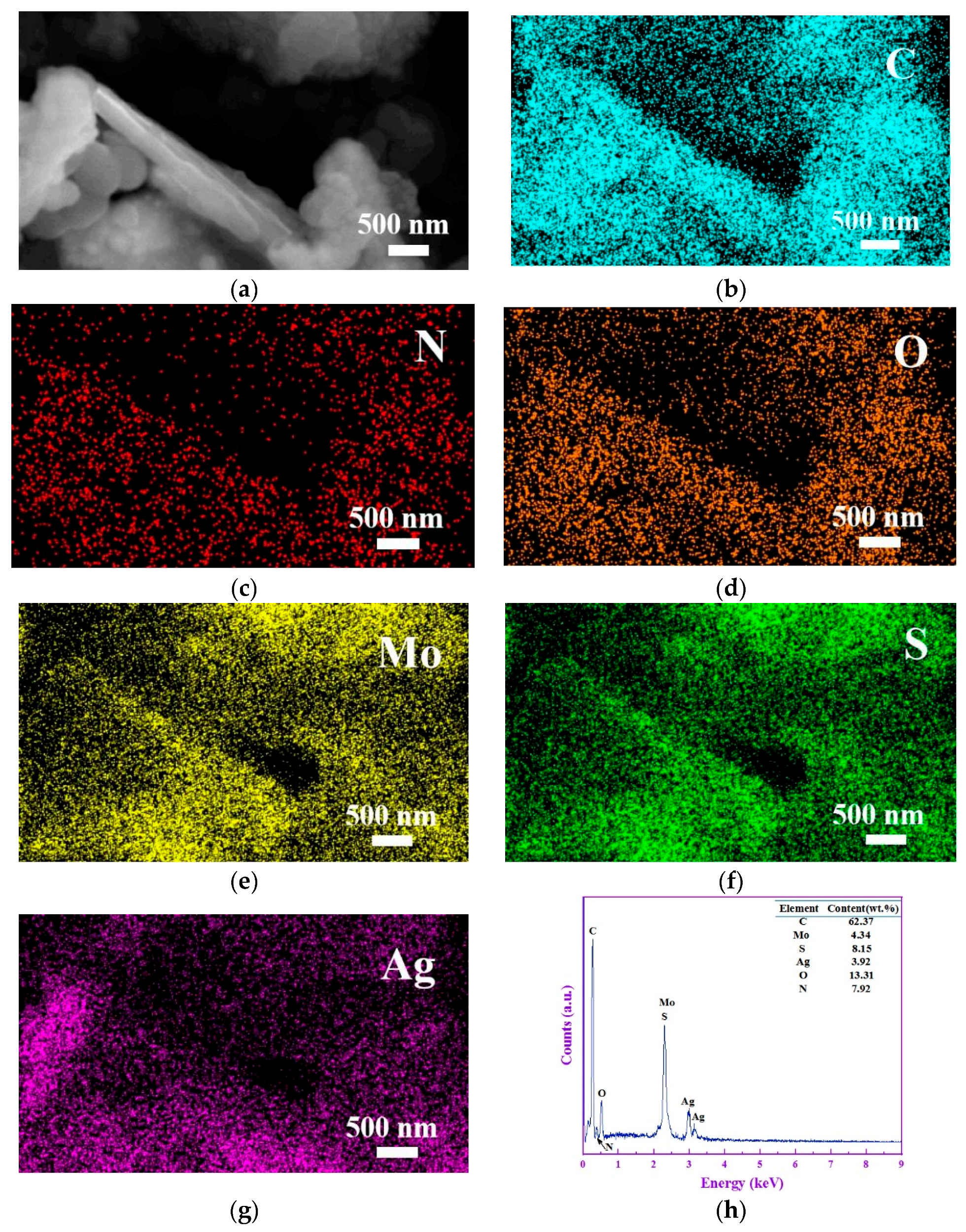
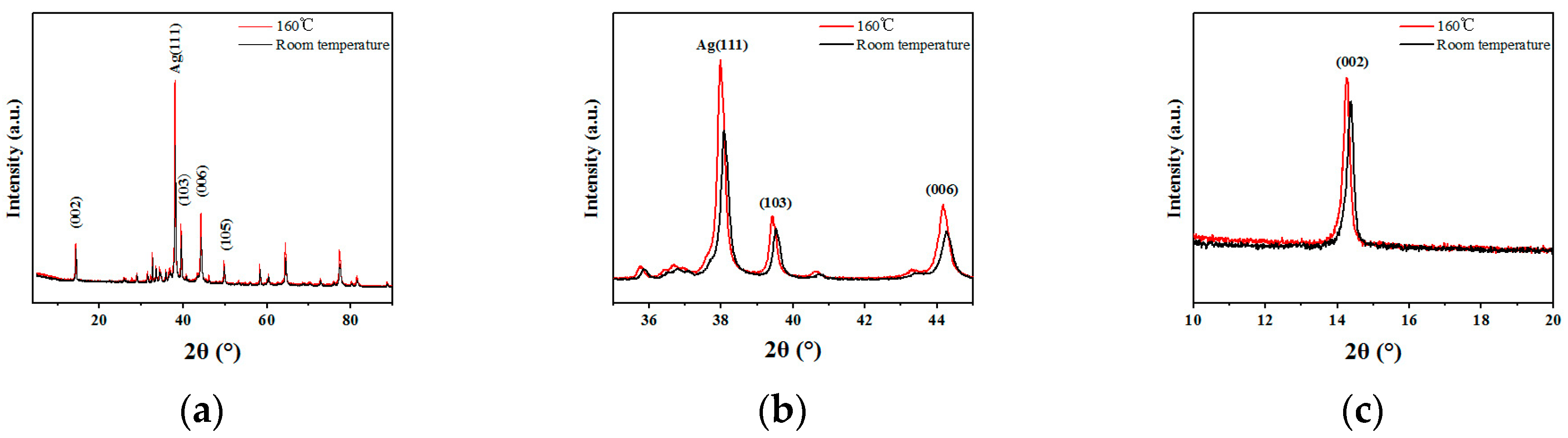
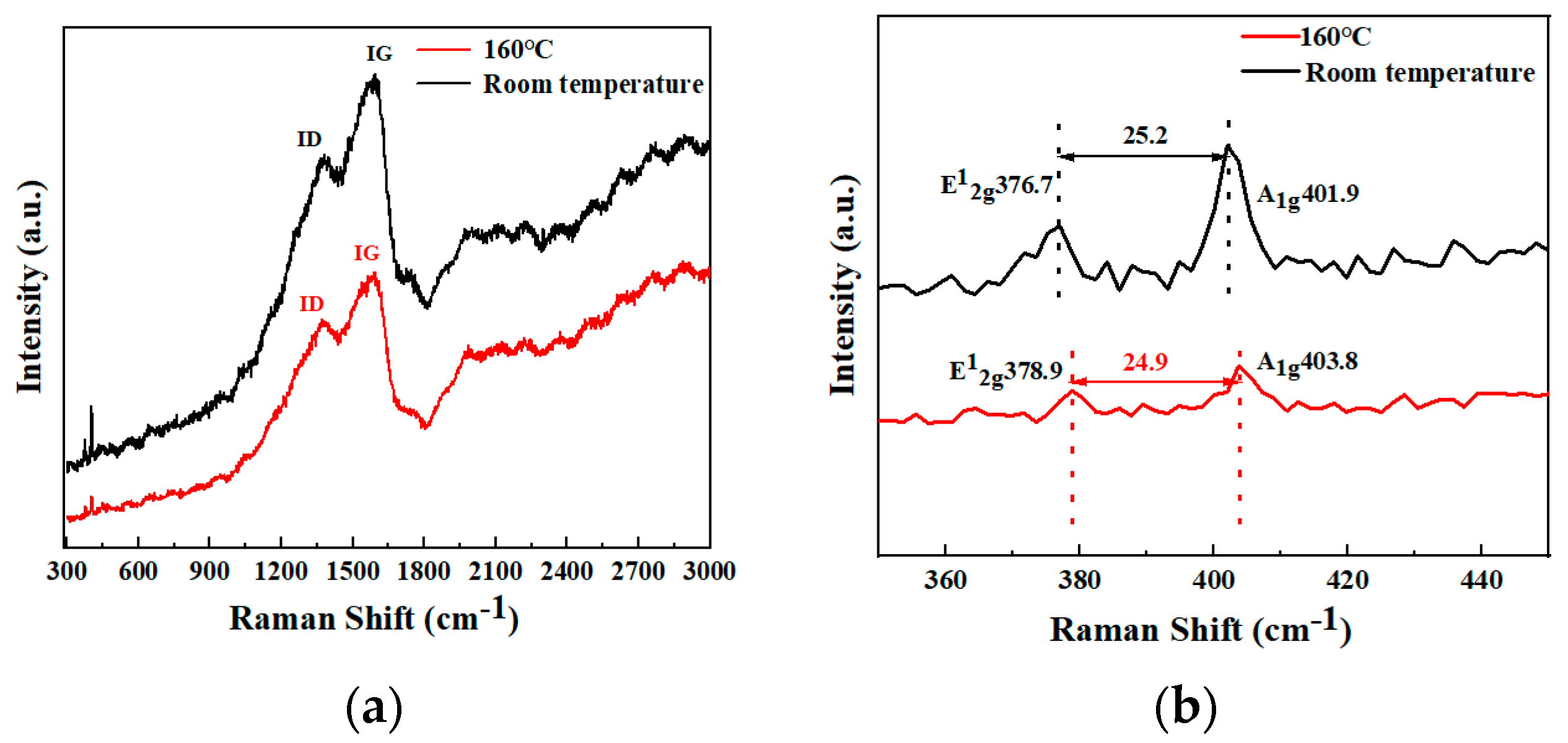
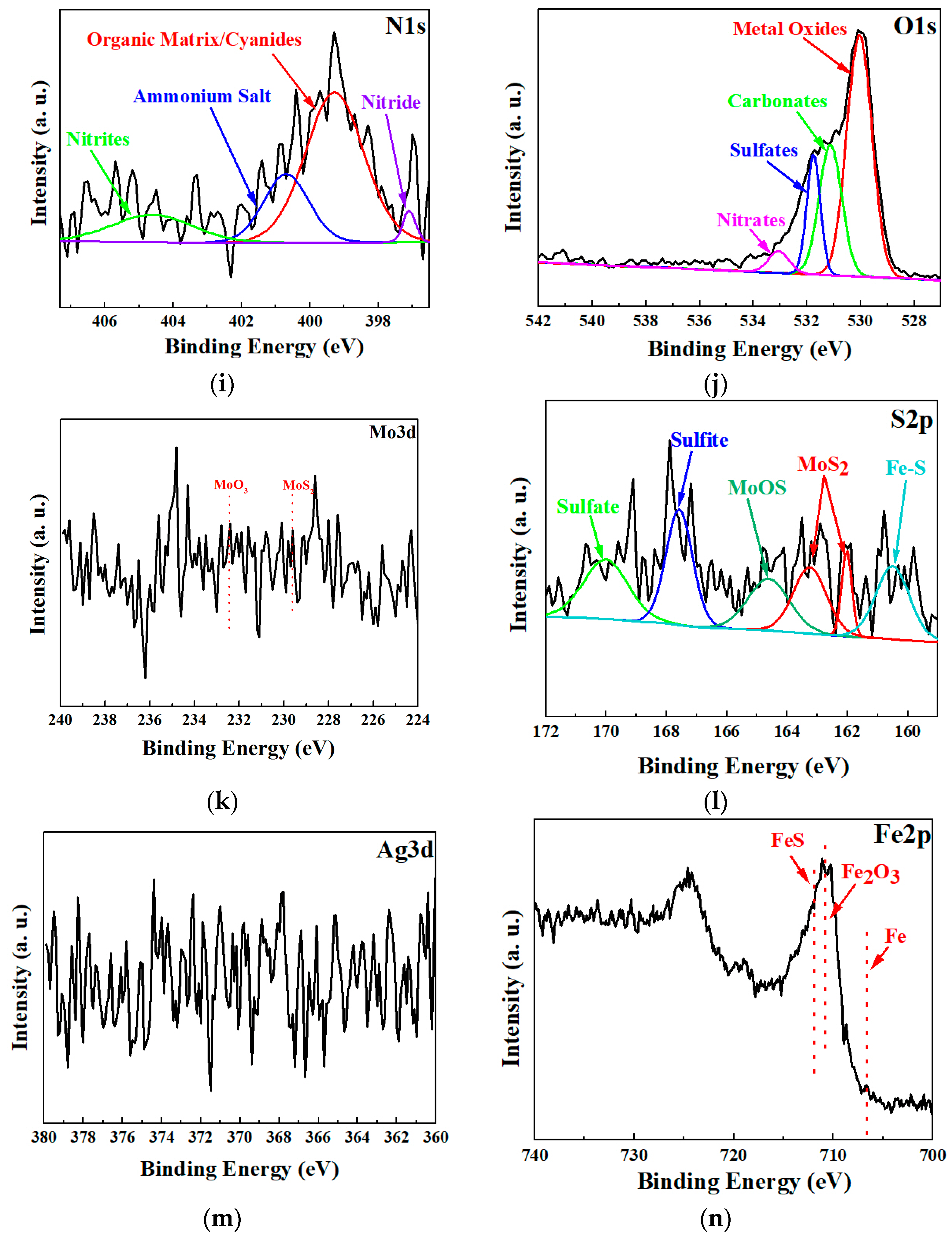
3.1. Structure
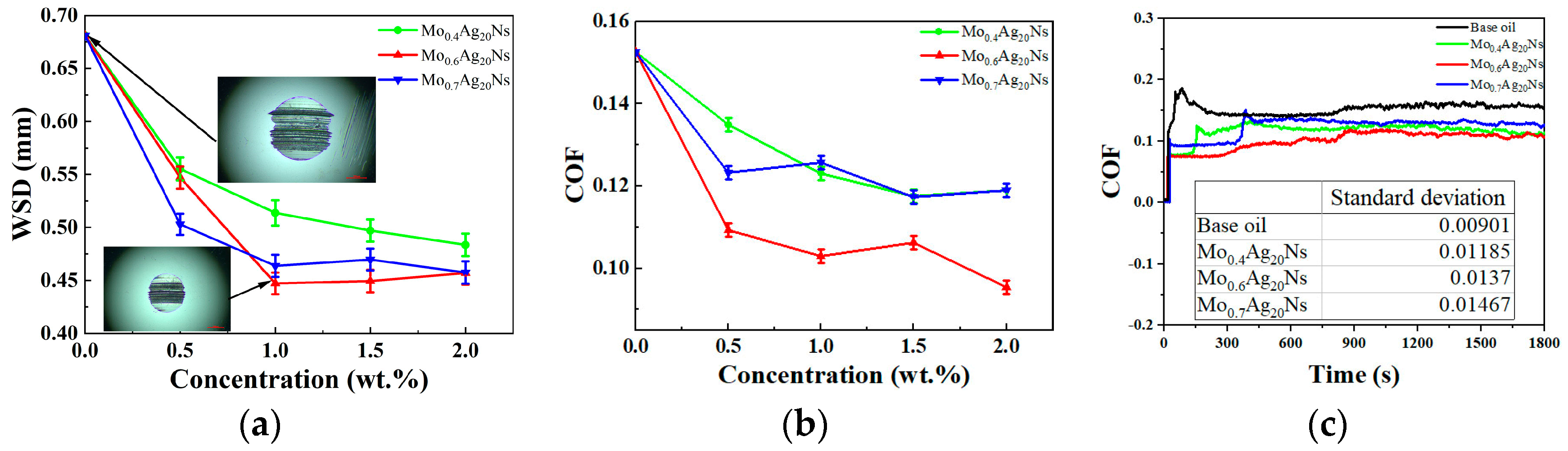
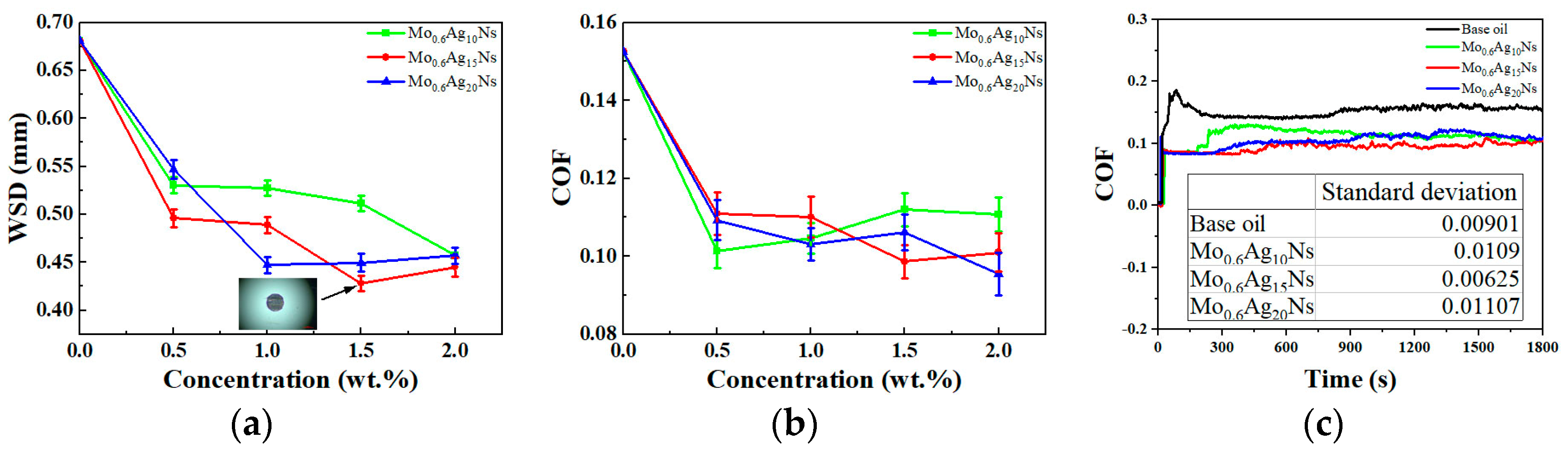
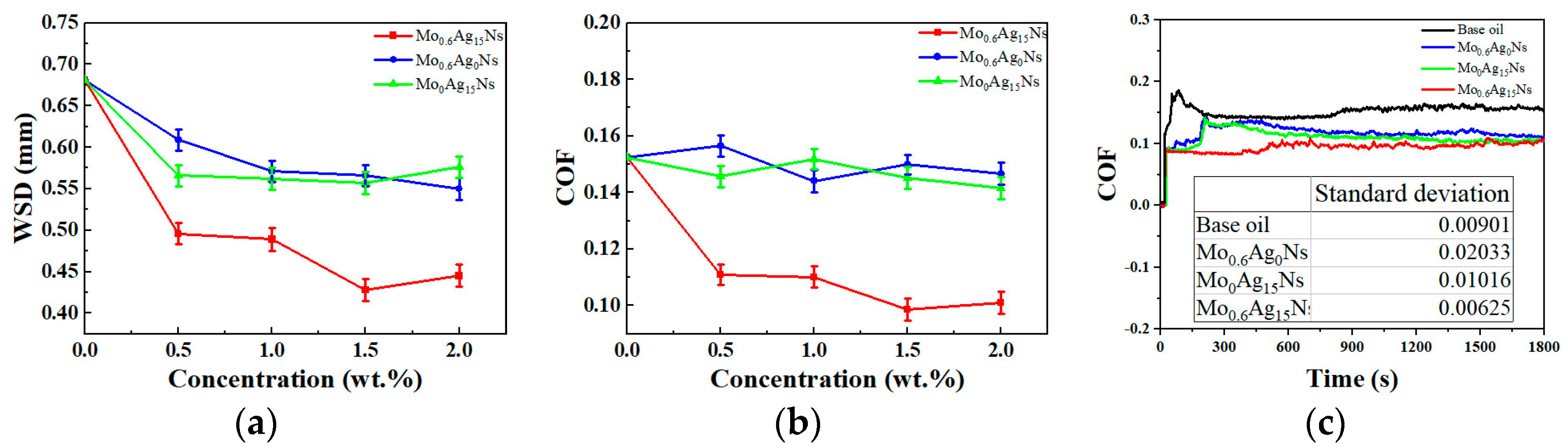
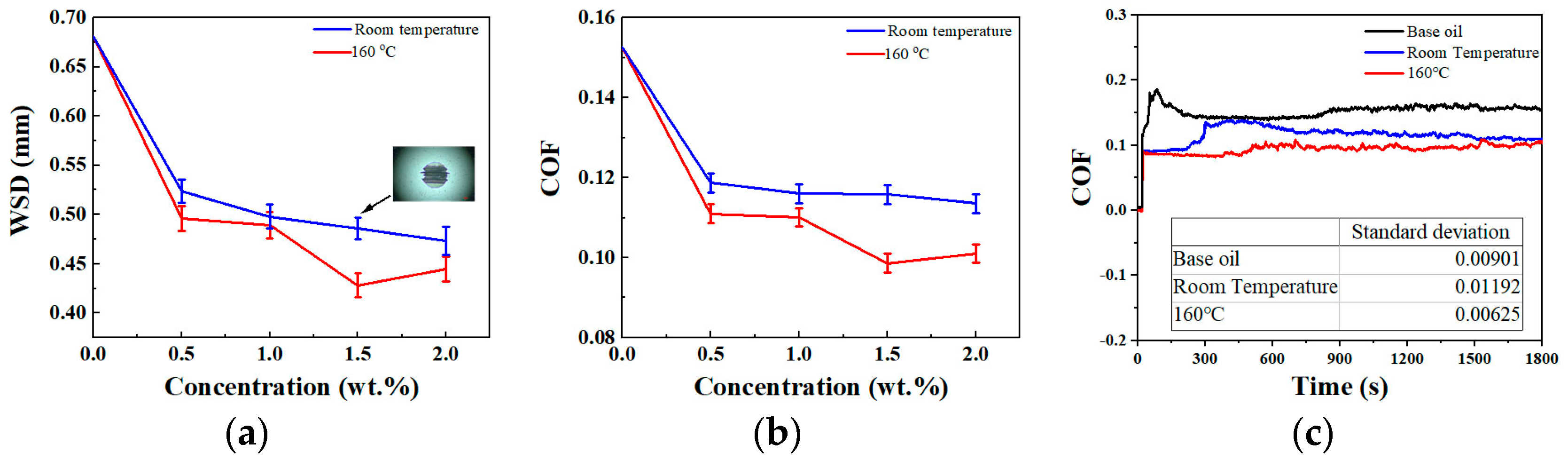
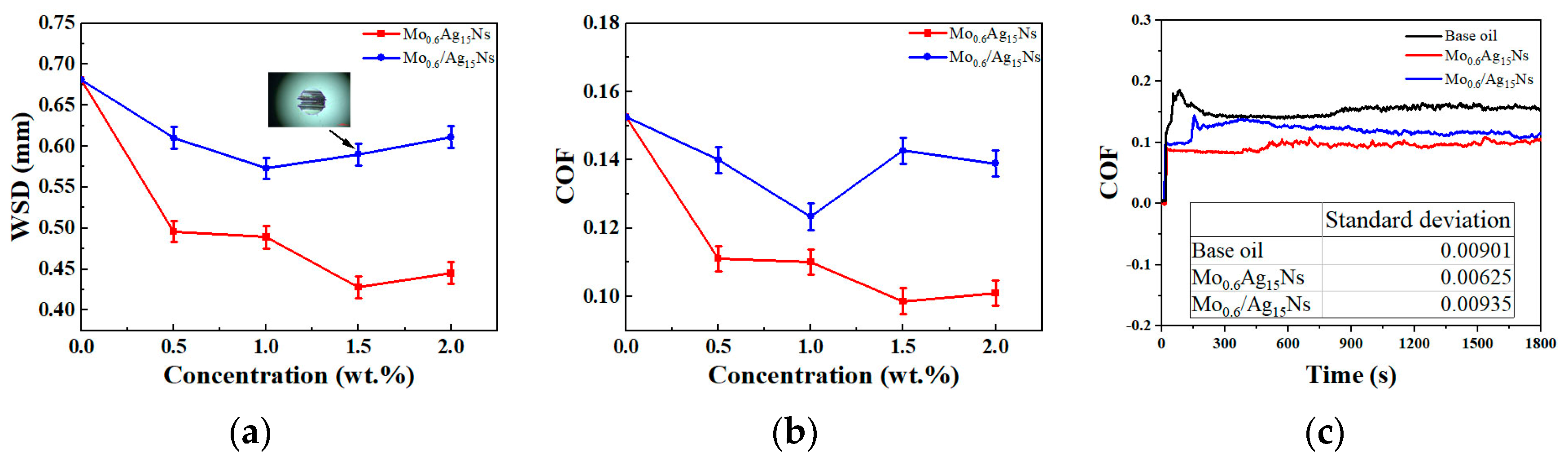
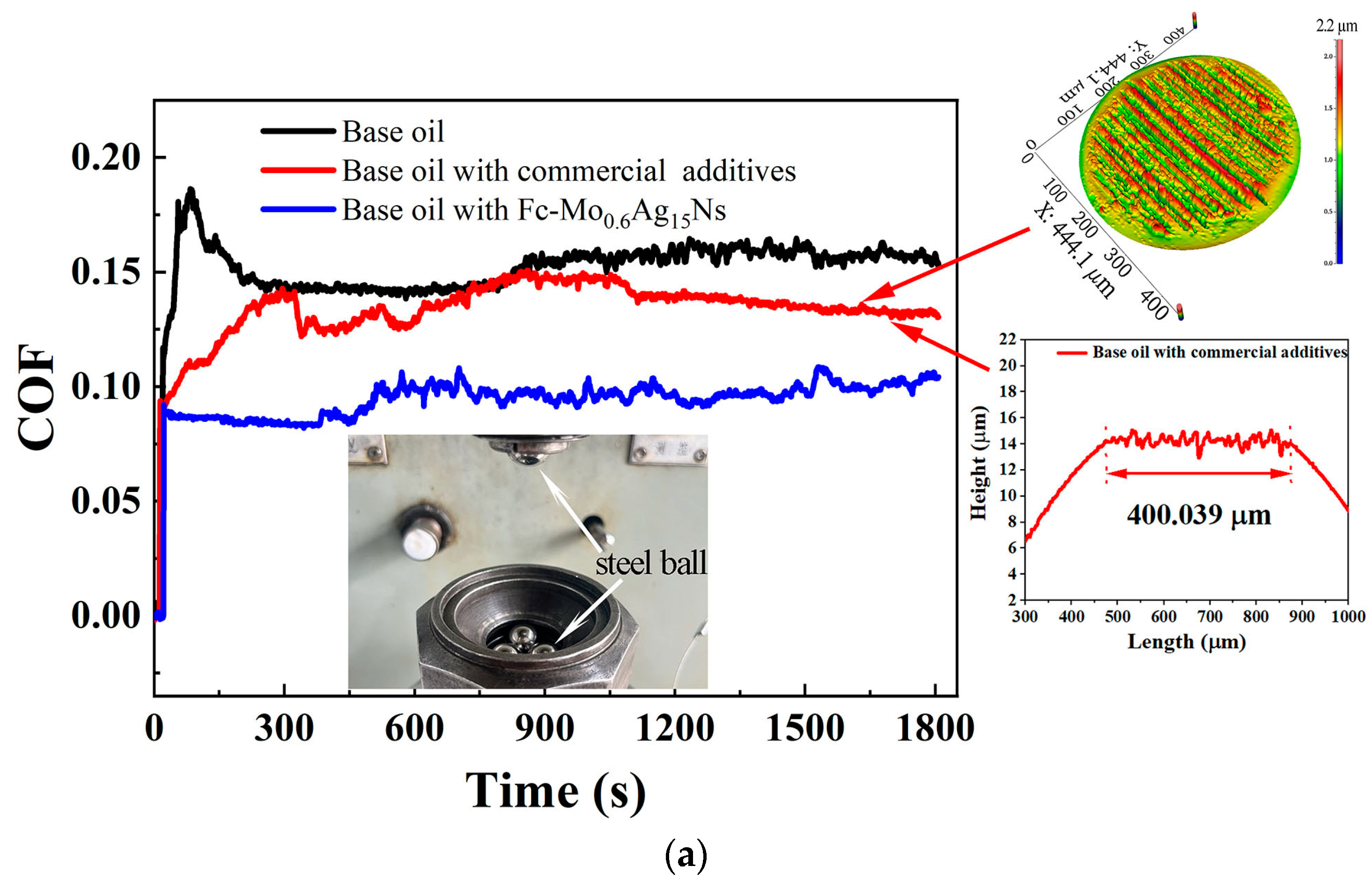
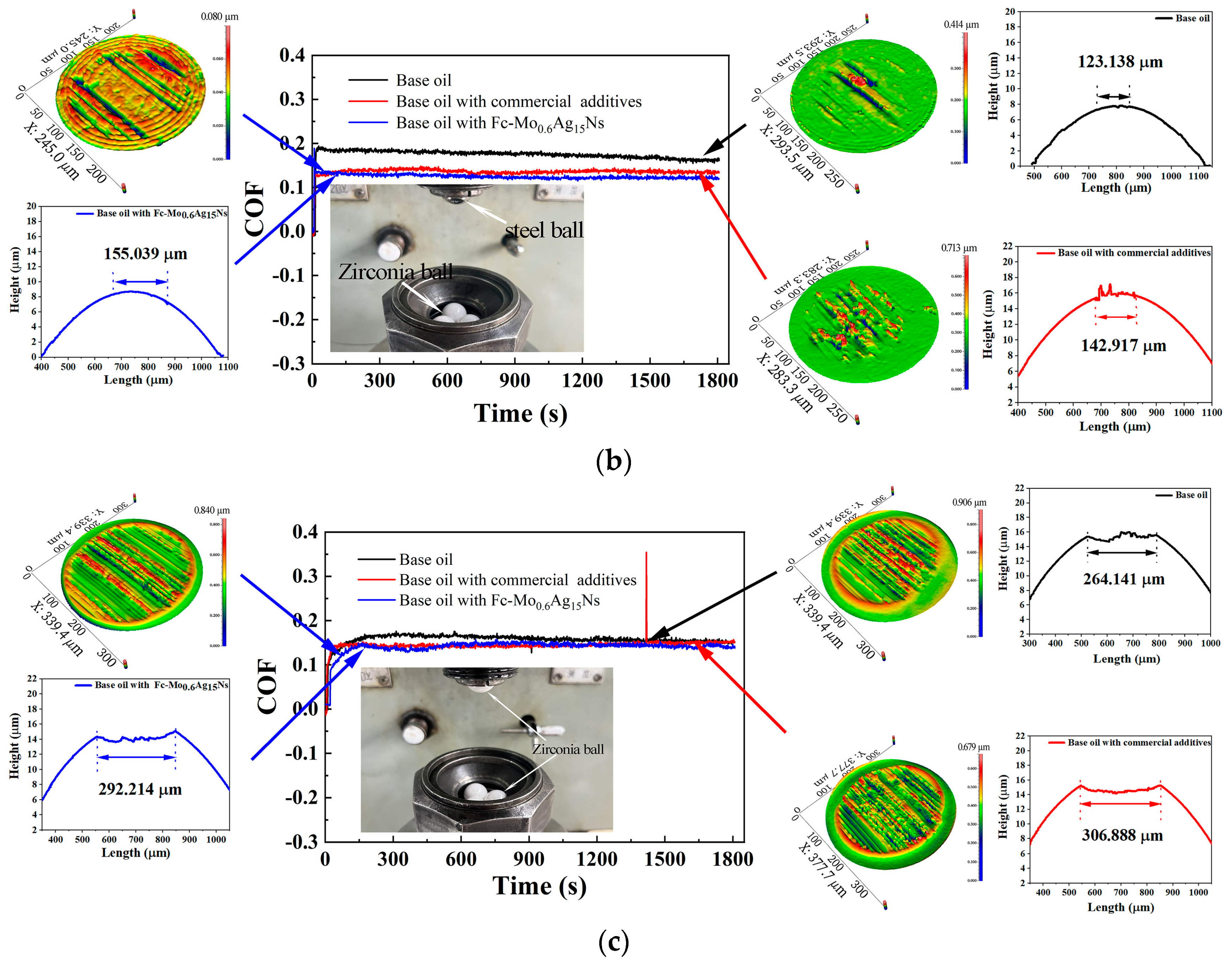
3.2. Tribological Behavior
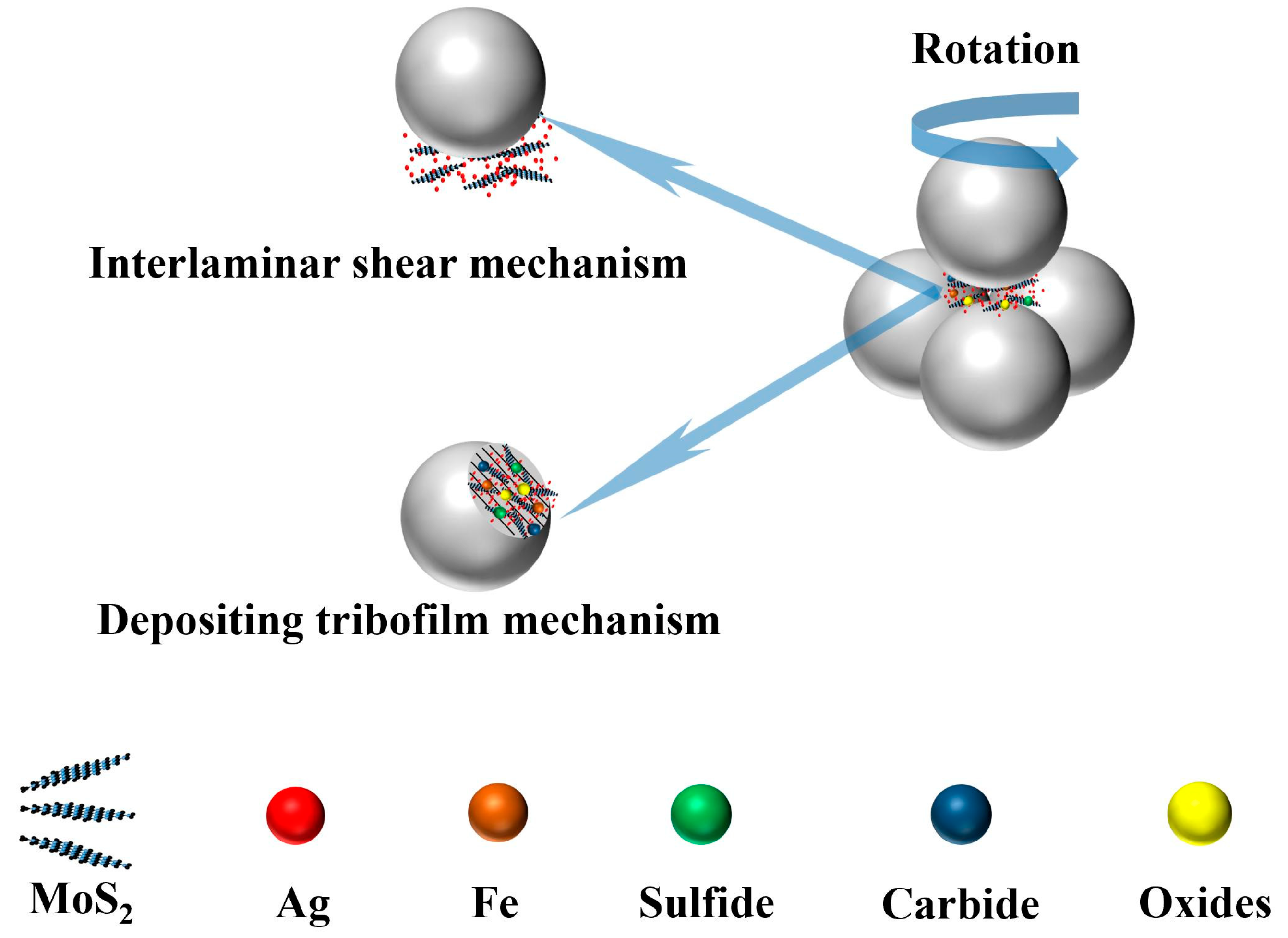
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumari, S.; Chouhan, A.; Siva Kumar Konathala, L.N.; Sharma, O.P.; Ray, S.S.; Ray, A.; Khatri, O.P. Chemically functionalized 2D/2D hexagonal boron nitride/molybdenum disulfide heterostructure for enhancement of lubrication properties. Appl. Surf. Sci. 2022, 579, 152157. [Google Scholar] [CrossRef]
- Xie, L.; Li, M.; Zhu, P.; Xiao, X.; Jia, Z.; Wei, Z.; Jiang, J. Novel combination of nickel-cobalt sulfide and oxide derived from Ni2CoS4@ZIF-67 for high performance supercapacitor. J. Alloys Compd. 2022, 898, 162861. [Google Scholar] [CrossRef]
- Zang, C.; Yang, M.; Liu, E.; Qian, Q.; Zhao, J.; Zhen, J.; Zhang, R.; Jia, Z.; Han, W. Synthesis, characterization and tribological behaviors of hexagonal boron nitride/copper nanocomposites as lubricant additives. Tribol. Int. 2022, 165, 107312. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Kumar, R.; Hussainova, I.; Antonov, M.; Maurya, H.S.; Ripoll, M.R. Temperature-induced wear micro-mechanism transition in additively deposited nickel alloys with different solid lubricants. Wear 2024, 552–553, 205452. [Google Scholar] [CrossRef]
