Abstract
A selective transfer is realized safely if the material pair and the lubricant are adequate, in the presence of a relative motion and an energy favoring the transfer. The paper highlights the mechanism of achieving the selective transfer phenomenon and analyzes the physiochemical aspects that take place in a suitably lubricated friction pair (here, bronze/steel lubricated with glycerin) that favor friction by reducing the friction force and implicitly reducing the wear in the selective transfer conditions. In addition, the paper seeks to highlight the effect that the servowitte layer (film) that appears on the friction pair surfaces following the selective dissolution of the superficial layer in the contact area has on the factors that influence the friction and wear process through the implications on the friction coefficient and the wear itself.
1. Introduction
The current trend is to use lubricants in friction pairs that accelerate oxidation processes as they heat up, which leads to their mechanical destruction and a catalytic and electrochemical effect on the friction surfaces. Experiments have shown that it is useful to quickly realize, in the initial stage of friction, thermodynamically unstable processes in the lubricant and on the friction pair’s contact surfaces. This presupposes that in the operating conditions, physiochemical processes favorable to friction unfold, such as polymerization, active substance formation at the contact surfaces, colloids, and other substances.
Thus, in refs. [1,2,3], it is defined that friction in such conditions bears the name of selective transfer and is used where the friction of the mixed and adherent layers is not safe enough or the service life of the friction pairs is not ensured.
The selective transfer phenomenon and the study of its features provide new possibilities for substantially increasing the wear resistance of metallic materials. The effect of selective transfer on the friction of the material is highly sensitive to its structure, and the formed layer is characterized by certain proportions of the structural transformations in the surface layers of the interacting materials [4]. Additionally, Rybakova and Kuksenova [4] showed that an analysis of the structural criteria of the phenomenon of selective transfer permits the conclusion that both at the initial stages and in a steady mode the composition of the material initiating this phenomenon is the decisive factor.
The requirements for the reliability and service life of friction pairs are becoming increasingly strict and, therefore, it is particularly important to use the internal structural reserves of metallic materials. One way to use structural factors to increase wear resistance is to modify the structure of strained surface micro volumes, which implies realizing the phenomenon of selective transfer by friction [3,4].
Polyakov and Ruzanov [5] presented an example of the formation of a dissipative structure in a deformable metal during cyclic friction and deformation of the metal’s surface layer. An analysis of the dissipative structure indicated [5] that inhomogeneity and stability (proximity to equilibrium of the system, the extent to which it is closed, and the lack of exchange with the external environment) were overcome during the kinetic phase transition from a closed system of friction with continuous lubrication to an open system of selective transfer.
Kuzharov [6] showed that definite tribochemical reactions are necessary for the formation of selective transfer, as well as a trajectory of irreversible transformations in the system–environment interface. As a result, tribosystems are capable of self-organization, in which the process of frictional interaction is passed to a nanocrystalline quasi-liquid [6], and thus provides the friction coefficient similar to hydrodynamic friction, forming nanoclusters with almost perfect crystals, which leads to an increase in load capacity and wear resistance of the friction surfaces. Thus, it has the ability to reduce the coefficient of friction and significantly eliminate wear as a result of the tribosynthesis of nano-coatings on the contact surfaces, and fully corresponds to the principles of green chemistry, engineering, and tribology [6].
On the other hand, Kuksenova and Savenko [7] showed that using dispersed systems with surface-plasticizing dispersed phases in friction pairs as lubricants creates a wear-resistant structure in the antifriction material and establishes a stable low-wear selective transfer mode during the friction process. For example, when bronze is rubbed in surface-plasticizing media, two crystallographically isostructural solid solutions form in the near-surface layer of bronze, one of which is enriched in copper.
Therefore, the conditions for selective transfer are complex and caused by the physiochemical processes in the contact areas due to pressure, sliding speed, temperature, lubricant’s thermodynamic instability and the material, collisions of asperities, tribodestruction—the catalytic effect on the lubricant of the oxide layers and the material. In ref. [1,2], it is shown that lubricant tribodestruction under selective transfer conditions at the friction start leads both to the oxidation problem resolution and a series of other phenomena:
- -
- the production of electric charges, through which particles of different charges are attracted, forming an electric double layer and other structural defects in the superficial layer from surface areas, which reduces tensions and causes an apparent softening, which favors deformations [2,8].
- -
- depolarization due to the sliding friction of the contact surfaces, having the effect of acceleration of corrosion processes and the destruction of oxide layers, ultimately reducing self-passivation;
- -
- electron emission, especially during oscillating movement, without oxides, of the friction pair elements;
- -
- formation of organometallic compounds, surfactant substances, and colloids, which allow the transport of metallic particles to the contact area until a balance with the friction surface areas is established, which inevitably leads to a reduction in friction and wear.
The processes listed above are justified for the pair considered in the present paper (bronze/steel lubricated with glycerin), which operates in selective transfer conditions. Thus, the transfer on the steel surface of copper particles, as well as the concentration reduction of the alloying elements in the steel layer, cleaned of oxides as a result of their dissolution, reduces the potential of the microelements that form in the alloy, as well as between the alloy and the steel, to values close to zero. Thus, Kargul and Konieczny [9] showed that after abrasion resistance tests on specimens of sintered composites based on copper powders and steel particles, the hardness increased compared to a pure copper sample, while the density and electrical conductivity decreased. Instead, Bouchoucha et al. [10] studied metal transfer and oxidation in a sliding copper–steel electrical contact, where they demonstrated the influence of the electric field and a high thermal gradient.
Changing external conditions (load, speed, and temperature) disrupts the equilibrium, inevitably leading to an increase in potential, while all the phenomena listed above contribute to a reduction in potential.
Therefore, it was possible to find that the potential between the contact area and the friction surface zone, where there is no contact, remains constant for the given operating conditions throughout the entire period and, thereby, certainly contributes to reducing friction and wear. However, there are other ways to reduce friction, such as lubrication, improving surface roughness, reducing the acting surface pressure, surface texturing, and reducing the contact area, as shown in ref. [11].
Tribodestruction products have a relatively high thermal stability, also contributing to a thermodynamically unstable compounds’ natural selection, which leads to more stable substances after oxidation. For example, with the use of lubricants to achieve selective transfer, it was found that the periods between oil changes could be three times longer than before, thereby reducing the time for changing the oil in the maintenance period and labor consumption [3]. The phenomena favorable to friction, which arise as a result of the lubricant tribodestruction, are caused by the interaction between the substances formed during the tribodestruction and the metal.
It is known that during dry and mixed friction, there is a dissipation of thermal energy, which ultimately leads to fatigue failure and destruction as a result of structural changes. The thin layer of oxide and the layer of lubricant absorbed by it, as well as the mixture of moisture and oxygen, do not protect the surface layer from deformations, encrustation, and destruction.
The cause of these deficiencies is the lack of regeneration after mechanical destruction in the friction surface areas, as well as the lack of balance in the processes that lead to the destruction. Aniołek et al. [12] demonstrated that the thermal oxidation process (with and without an anti-wear oxide layer) makes it possible to control, over a wide range, the friction–wear characteristics of an alloy. These deficiencies, which appear in the friction of the adherent layers and in dry friction, are the result not only of the thermodynamic instability of the lubricant, but also of the instability of the materials (metals). Some noble metals are an exception, at rest and especially during the friction process, as well as glass.
During selective transfer, the metals tend to reduce oxides to create a special layer on the friction surfaces and protect them against oxidation. This special layer takes the shear stress without being destroyed and, thus, also protects the base metal against wear. Thus, selective transfer avoids both metal and lubricant oxidation to reduce friction and wear.
Research on the physiochemical mechanism for reducing friction and wear under selective transfer conditions [1,2,8] and on factors that increase wear resistance led to the conclusion that these are the result of the self-regulation of the balance phenomena disturbed in the friction process, as well as of the frictional force. In practice, those lubricants should be used that have self-regulating capacity and operate not only under selective transfer conditions, but also under friction conditions between the mixed and adherent layers, such as the polymerization of contact areas. In ref. [13], an analytical, numerical, and experimental analysis of adhesive contacts subjected to unstable and jerky tangential motion of the adhesive contact boundary area and its dependence on surface roughness was presented. Parkatzidis et al. [14] presented a perspective on the most recent developments in controlled radical polymerization in recent years, critically highlighting the strengths and weaknesses of the recent achievements and also how these discoveries had extended the scope of application of personalized polymer materials.
Therefore, the basis of selective transfer is the tribodestruction’s physiochemical processes and the electrochemical reactions of the lubricant in friction pairs. These lead to self-regulation of the balance processes disturbed by the appearance of wear, as well as to a friction force reduction [15]. For this reason, selective transfer is characterized by the following processes: real pressure reduction in the contact area, deformation compensation, shear resistance reduction in the superficial regions, returning the removed particles to the contact area from the friction surface areas, and the formation of a polymerized protective layer, called servowitte [1,2,3].
The servowitte layer is formed due to the relative motion-induced energy flow that emerges in the friction process of a suitable material pair (the bronze/steel pair) and a suitable lubricant (glycerin). Depending on the material pair involved in the friction process and the friction conditions, the servowitte film (layer) formation mechanism on the friction surfaces can vary [1,2,8]. Glycerin was used because, as was shown in refs. [1,2,3,9,16], it is a model liquid that achieves the selective transfer mode more easily than other liquids.
Thus, the paper aims to highlight the physical and chemical mechanisms of reducing friction and wear through the selective transfer phenomenon under specific conditions. To achieve this, it analyzes the friction behavior of glycerin-lubricated bronze/steel materials through experimental studies and discusses the impact of the selective transfer layer (servowitte) on the friction and wear coefficient.
2. Materials and Methods
2.1. Mechanism of the Formation of the Selective Layer (Servowitte)
To have the certainty of achieving selective transfer in the friction process, it is necessary to use a pair of materials and a lubricant that favors the selective transfer process. As a result, bronze and steel lubricated with glycerin were chosen as the friction pair, in the presence of relative motion and energy favoring the transfer.
The selective transfer was highlighted on a test machine that works on the Amsler tribometer principle (roll/roll), with one roll made of a copper alloy (CuSn12T bronze, equivalent to UNS-C90800) and the other roll made of steel (OLC 45 steel, equivalent to AISI-SAE 1045), with a sliding speed of 0.2 m/s.