- Gong, K.; Lou, W.; Zhao, G.; Wu, X.; Wang, X. MoS2 nanoparticles grown on carbon nanomaterials for lubricating oil additives. Friction 2021, 9, 747–757. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Choi, J.; Wang, C.; Su, D. Synthesis of N-doped carbon quantum dots as lubricant additive to enhance the tribological behavior of MoS2 nanofluid. Friction 2023, 11, 441–459. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, Y.; Geng, J.; Peng, Y.; Olson, D.; Hu, X. Tribological behavior of Fe3O4/MoS2 nanocomposites additives in aqueous and oil phase media. Tribol. Int. 2016, 102, 79–87. [Google Scholar] [CrossRef]
- Xuan, D.; Zhou, Y.; Nie, W.; Chen, P. Sodium alginate-assisted exfoliation of MoS2 and its reinforcement in polymer nanocomposites. Carbohyd. Polym. 2017, 155, 40–48. [Google Scholar] [CrossRef]
- Subitha, M.; Sasikanth, S.M.; Bindhu, B. Ionic liquid assisted exfoliation and dispersion of molybdenum disulphide: Synthesis and characterization. AIP Conf. Proc. 2019, 2100, 020098. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. Available online: https://www.science.org/doi/10.1126/science.1194975 (accessed on 13 August 2024). [CrossRef]
- Chen, Y.; Sun, J.; Liu, Y.; Li, Q.; Xiao, S.; Su, F. Microstructure, mechanical and high-temperature tribological properties of MoS2-Cr-Ag composite films. Surf. Coat. Technol. 2023, 452, 129135. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Paudel, Y.; Simonson, W.J.; Ge, Q.; Kohli, P.; Muratore, C.; Voevodin, A.A. Tribological investigation of adaptive Mo2N/MoS2/Ag coatings with high sulfur content. Surf. Coat. Technol. 2009, 203, 1304–1309. [Google Scholar] [CrossRef]