It is mentioned that the rollers tested on the Amsler installation/tribometer both have rotational movements but with different speeds to slide/rub against each other. Thus, there is a combined friction of rolling with sliding. The diameters of the rollers are also different, 25 mm (roll of copper alloy) and 40 mm (roll of steel), with the same thickness of 10 mm. The rotation speed of the copper alloy roller was 160 rpm, while that of the steel roller was 100 rpm, so the resulting sliding/friction speed was approximately 0.2 m/s. Thus, the sliding ratio is the sliding/friction speed ratio (the linear speed at the periphery of the rollers, which is the same), i.e., 0.2/0.2 = 1. Meanwhile, the rolling ratio is the ratio of the roller’s revolutions, i.e., 100/160 = 0.625, and their ratio (slip ratio/rolling ratio) is 1/0.625 = 1.6, a significant difference, which proves that there is sliding friction, although the rollers have rotational movement. The test temperature was room temperature (around 20 °C in the morning and around 30 °C in the afternoon; the tests were performed during the summer under the same conditions for all tribo-tests).
Then, on the same Amsler tribometer, the roller/shoe friction pair was tested, both being 10 mm thick: the steel roller with a diameter of 40 mm and the bronze shoe. In this case, the roller had the rotational movement, and the shoe was fixed, resulting in sliding friction. Further, selective transfer was highlighted on the shaft-bearing installation/tribometer. The friction pair was of the shaft-bearing type, with the shaft made of steel and the bearing made of bronze. The nominal diameter of the shaft (outer) was equal to the nominal diameter of the bearing (inner) at 30 mm (the diameters in the contact area), and the contact width (of the bearing) was 10 mm. Here, the shaft had rotational movement, and the bearing was fixed, so there was sliding friction as well. It is worth noting that the same pair of materials as above was used for each of the friction pairs tested and lubricated with glycerin. In all the tests, the specimen’s contact with the friction pairs was linear. The contact surfaces of the specimens were polished with 600 grit sandpaper and thoroughly cleaned with acetone, isopropanol, and distilled water before the experimental tests.
Additionally, for the roller/shoe and shaft-bearing specimens, COFs were determined at different sliding/friction speeds, the variations of which depending on the contact pressure are shown below in Section 3.2. COFs were determined by reading/recording the frictional moment on the installation reader indicating its value at any time and relating it to the product of the normal load in the contact area (known/imposed) and its arm (the steel roller radius). At the same time, the temperature in the contact area was measured by placing temperature sensors (thermocouples) very close to the contact surface, in holes drilled in the shoe and the bearing at a depth of ca. 0.3 mm. The starting temperature and the one during operation (outside) were the room temperature where the installations (Amsler and shaft-bearing specimens) were placed.
In the friction process, the contact surface heats up over time, depending on the normal load and sliding speed. This heating was recorded by the thermocouples (with a tiny difference due to the distance from the contact surface) and modeled in the form of an electrical signal (electrical voltage), measured using a millivoltmeter, to which the thermocouples were connected. Based on the calibration curve (voltage versus temperature), the temperature was determined at any time, at constant load and different sliding speeds, or at constant speed and different loads.
Both the bronze/steel pair and glycerin were used because, as was shown in refs. [1,2,3,9,16], they are model friction pair and lubricant that achieve the selective transfer mode more easily than other friction pairs and lubricants.
Then, to capture images of the formation of the protective layer obtained in the friction polymerization process, a scanning electron microscope (SEM, JEOL GmbH, Freising, Germany) was used.
Thus, in the bronze/steel friction pair lubricated with glycerin, the bronze friction surface begins to dissolve in the first period of operation when the glycerin acts in the friction process as a weak acid. Then, in the lubricating liquid (glycerin), atoms of the component elements of the bronze (Sn, Al, Zn, Fe, etc.) are transferred, and the copper atoms enrich the bronze friction surface [17,18,19].
After this, in the friction process, the surface of the bronze provokes by deformation a diffusion flow [20] of new atoms (Sn, Al, Zn, Fe, etc.) of the bronze towards the surface and, thus, they reach the lubricating liquid (glycerin). Thus, the surface of the bronze becomes predominantly rich in copper by releasing these elements. In this way, in the bronze layer from the surface, a large number of vacancies is created; some by their union form pores, in which glycerin molecules enter.
Since glycerin redoes copper oxides, the copper layer’s friction surface is released by the oxide films, it is very active and able to adhere to the layer from the steel surface because of free valences. As a result, the steel surface is gradually covered with a thin layer of copper. The copper layer, as a result of its transfer to the steel surface, thins out, so a continuous dissolution is produced of bronze. This process takes place until a copper layer is formed on the bronze and steel surfaces in contact, with an optimal thickness of 1–3 μm on average. After the copper film on the surfaces of steel and bronze reaches the optimal thickness, the bronze dissolution process stops. The glycerin molecules no longer react with the bronze, but they attract the atoms of the bronze component elements, and thus the selective transfer begins to unfold [1,2].
In the case of a small amount of lubricating material, copper particles (micelles) that can form are surrounded by a dense ring of lubricant molecules. The particles have an electric charge, which keeps them in the interstitium, and under the electric field action, they move into the cracks of the surfaces.
The formation of the copper layer occurs on the bronze surface due to the electro-chemical process of metal dissolution [15]. As a result of the servowitte film formation between the portions of anodes and cathodes on the bronze surface, the dissolution process can stop completely, and it follows that the friction mode begins to become stable. If for some reason the copper layer is destroyed, the dissolution of the bronze occurs again, accompanied by some catalytic transformations, and the surface is enriched in copper until it no longer passes to the passive state.
The copper surface, in the absence of the oxide film, can cause dehydrogenation of the liquid. As a result, free hydrogen is eliminated, which plays an active role in the friction process—it reduces the oxide layers on the copper–steel mixture, supporting the non-oxidizing friction process. At temperatures higher than 65 °C, the amount of removed hydrogen increases, and the selective transfer mode in the bronze/steel pair in the case of glycerin lubrication passes into hydrogen wear [1,2,21].
The surface of the steel, to a greater extent, is saturated with hydrogen; as a result, it cracks and, in the form of powder, is transferred to the surface of the bronze. The mechanism of hydrogen generation from lubricants was studied by Kohara et al. [21] using sliding tests on several types of bearings. They concluded that the lubricant is decomposed and hydrogen is generated (quantitatively) through a catalytic reaction with the fresh steel surface. The amount of hydrogen generated by lubricants depends not on their chemical structure, but on their lubrication degree.
Thus, at the bronze/steel friction pairs lubricated with glycerin, the servowitte film is formed on the friction surfaces by the copper melt decomposition [1,2,3,22,23] (solid solution) at low temperatures, easing in the diffusion process the shear deformation (there were situations where tap water was used as a lubricant for selective transfer to clarify some observations and findings during experimental tests and to make some comparisons). However, Winkel et al. [22] demonstrated that the absence of oxygen in combination with high decomposition temperatures favors the removal of metal impurities by volatilization in the case of three synthetic copper sulfides and two commercial copper alloys.
Physiochemical investigations of the structure of the servowitte film [1,3,24] allowed us to assume that the film material was in a melt-like state. The film was not able to adsorb, it was porous, presented low sliding forces, had the top layer without oxides, and could transfer from one friction surface to another, i.e., adhere without damage and an increase in the friction force.
Friction in a bronze/steel friction pair under selective transfer conditions can be compared to the sliding of a body on ice, during which the low coefficient of friction is ensured by the copper film.
2.2. Physical Basis of the Selective Transfer Mechanism
- (a)
- Contact realization of the friction surfaces through a thin layer of deformable plastic copper
Both during normal friction without a lubricant and with the presence of the lubricant film at the end of the contact surfaces, the contact occurs on a very small surface of the nominal contact surface. As a result, the areas of real contact are subjected to high stresses, which lead to interpenetration, and plastic deformations and, thus, to the intensification of wear [3,25].
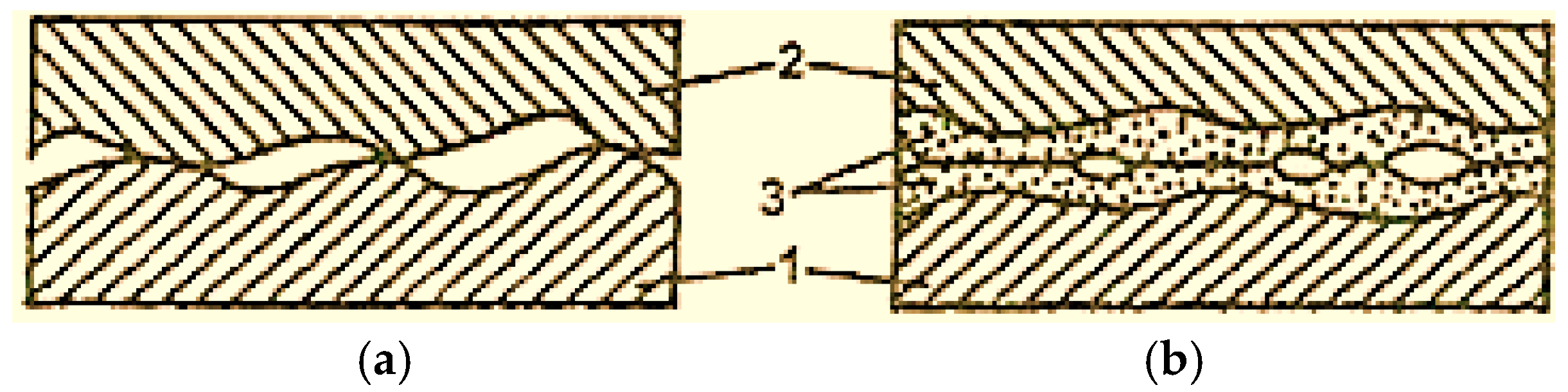
From Figure 1 [2], it can be observed that during boundary lubrication, the contact of the steel 1 and bronze 2 surfaces occurs only at certain points (Figure 1a), and in the selective transfer conditions, the contact is made through the thin copper layer, malleable and deformable plastic (Figure 1b) [2].
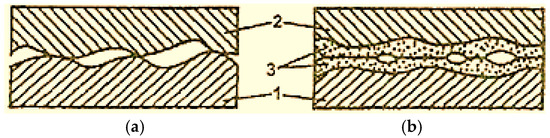
Figure 1.
Surface contact scheme with boundary lubrication (a) and selective transfer (b): 1—steel; 2—bronze; 3—copper film [2].
Therefore, the real contact surface increases tens of times, and the materials of the friction pair elements are only additionally subjected to elastic deformations. The thickness of the copper film is 1–5 μm and corresponds to the dimensions of the asperities or exceeds them. Through boundary friction, the interaction of surface asperities leads to fatigue wear. Through selective transfer, the friction is uninterrupted, and the real contact surfaces are increased.
- (b)
- Avoiding the oxidation process of the material on the friction surfaces
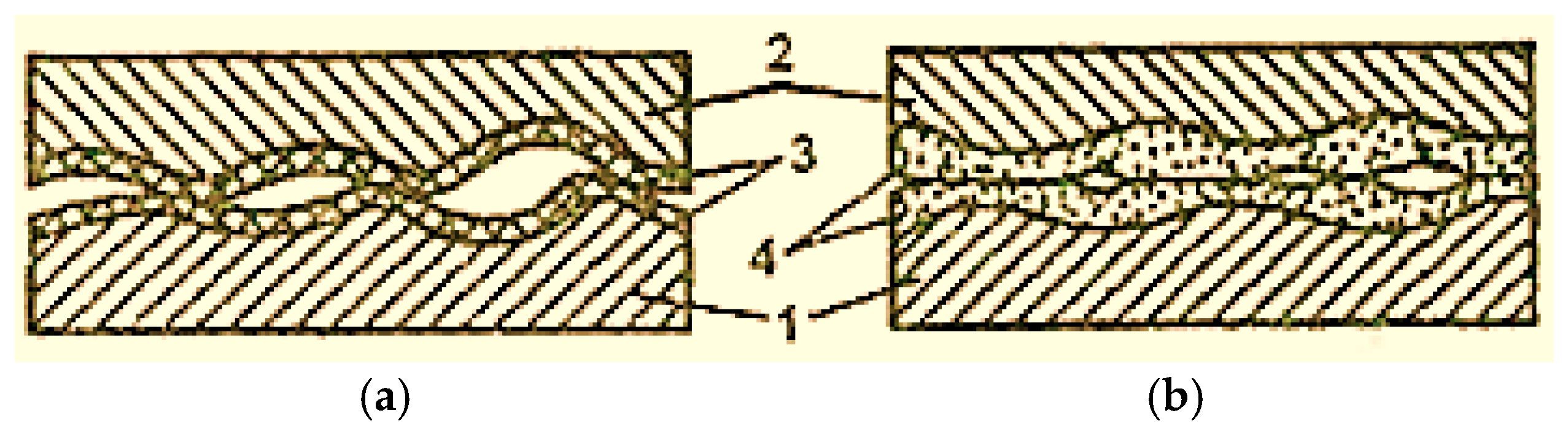
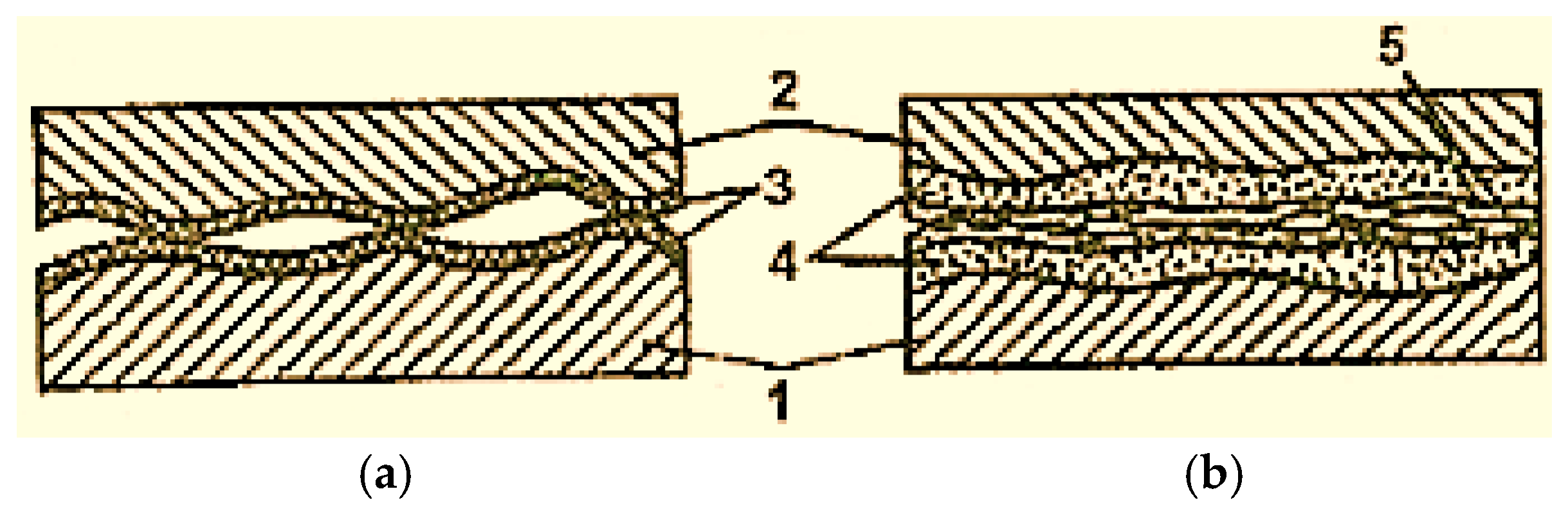
In the case of dry and boundary friction, the surfaces of the elements of the friction pairs are always covered with films of oxides (Figure 2 [2]), which avoids the metal surfaces’ direct contact and their micro-welding. However, oxide films are fragile and cannot be deformed multiple times and, for this reason, in the friction process, they are the first to be destroyed [2].
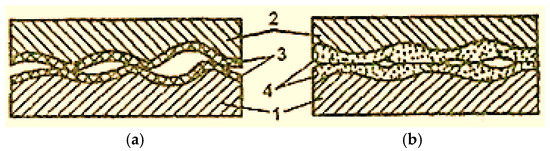
Figure 2.
Scheme of oxidized surfaces’ contact with boundary lubrication (a) and selective transfer (b): 1—steel; 2—bronze; 3—oxide film; 4—servowitte film [2].
As the temperature in the friction zone increases, the oxide film increases (thickens), but the amount of destroyed film also increases. In the conditions of selective transfer, friction occurs without oxidation of the contact surfaces and is therefore not accompanied by oxide film formation.
The surfaces are protected against oxidation by compact layers of positively charged substances, active and adsorbing on the surface, which are formed in the friction process and thus avoid the penetration of oxygen, O2, into the servowitte film. The lack of an oxide film leads to the formation of chemisorption processes, which provide additional wear resistance. Under normal or boundary friction, oxide films prevent dislocations from coming to the surface, which accelerates the surface layer hardening and its destruction. The servowitte film is not subject to encrustation and can be deformed multiple times without being destroyed, and in the absence of oxide films, dislocations pass through it easily.
- (c)
- Achieving the Rehbinder effect
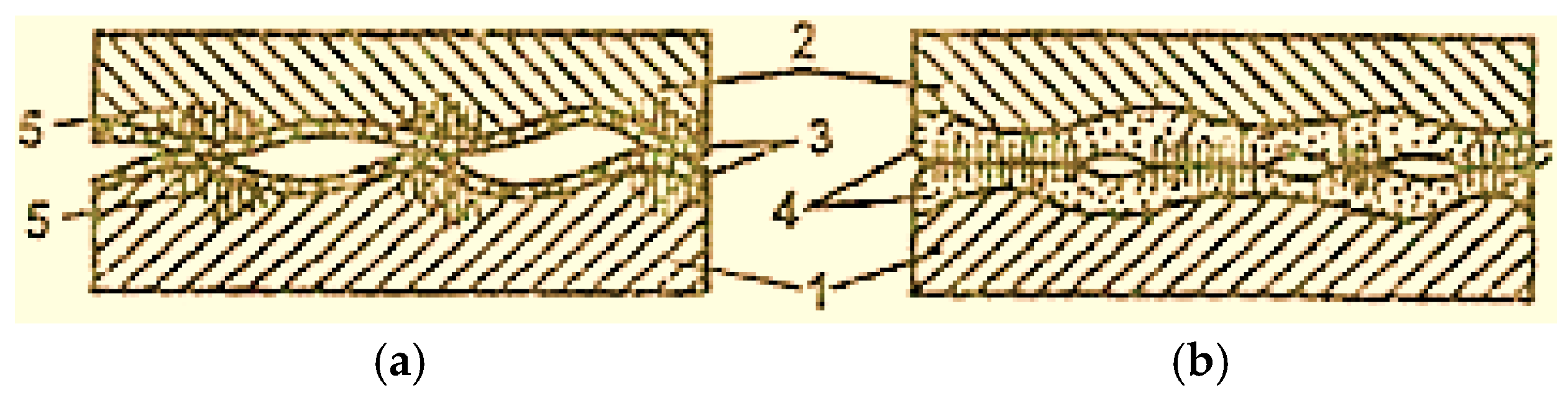
Almost all lubricating materials contain active substances on the surface, which determines the possibility of plasticization of the surface layers, of the materials of the friction pair elements, as a result of the Rehbinder effect (the effect of plasticization by adsorption) and the decrease in the frictional forces between them (Figure 3 [2]).
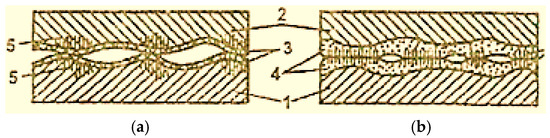
Figure 3.
Scheme of deformation propagation in the contact points of surfaces with boundary lubrication (a) and with selective transfer (b): 1—steel; 2—bronze; 3—oxide film; 4—servowitte film; 5—deformations [2].
In normal friction, oxide films prevent the penetration of the medium into the metal, which causes the Rehbinder effect to decrease. As a result, the plastic deformations of the contact areas include deeper layers (Figure 3a) [2].
In the case of selective transfer, oxide layers are missing, and the action of the Rehbinder effect is fully realized, and only the servowitte film is deformed; the layers beneath the metal surface do not deform (Figure 3b) [2].
Since the molecules of surface-active substances are in the pores of the servowitte layer, sliding inside the film is not excluded due to the diffusion mechanism, but with low energy consumption, which leads to a decrease in friction and wear.