- Kumar, R.; Torres, H.; Aydinyan, S.; Antonov, M.; Varga, M.; Hussainova, I.; Ripoll, M.R. Tribological behavior of Ni-based self-lubricating claddings containing sulfide of nickel, copper, or bismuth at temperatures up to 600 °C. Surf. Coat Technol. 2023, 456, 129270. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, R.; Song, H.; Jia, X. Improved tribological properties of the synthesized copper/carbon nanotube nanocomposites for rapeseed oil-based additives. App. Surf. Sci. 2018, 428, 630–639. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Z.; Liu, C.; Zhao, L.; Ni, J.; Li, Y.; Shao, X.; Wang, C. The synthesis and tribological properties of Ag/polydopamine nanocomposites as additives in poly-alpha-olefin. Tribol. Int. 2017, 114, 282–289. [Google Scholar] [CrossRef]
- Wu, Y.; He, Z.; Zeng, X.; Ren, T.; De Vries, E.; van der Heide, E. Tribological properties and tribochemistry mechanism of sulfur-containing triazine derivatives in water-glycol. Tribol. Int. 2017, 109, 140–151. [Google Scholar] [CrossRef]
- Wadi, V.S.; Jena, K.K.; Halique, K.; Alhassan, S.M. Enhanced mechanical toughness of isotactic polypropylene using bulk molybdenum disulfide. ACS Omega 2020, 5, 11394–11401. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Shen, Q.; Zhao, W.; Tan, Q.; Zhang, N.; Li, P.; Jiao, L.; Qu, X. Sandwich-like heterostructures of MoS2/graphene with enlarged interlayer spacing and enhanced hydrophilicity as high-performance cathodes for aqueous zinc-ion batteries. Adv. Mater. 2021, 33, 2007480. [Google Scholar] [CrossRef]