Changes in microstructural characteristics during deformation by friction of bronze under conditions of the Rehbinder effect are considered as an example. This determines the possibility of achieving the selective transfer mode during the operation of tribological interfaces. When bronze is rubbed in surface-plasticizing media, two crystallographically isostructural solid solutions form in the near-surface layer of bronze, one of which is enriched in copper [7].
- (d)
- Transfer of particles from one surface to another and their maintenance in the area of contact with the electric field
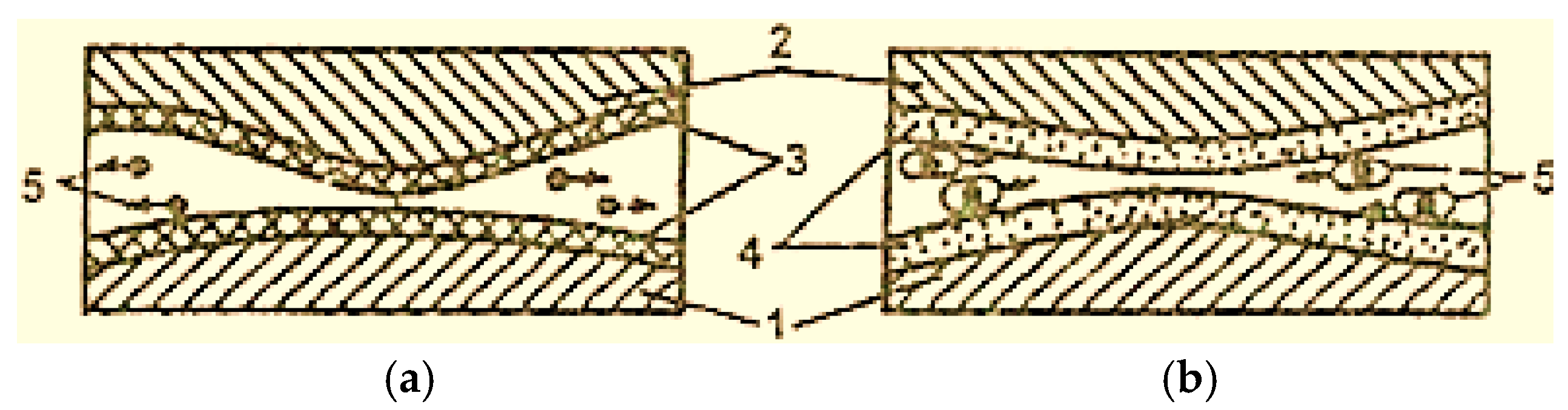
The wear products during boundary friction are mainly oxides, which have no electrical charge and freely leave the friction zone, moving between the contact surfaces (Figure 4 [2]), having an abrasive action on these surfaces (Figure 4a) [2]. It is necessary to take measures to remove worn products from the lubrication layer. Thus, the wear products are represented by copper particles due to the presence of the servowite film on the friction surfaces. Their surface is porous and very active because copper particles become covered with an adsorption layer of active substances on the surface. These types of particles (micelles) are electrically charged, and, under the action of electric charges, they concentrate in the interstitium (Figure 4b) [2].
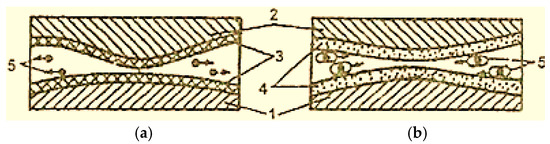
Figure 4.
Scheme of the movement of wear particles in the contact area of surfaces with boundary lubrication (a) and with selective transfer (b): 1—steel; 2—bronze; 3—oxide film; 4—servowitte film; 5—wear particles [2].
In addition, during selective transfer, wear particles can transfer from one friction surface to the other and become trapped between them, without causing defects in the respective surfaces.
- (e)
- Formation of the polymerization products of the lubricating material on the servowitte film surface
To increase the bearing capacity of the servowitte film, during the friction process, special substances are introduced into the lubricating material (e.g., a mixture of hetero-metallic acids with many bases and polyamides), which, during the friction process, polymerize and create an additional protective layer on the friction surfaces to prevent their direct contact (Figure 5 [2]). However, under the conditions of boundary friction, such a film is difficult to form, because the oxide film, being inactive, prevents the poly-condensation and polymerization reactions (Figure 5a) [2].
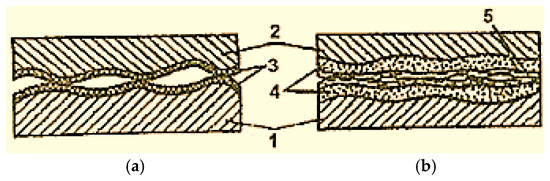
Figure 5.
Scheme of the contact of the surfaces between which there is a lubricant with special additives and with lubrication at the boundary (a) and with selective transfer (b): 1—steel; 2—bronze; 3—oxide film; 4—servowitte film; 5—polymer film [2].
During selective transfer, the servowitte film is a very strong polymerization catalyst because oxide films are absent.
The polymerization film is formed from the organic substances’ free radicals that appear in the tribodestruction process of the lubricating material (Figure 5b [2]), preventing direct contact with the metal surfaces, and, as a result, the contact pressure decreases [26,27,28,29]. The source of free radicals comes from the lubricant’s thermodynamic instability and the material due to the catalytic effect of oxide layers and the material on the lubricant. As a result of oxidation, a series of products derived from glycerin (aldehyde, glycerin acid, acrolein, formic acid aldehyde, etc.) is formed in the contact area of bronze and steel by friction, which are also sources of free radicals. This has been demonstrated by mass spectroscopy and chemical analysis [16,30]. However, the main source is related to the stability of selective transfer, which is mainly influenced by acrylic acid aldehyde called acrolein. Acrolein is formed at the very beginning of friction due to oxidation, variations in contact conditions, and other chemical transformations, which change the operating properties of glycerin and, at the same time, influence selective transfer.
3. Results and Discussion
3.1. Reduction of the Real Contact Area Pressure and the Physiochemical Implications
One of the possibilities for reducing wear is decreasing the real pressure in the contact area of the friction pairs under selective transfer conditions. It is known that the real contact surface is about 10–100 times smaller than the nominal friction surface [3,31,32]. It changes very little during the working process if it is properly run because it is related to the self-adjustment mechanism and obtaining optimal roughness during operation [31,33]. Along with the conditions that lubricants must meet in real contact stress, the materials of the friction pairs and many other factors have an influence. In addition, during the operation of a friction pair, a large part of the friction surface is not used, which means that there is reserve capacity.
In the contact areas, high real pressures arise, which act on the surface even at low nominal loads, which can lead to elastic and plastic deformations of certain areas. For this reason, to decrease the value of the real contact pressure, the nominal contact surface must be increased in several cases [3,31,32,33], for example, in the case of a bearing. This is in contradiction with the requirements and efforts of designers to reduce the mass of a construction while simultaneously increasing its resistance.
The friction process under the conditions of a transfer takes place through the activation of electrochemical processes, with the dissolution of anodic alloying elements at high tensions in the areas of the contact surfaces. By dissolving the anodic components of the metal, surfactant substances are formed, which are adsorbed onto the areas that play the role of cathodes. As a result, the resistance is reduced, and the formation of colloidal particles is favored because surfactant substances and colloids are very good lubricants.
It is known that under the influence of surfactant substances during friction, selective anodic dissolution of some components contained in antifriction copper alloys occurs [34]. Atoms of such components enter the lubricant, and the concentration of porous microinclusions increases in the surface layer of antifriction copper alloys.
Colloidal particles are the smallest particles into which matter can be decomposed without losing its specific properties, and the next level of decomposition would be the atom itself. These particles are dispersed in the selective solution (mixture of glycerin with ions/nanoparticles and other submicroscopic particles formed by chemical transformations (also presented above)) and are electrically charged. Since electrical charges of the same type repel each other, they are maintained in a state of suspension. By breaking down into submicroscopic particles, the total interaction surface increases by an enormous factor, implicitly increasing the effects produced. Additionally, the possibility of penetrating the surface even in the most isolated places is also greatly increased.
It is expected that when the real contact surface increases and when the stresses from the plastic deformation range pass to lower values, the process of increasing the surface will slow down. However, the joint influence of the selective solution of the structural components and the reduction of resistance by adsorption, together with a residual portion from the solution of the cathode alloying components, leads to the formation of a dense layer of these components. From the point of view of density, the layer is similar to a liquid, as demonstrated by Kuksenova and Savenko in ref. [8] and confirmed by Ilie in ref. [35]. The fact that this layer is in a special structural state also determines its lubrication capacity. This makes it possible for the friction to take place under conditions of a much higher pressure than with the friction of mixed or adherent layers.
Increasing the real contact surface and the corresponding reduction of the real contact stress can reduce friction and wear and increase the load-carrying capacity. This is necessary for the protection of the layer against breakage or a change in its deformation during the friction process.
Therefore, research has shown that reducing the actual contact pressure increases the safety of friction pairs, as well as their bearing capacity.
3.2. Reduction of Shear Resistance and Deformation of Superficial Layers
The reduction of the real contact pressure is the result of the formation of the servowitte film through the selective dissolution of a thin superficial layer on the friction surfaces during the friction process. This film (layer) behaves similarly to malleable materials during deformation processes, causing an agglomeration of dislocations and, thus, protecting it against destruction. In the presence of organic compounds and surfactant substances, this layer allows obtaining a coefficient of friction (COF) comparable to that of fluid friction.
From the experimental research presented in [4,25], it was found that this layer has a high density of point defects (vacancies) of 1021 atoms/cm3, which exceeds the number of vacancies (1018–1019 atoms/cm3) that are observed under normal heating or deformation conditions.
The alloying components’ selective dissolution in a copper alloy induces a surplus of defects in the crystalline network of this solid solution as well as in the chemical compounds. Apart from this, defects also appear in the deformation of the superficial areas and, at the same time, at the exit of dislocations on the surface. The layer thickness is ca. 1–3 μm (on average), and it is extremely porous, with its dimensions comparable to the stress fields of dislocations.
The surfactant substances that are in the pores of this layer reduce the resistance of the pore walls, and the high mobility of dislocations in the layer is caused by the following factors: the high density of defects, the Rehbinder effect, and the reduced thickness of the pore walls.
Increasing the real contact area to approximately the nominal contact area size and reducing the coefficient of friction led to the assumption that friction does not unfold between the solid areas of the surface but rather between the boundary areas, with very low interaction within these areas. Researching this state of the layers is difficult because it exists only during the friction process under conditions of very high pressure, at a well-established temperature, and through the development of special tribotechnical processes. When the friction process ends, this layer ceases to exist.
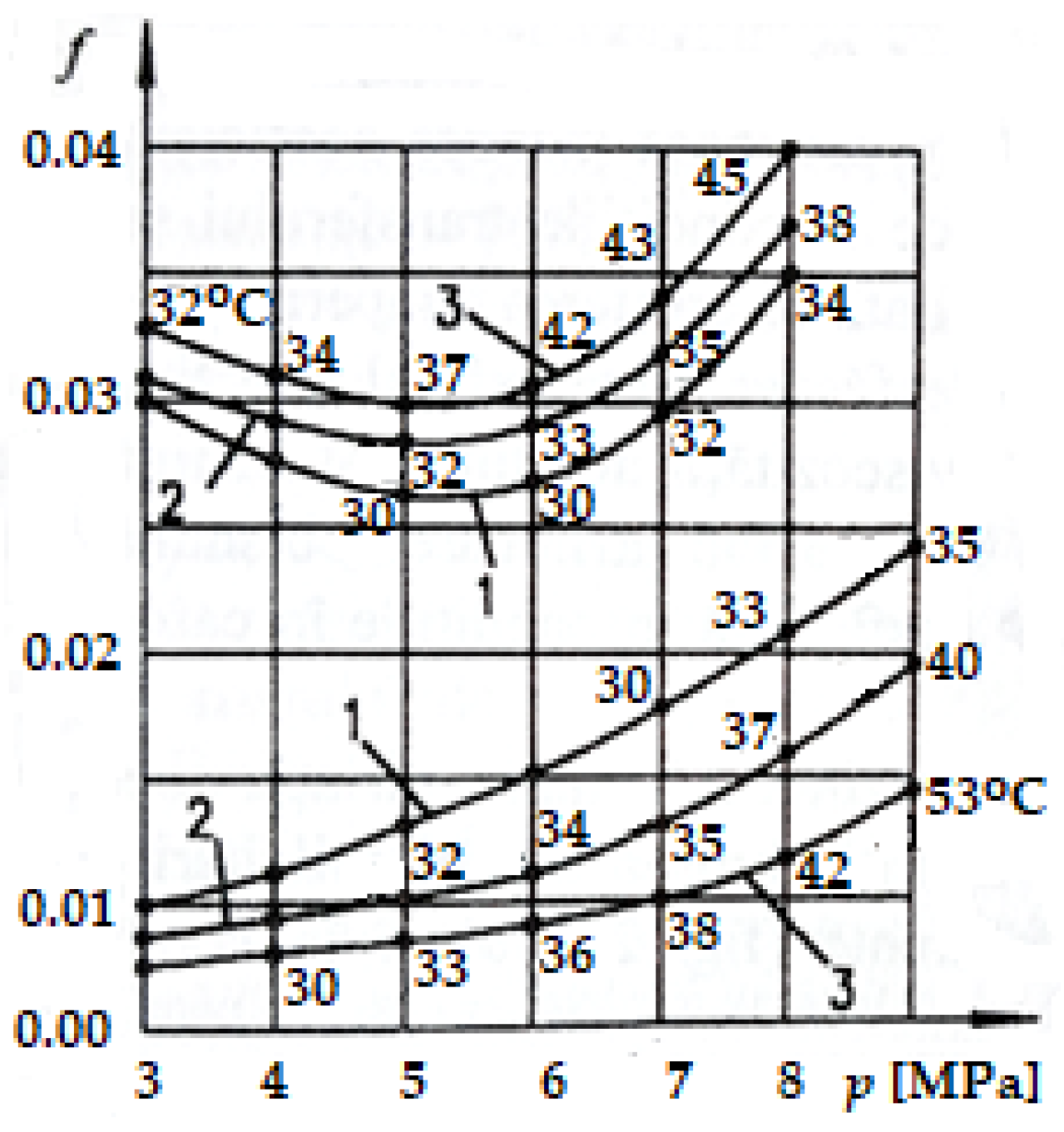
Figure 6 shows the variation of COFs with pressure (p) at different sliding speeds under the friction conditions between the adherent layers (upper curves) and selective transfer (lower curves) [1,2]. The average temperature values are shown on the curves, which resulted in the COF values corresponding to the given pressures (see Figure 6) and determined experimentally on copper-based alloy specimens (CuSn12T, equivalent to UNS-C90800) in contact with steel specimens (OLC 45, equivalent to AISI-SAE 1045) [2,36] of the roller–shoe type, tested on the Amsler installation.
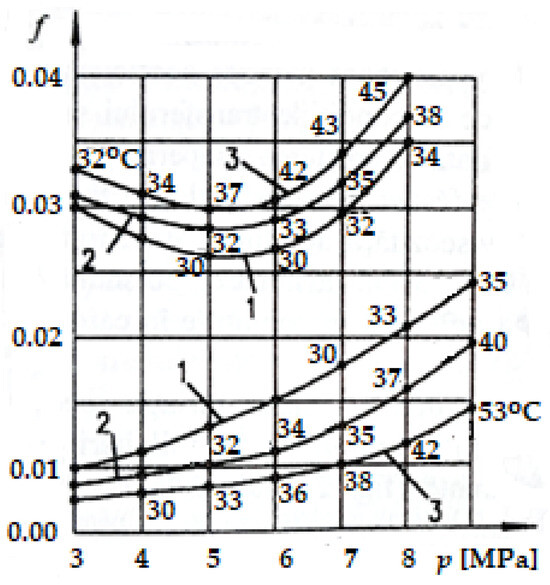
Figure 6.
Friction coefficient variation (f) depending on the contact pressure (p) for three sliding speeds on the Amsler installation: curve 1 at 0.6 m/s; curve 2 at 1 m/s; curve 3 at 2 m/s [2,37].
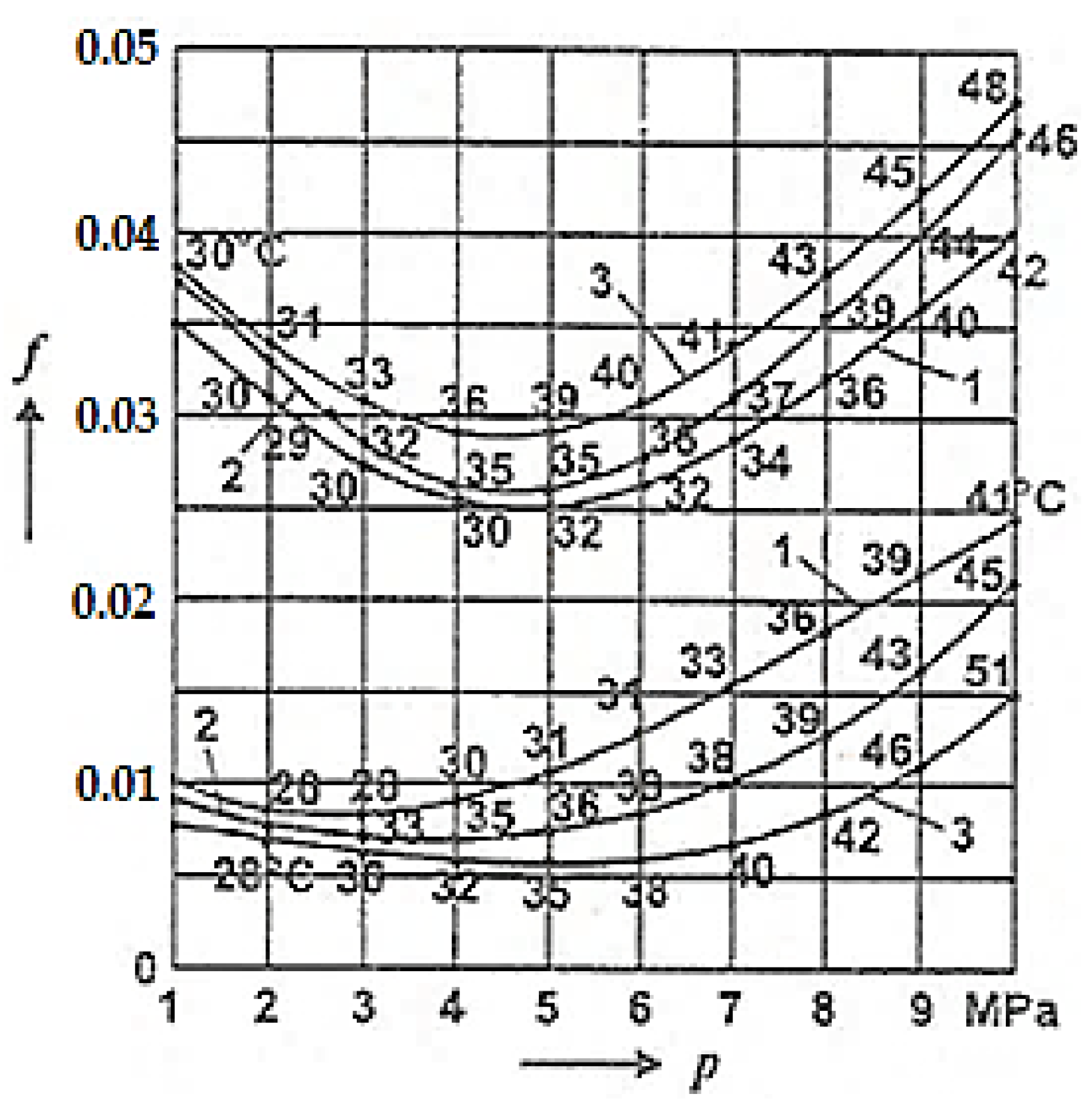
For comparison, Figure 7 presents the COF variation with the average pressure (p) under conditions of selective transfer between the adherent layers (upper curves) and selective transfer (lower curves) [2] at different sliding speeds in the shaft–bearing installation (shaft made of OLC 45 (AISI/SAE 1045), bronze bearing made of CuSn12T (UNS-C90800)) lubricated with glycerin, with the friction coefficient obtained experimentally [2].