- Chaudhari, A.B.; Anand, A.; Rajput, S.D.; Kulkarni, R.D.; Gite, V.V. Synthesis, characterization and application of azadirachta indica juss (neem oil) fatty amides (AIJFA) based polyurethanes coatings: A renewable novel approach. Prog. Org. Coat. 2013, 76, 1779–1785. [Google Scholar] [CrossRef]
- Pooja; Yadav, S.; Pawar, R. Ternary heterostructures of GO, MoS2, and g-C3N4: Synthesis, stability and properties. Results Surf. Interfaces 2023, 11, 100115. [Google Scholar] [CrossRef]
- Akintayo, C.O.; Akintayo, E.T.; Ziegler, T. Studies on newly developed urethane modified polyetheramide coatings from Albizia benth oil. Prog. Org. Coat. 2011, 71, 89–97. [Google Scholar] [CrossRef]
- Narayana, T.; Saleem, S.S. Enhancing fretting wear behavior of Ti64 alloy: The impact of surface textures and CrN-MoS2-Ag composite coating. Tribol. Int. 2024, 193, 109346. [Google Scholar] [CrossRef]
- Quan, X.; Zhang, S.; Hu, M.; Gao, X.; Jiang, D.; Sun, J. Tribological properties of WS2/MoS2-Ag composite films lubricated with ionic liquids under vacuum conditions. Tribol. Int. 2017, 115, 389–396. [Google Scholar] [CrossRef]
- Hu, K.H.; Liu, M.; Wang, Q.J.; Xu, Y.F.; Schraube, S.; Hu, X.G. Tribological properties of molybdenum disulfide nanosheets by monolayer restacking process as additive in liquid paraffin. Tribol. Int. 2009, 42, 33–39. [Google Scholar] [CrossRef]