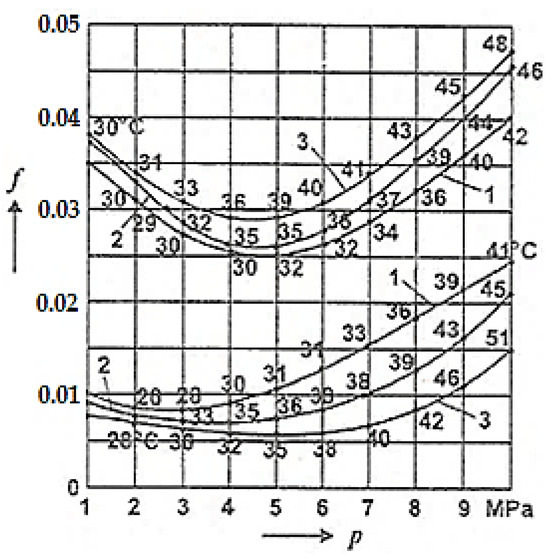
Figure 7.
Friction coefficient variation (f) depending on the contact pressure (p) for three sliding speeds on the shaft–bearing installation: curve 1 at 0.65 m/s; curve 2 at 1.18 m/s; curve 3 at 2.28 m/s [2].
It can be observed that the values of these coefficients are similar, having approximately the same shape, with slightly higher values at the same pressures than in the case presented in Figure 6. At the same time, it is noted (in both situations) that the temperature increase due to the friction of the adherent layers caused an increase in the friction coefficient with the increase in speed, while in the conditions of selective transfer, there was a reduction of the friction coefficient with the increase in temperature. The values of the COFs and temperature in Figure 6 and Figure 7 were determined according to the methodology presented above in Section 2.1.
It is important to note that to differentiate between the adherent layers and selective transfer, COF measurements and temperature recordings were initiated at the start of the Amsler tribometer and the shaft–bearing installation, within a time interval from 0 to 5 min (considered the minimum running-in time), depending on the contact pressure and at different sliding speeds (see Figure 6 and Figure 7). During this time, selective transfer was also triggered, and friction occurred between the adherent layers. After 5 min, the selective layer (servowitte) was created, and friction occurred under the conditions of selective transfer. A similarly procedure was followed for determining the COF and the measuring temperature.
The reactions that take place during selective transfer lead to an improvement in lubrication and a reduction in the viscosity of this layer due to chemisorption processes. This is not possible under typical friction between the adherent layers. Similar reactions unfold under conditions where high stresses suddenly appear during selective transfer.
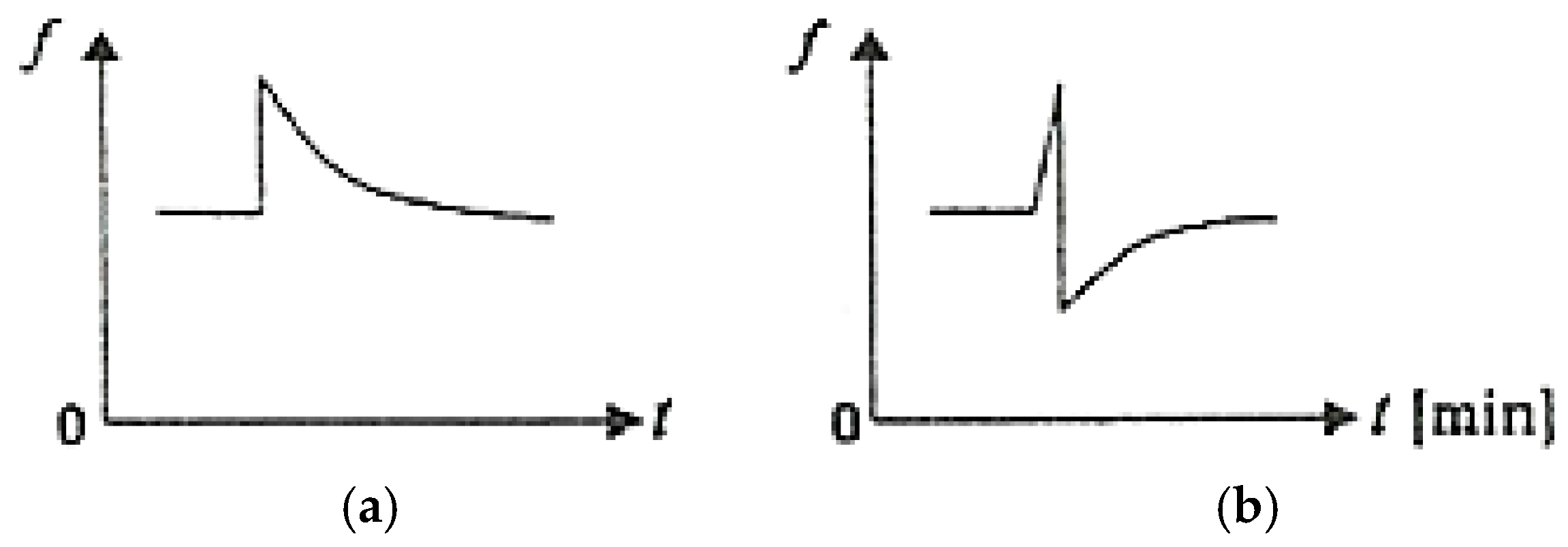
Figure 8 schematically illustrates the variation of COFs as a function of time (t) upon the sudden appearance of an additional stress under conditions of friction between the adherent layers (Figure 8a) and under conditions of selective transfer (Figure 8b) [1,2,37].
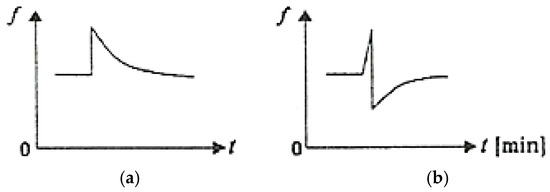
Figure 8.
COF variation as a function of time (t) when an additional load is suddenly applied: (a) under conditions of friction between the adherent layers; (b) under conditions of selective transfer [2].
After removing the additional load, the COF value gradually returns to the initial value under the friction conditions between the adherent layers, while under selective transfer conditions, the COF decreases greatly, then gradually returns to the initial value.
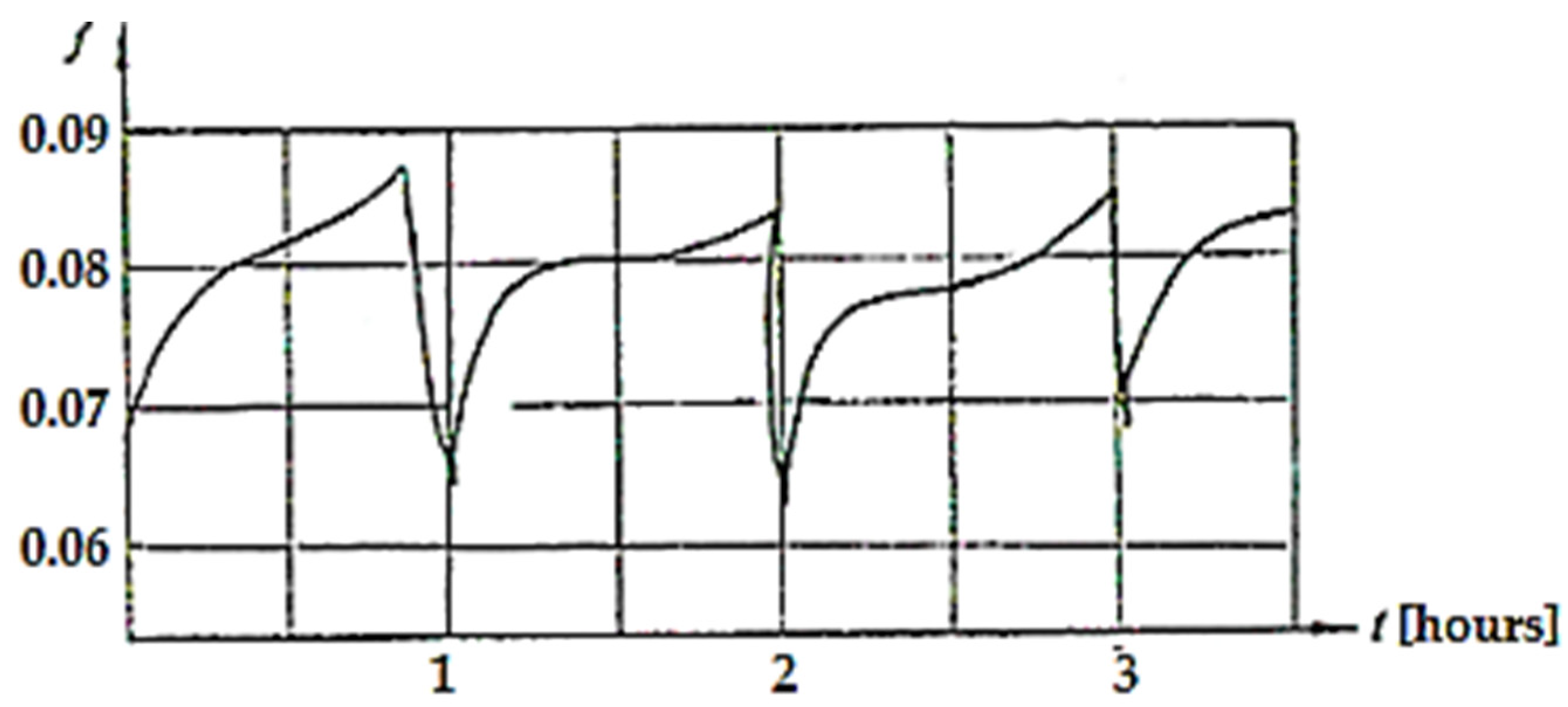
The way the COF varies over time for a pressure of 4.5 MPa and a speed of 0.2 m/s is shown in Figure 9 [2].
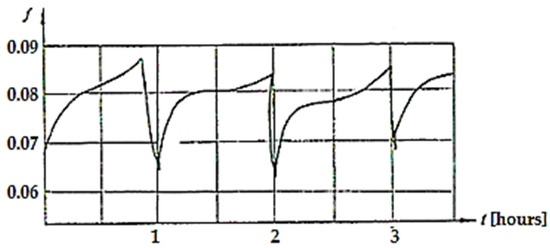
Figure 9.
COF variation over time (t) when the load is 4.5 MPa and the sliding speed is 0.2 m/s [2].
It was observed that the COF varied around the value of 0.08 after an almost repeatable cycle, with the duration of the cycle being approximately 1 h. The typical relaxation pattern of the oscillations is due to the unloading of dislocations clusters and the accumulation of vacancies. This phenomenon was described by Poliakov [38] who presented the dislocation–vacancy mechanism in selective transfer, while Ilie and Ipate [39] showed that during the formation of a selective layer, its crystalline network presents an excess of defects (vacancies), which cause dislocations to emerge on the surface.
The surface reactions are the same as those occurring when the temperature increases, namely the transition from adsorption to chemisorption, with additional dissolution and formation of surface-active lubricants.
The servowitte layer depends (in terms of the protective effect against abrasive, corrosive wear, as well as in terms of the effect of reducing the friction force) on the characteristics of the copper alloy, such as the properties and qualities of the alloying elements, the limit of solubility, and the properties of surfactant substances that arise during friction.
If friction takes place only in a single medium composed of dissolved mineral salts and metal particles, then the layer that is formed provides protection against wear, but the protective effect is considerably reduced, and the COF value is, therefore, higher than with the servowitte layer which contains surface-active lubricants, called “dividal” (a layer formed by the combination of surface-active substances from the base lubricant and metal ions arising through electrochemical processes—ionic lubrication—in the friction zone) [1,2].
In the absence of organic hydrocarbon molecules in the lubricant, which facilitate selective transfer, there are no longer processes compensating for the deformation of the superficial layer or polarization. In addition, the absence of obstacles in the superficial layer prevents the penetration of oxygen due to the surfactant substances, thus preventing oxidation on the surface where this layer is not in a state of contact. Wear is continuously reduced, as the friction surfaces are separated by this layer in the friction process.
3.3. Electrical Phenomena Under Conditions of Selective Transfer
Under conditions of selective transfer, electrical phenomena also occur in the contact area, leading to the formation of a double layer with electrical charges. Additionally, tribochemical processes and metal dispersion during the friction process form inorganic layers, complex and metalloorganic compounds, colloids, and simple electrically charged particles, which are, in fact, the cause of electrokinetic and electrochemical phenomena [40,41,42,43,44].
The considerable potentials that develop during frictional stress in the dispersion medium cause an electrophoretic movement and the precipitation of particles in the area of the frictional contacts (electrophoresis is the directed movement of particles in a solution, which arises from the effect of an electric field). The processes of electrophoresis were also confirmed experimentally [7,40] and practically implemented in different forms of selective transfer [40].
Thus, the transfer of copper particles to the steel surface, as well as the gradual reduction of the alloying components in the superficial layer, led to a decrease in the potential, starting from the initial stage of friction to a value approaching zero due to the potential difference between the contact area and the non-contact area.
Therefore, the interaction surface of the bronze transfers copper particles to the interaction surface of the steel, facilitating selective transfer. This means that the bronze surface loses electrons and becomes positively charged, resulting in a surplus of protons in the atomic nuclei, i.e., a positive polarity. Conversely, the interaction surface of the steel gains electrons and becomes negatively charged, resulting in a surplus of electrons in the atomic nuclei, i.e., a negative polarity.
In cases where one of the friction surfaces, or both, of a friction pair are dielectric, the electrically charged double layer can form due to the so-called triboelectrification. For this reason, in the lubrication gap, between the friction surfaces, both larger and smaller forces act due to the electric field, which also causes electrokinetic phenomena. The deposition of colloidal particles or particles of the cathode metal leads to a reduction in frictional force and erosion. Such particles are present in the lubricant and, therefore, also in the field of the electrically charged double layer. An exception occurs when tribodestruction of the lubricant is lower, and friction occurs between the same metals, which is typical in the friction of adherent layers.
Under operating conditions, the process in a friction pair also has a depolarizing effect on the polarized surface layers and thereby facilitates a kind of “cleaning” of the surfaces, which leads to the integration of the servowitte layer into the metal. During this process, they are entrained together with the particles and molecules of the surfactant lubricant, which are then adsorbed on the surface of these particles. These molecules cause a certain porosity of the layer, an additional lubrication effect, and, in particular, an adsorbing effect on the layer, creating the possibility that they prolong the existence of adsorption defects. A typical example of electrokinetic deposition and precipitation of copper particles in the contact zone by friction could be observed in refrigeration units. This is where copper is removed through the freon solution, which has a slightly corrosive effect, from copper pipes and deposits in the friction areas of the compressor.
This example is very interesting because there is no copper in the friction pair’s contact area. Here, the friction pair is steel/steel and, for this reason, the transfer of copper particles and the formation of a servowitte layer from these particles are of particular interest [45].
In practice, pairs of friction surfaces made of different materials with different lubricants are used, and therefore, there are different conditions for selective transfer. Table 1 shows some examples of the use of different materials under appropriate conditions, where electrokinetic phenomena have been observed and the operation occurs with selective transfer [1,40].

Table 1.
Examples of different materials where electrokinetic phenomena occur under selective transfer conditions.
It should be noted that the precipitation of particles in the lubrication gap is not a sufficient condition for the formation of a servowitte layer. The layer deforms without being destroyed because the lubricants contain organic compounds and surfactant substances. Otherwise, a dividing layer must be created, because it also favors the electrokinetic capture of particles.
3.4. Protection Against Metal Oxidation to Frictional Stress
In the friction pairs contact area, due to friction, the temperature increases. This causes the acceleration of metal oxidation reactions. A separate cause of the wear of metal surfaces through the friction of adherent layers and friction without a lubricant is the wear of oxide layers.
In friction pairs that work at high pressures, an increase in wear is observed as a result of the oxide layer destruction and the formation of some local welding bridges. When the temperature of the friction pair elements increases as a result of friction (through external heating or improper heat removal), an increase in the oxide layer thickness is observed, and implicitly, an increase in wear, an inevitable phenomenon in the mixed friction or the friction of adherent layers.
Under conditions of selective transfer, the adsorbed layer, which also contains surface-active substances, assumes the function of protection against oxidation and micro-welding [1,2,3]. Surface-active substances are surfactant substances or, in short, surfactants, which adhere to contact surfaces by transforming glycerin into value-added molecules, such as propylene glycol, acrolein, ethanol, and epichlorohydrin. These molecules, together with copper particles, heterometallic acid mixtures with many bases, polyamides, metal ions, and other simple electrically charged particles from glycerin, polymerize and create an additional protective layer on the friction surfaces during the friction process, preventing their direct contact.
Surfactant substances are formed in the initial stage of friction, during selective dissolution, through the destruction of the anodic components of an alloy [1,3,10]. Because the layer moves onto the cathode surfaces, it blocks them against the deposition of oxygen molecules. At the same time, the resistance decreases as a result of the adsorption effect, which facilitates the dispersion of the metal.
By dispersion, colloidal particles are formed, which, due to the electrically charged double layer, are attracted to the contact area, where they discharge and combine with the metal, forming a layer. In this situation, the areas of friction surfaces exhibit reducing properties. During the tribodestruction and oxidation of the lubricant, a series of substances with reductive action can form [1,12].
Colloidal particles are the smallest particles into which matter can be decomposed without losing its specific properties, and the next level of decomposition would be the atom itself. These particles are dispersed in the selective solution (mixture of glycerin with ions/nanoparticles and other submicroscopic particles formed by chemical transformations (as described above)) and are electrically charged. Since electrical charges of the same type repel each other, they are maintained in a state of suspension. By breaking down into submicroscopic particles, the total interaction surface increases by an enormous factor, implicitly increasing the effects produced. Additionally, the possibility of penetrating the surface even in the most isolated places is also greatly increased.
If the lubricant lacks organic compounds, a dividing layer that forms in the friction process remains under reducing conditions and does not oxidize, whereas in the absence of friction, oxidation proceeds normally.
All these statements are justified by experimental studies. In general, during oxidation, pairs of materials (bronze/steel) in contact and under relative motion are subjected to sliding stresses. On the one hand, oxide formation causes bronze–steel interaction and sometimes slight wear, and on the other hand, oxidation due to frictional heat at room temperature leads to unidirectional sliding.
Lim [49] showed that is necessary to have a sliding speed of at least 1 m/s for oxidation to become important because it provides sufficient frictional heat, up to a flash temperature approaching 700 °C. At lower speeds, the decrease in the wear rate is associated with a modification of the oxide debris, which is mainly metallic, but may change to oxide debris, as shown/demonstrated by Mishra [50]. At speeds greater than ca. 10 m/s, as proposed by Lim [49], wear is caused by oxide removal in the form of wear debris [50]. Oxides form on the asperities of materials both due to oxidation and through contact with other asperities, and the oxidation extent depends on the temperature developed at the contact points. Oxidation of metal scrap can also be viewed as a source of oxides. The oxidation process deepens during sliding due to the heat of deformation and the particles’ increased energy mainly due to the increase in surface energy. Oxides developed during sliding can only be partially removed and can thus further protect an alloy or metal against wear, and the formed oxide debris can also act as a protective layer.