- Long, L.N.; Quang, N.T.; Khuong, T.T.; Kien, P.T.; Thang, N.H.; Khai, T.V. Controllable synthesis by hydrothermal method and optical properties of 2D MoS2/rGO nanocomposites. J. Sol-Gel Sci. Technol. 2023, 106, 699–714. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, H.; Dargusch, M.; Huang, Y. A reliable and highly efficient exfoliation method for water-dispersible MoS2 nanosheet. J. Colloid Interf. Sci. 2018, 514, 642–647. [Google Scholar] [CrossRef]
- Ji, S.; Yang, Z.; Zhang, C.; Liu, Z.; Tjiu, W.W.; Phang, I.Y.; Zhang, Z.; Pan, J.; Liu, T. Exfoliated MoS2 nanosheets as efficient catalysts for electrochemical hydrogen evolution. Electrochim. Acta 2013, 109, 269–275. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. App. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Zhang, J.; Wang, K.; Shen, M.; Zeng, S.; Dayeh, S.A.; Feng, L. Lattice strain effects on the optical properties of MoS2 nanosheets. Sci. Rep. 2014, 4, 5649. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Q.; Lin, Z.; Li, Y.; Jia, X.; Song, H. Growth of ultra-dense MoS2 nanosheets on carbon fibers to improve the mechanical and tribological properties of polyimide composites. Friction 2021, 9, 1150–1162. [Google Scholar] [CrossRef]
- Wu, M.; Zhan, J.; Wu, K.; Li, Z.; Wang, L.; Geng, B.; Wang, L.; Pan, D. Metallic 1T MoS2 nanosheet arrays vertically grown on activated carbon fiber cloth for enhanced Li-ion storage performance. J. Mater. Chem. A 2017, 5, 14061–14069. [Google Scholar] [CrossRef]
- Gao, M.R.; Chan, M.; Sun, Y. Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production. Nat. Commun. 2015, 6, 7493. [Google Scholar] [CrossRef]
- Liu, H.; Su, D.; Zhou, R.; Sun, B.; Wang, G.; Qiao, S.Z. Highly ordered mesoporous MoS2 with expanded spacing of the (002) crystal plane for ultrafast lithium ion storage. Adv. Energy Mater. 2012, 2, 970–975. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Han, Y.; Li, J.; Yang, L.; Benamara, M.; Chen, L.; Zhu, H. Two-dimensional water-coupled metallic MoS2 with nanochannels for ultrafast supercapacitors. Nano Lett. 2017, 17, 1825–1832. [Google Scholar] [CrossRef]
- Qin, P.; Yi, D.; Hao, J. Bi-layer molybdenum disulfide obtains from molybdenum disulfide-melamine cyanurate superlattice with a thermal shock. Adv. Powder Technol. 2021, 32, 1594–1601. [Google Scholar] [CrossRef]
- Ghatak, S.; Pal, A.N.; Ghosh, A. Nature of electronic states in atomically thin MoS2 field-effect transistors. ACS Nano 2011, 5, 7707–7712. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, Q.; Yang, M.; Zang, C.; Zhang, B.; Hou, Y.; Zhen, J.; Zhang, R.; Han, W.; Jia, Z. The synthesis and wear behavior of reduced graphene oxide/Ag nanocomposites as additives in liquid paraffin. J. Mater. Eng. Perform. 2023, 32, 6665–6676. [Google Scholar] [CrossRef]
- Gao, D.; Si, M.; Li, J.; Zhang, J.; Zhang, Z.; Yang, Z.; Xue, D. Ferromagnetism in free standing MoS2 nanosheets. Nanoscale Res. Lett. 2013, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Pan, B.; Liu, H.; Li, N.; Chen, Z.; Yan, J.; Fu, Z.; Guo, S.; Wang, H. One-step synthesis of carbon sphere@1 T-MoS2 towards superior antiwear and lubricity. Tribol. Int. 2022, 176, 107927. [Google Scholar] [CrossRef]
- Yang, M.; Jia, Z.; Pang, X.; Shao, X.; Zhen, J.; Zhang, R.; Ni, J.; Jiang, J.; Yu, B. The synthesis and tribological properties of carbonized polydopamine/Ag composite films. J. Mater. Eng. Perform. 2019, 28, 7213–7226. [Google Scholar] [CrossRef]
- Jia, Z.; Li, H.; Zhao, Y.; Frazer, L.; Qian, B.; Borguet, E.; Ren, F.; Dikin, D.A. Electrical and mechanical properties of poly(dopamine)-modified copper/reduced graphene oxide composites. J. Mater. Sci. 2017, 52, 11620–11629. [Google Scholar] [CrossRef]
- Jia, Z.; Li, H.; Zhao, Y.; Dikin, D.A.; Ni, J.; Zhao, L.; Zhen, J.; Ge, B.; Shao, X.; Ren, F. Preparation and electrical properties of sintered copper powder compacts modified by polydopamine-derived carbon nanofilms. J. Mater. Sci. 2018, 53, 6562–6573. [Google Scholar] [CrossRef]
- García, I.; Galipaud, J.; Kosta, I.; Grande, H.; Garcia-Lecina, E.; Dassenoy, F. Influence of the organic moiety on the tribological properties of MoS2: Glycol hybrid nanoparticles-based dispersions. Tribol. Lett. 2020, 68, 104. [Google Scholar] [CrossRef]
- Wei, X.; Li, W.; Fan, X.; Zhu, M. MoS2-functionalized attapulgite hybrid toward high-performance thickener of lubricating grease. Tribol. Int. 2023, 179, 108135. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Liu, Z.; Cheng, Z. Preparation and tribological properties of oleicacid-decorated MoS2 nanosheets with good oil dispersion. J. Dispers. Sci. Technol. 2018, 39, 1742–1751. [Google Scholar] [CrossRef]
- Nagarajan, T.; Khalid, M.; Sridewi, N.; Jagadish, P.; Shahabuddin, S.; Muthoosamy, K.; Walvekar, R. Tribological, oxidation and thermal conductivity studies of microwave synthesised molybdenum disulfide (MoS2) nanoparticles as nano-additives in diesel based engine oil. Sci. Rep. 2022, 12, 14108. [Google Scholar] [CrossRef]



| Viscosity (mm2/s, 40 °C) | Viscosity (mm2/s, 100 °C) | Flash Point °C | Pour Point °C | |
|---|---|---|---|---|
| Base oil (denoted as BO) | 263.56 | 23.8 | 289 | −15 |
| Commercial additives | — | 115–135 | ≧180 | — |
| BO containing commercial additives (11.5 wt.%) | 315.23 | 28.53 | 253 | −13 |
| BO containing nano composites. | 285.12 | 25.1 | 283 | −14.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Liu, Z.; Zhang, W.; Jia, Z.; Han, W.; Zhen, J.; Zhang, R. The Preparation of MoS2/Metal Nanocomposites Functionalized with N-Oleoylethanolamine: Application as Lubricant Additives. Lubricants 2024, 12, 319. https://doi.org/10.3390/lubricants12090319
Xing Y, Liu Z, Zhang W, Jia Z, Han W, Zhen J, Zhang R. The Preparation of MoS2/Metal Nanocomposites Functionalized with N-Oleoylethanolamine: Application as Lubricant Additives. Lubricants. 2024; 12(9):319. https://doi.org/10.3390/lubricants12090319
Chicago/Turabian StyleXing, Yaping, Zhiguo Liu, Weiye Zhang, Zhengfeng Jia, Weifang Han, Jinming Zhen, and Ran Zhang. 2024. "The Preparation of MoS2/Metal Nanocomposites Functionalized with N-Oleoylethanolamine: Application as Lubricant Additives" Lubricants 12, no. 9: 319. https://doi.org/10.3390/lubricants12090319
APA StyleXing, Y., Liu, Z., Zhang, W., Jia, Z., Han, W., Zhen, J., & Zhang, R. (2024). The Preparation of MoS2/Metal Nanocomposites Functionalized with N-Oleoylethanolamine: Application as Lubricant Additives. Lubricants, 12(9), 319. https://doi.org/10.3390/lubricants12090319