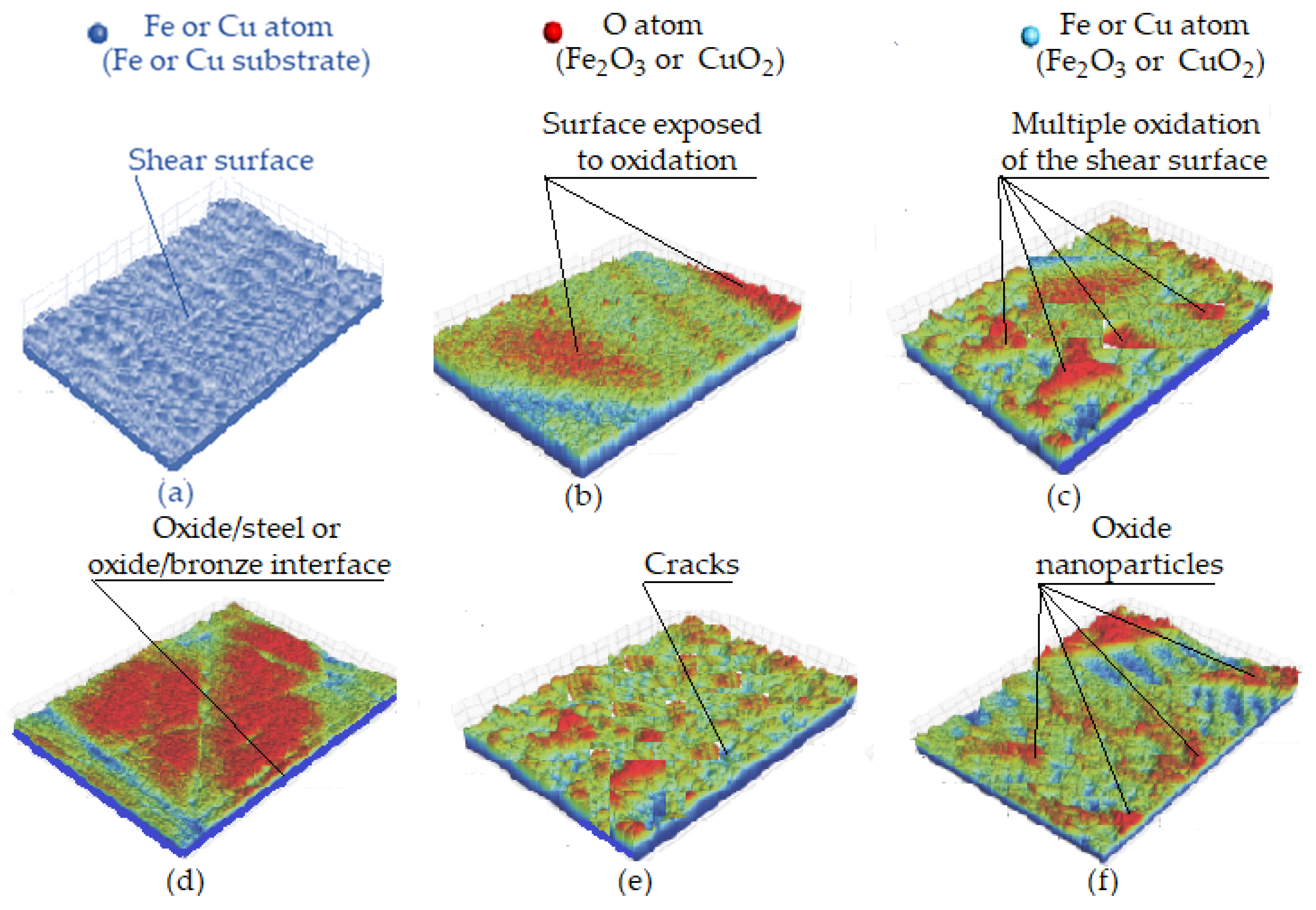
Figure 10 shows the oxide nanoparticle formation mechanism. Initially, several nanoparticles with high-density dislocations are formed within the selective layer that presents very small and dense channels for oxygen adsorption. During the friction process caused by shear stress, the steps with a geometric shape further accelerate oxygen adsorption because they are exposed on the surface, as seen in Figure 10a. Oxygen in the environment/air penetrates the high-density dislocation structure (the surfaces and steps of shear) continuously, which, after frictional shear surface exposure, begins to form oxides (see Figure 10b). Figure 10c shows how oxygen in the oxides continues to form oxides with copper or iron atoms exposed on the shear surfaces and steps. Once the oxide layer reaches its maximum thickness, it is constantly broken down and refined. Oxygen further diffuses into nanoparticles, leading to the oxide/metal interface formation and the development of oxide nanoparticles, which cover the wear surface (see Figure 10d) and play a protective role for the metallic structures. Next, cracks form, leading to oxide fragmentation and refinement (see Figure 10e), and finally, oxide nanoparticles form (Figure 10f) [51].
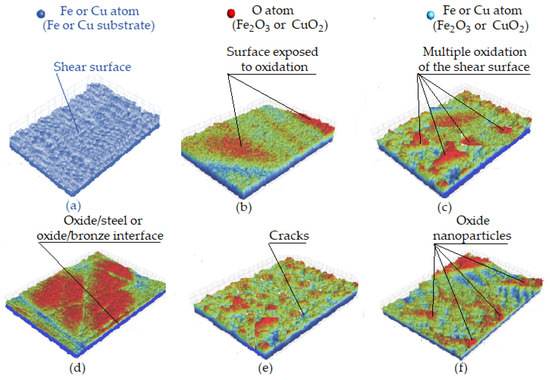
Figure 10.
Oxide nanoparticle formation mechanism: (a) exposure of the shear surface; (b) exposure of the oxidized shear surface; (c) oxidation of multiple shear surfaces; (d) oxide/metal interface formation when oxygen diffuses into nanoparticles; (e) oxide fragmentation and refinement; (f) formation of oxide nanoparticles [51].
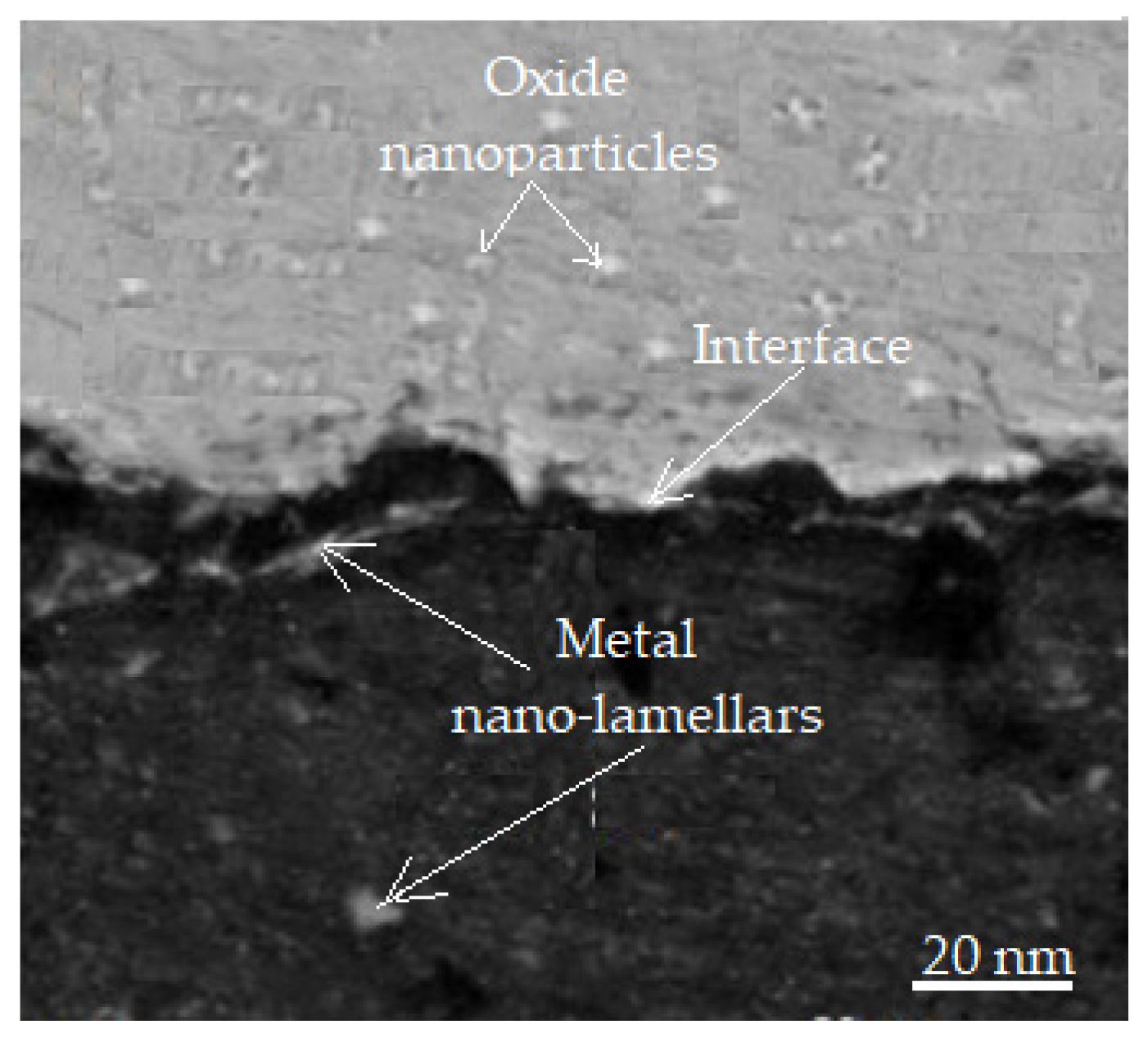
The thickness of the oxide layer is less than 2 μm when it is generated during the sliding of bronze or steel, i.e., it is within the range of the critical oxide thickness of metals [51]. When the oxide layer thickness exceeds the thickness that is considered to be critical, the layer becomes mechanically unstable, and the surface is continuously worn away in the friction process, which is favorable for its integration into wear. The oxide layer is brittle, and due to its brittleness, the brittleness of the interfaces in metallic nanoparticles can, during sliding, further promote breaking and finally form oxide nanoparticles, which accumulate on the wear surface to form a protective layer. The bright field image in Figure 11 shows that the metallic nanoparticles (nano-lamellae) and oxide nanoparticle regions have an obvious interface.
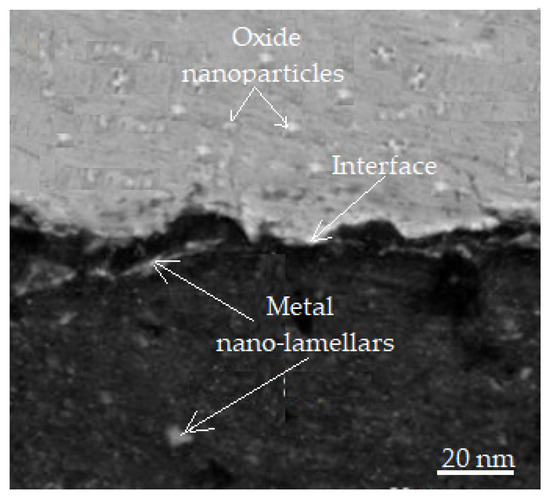
Figure 11.
Differences between metal nanoparticles and oxide nanoparticles [51].
The fully oxidized metal nanoparticles are no longer visible. The formation of an oxide nanoparticle compact protective layer is still necessary, and the decomposition of the oxidized metallic nanoparticles is consistent with the views promoted in the literature [52].
3.5. Formation of a Polymer Protective Layer
It is known that when lubricating with mineral oils, the mixed or adherent layer does not provide sufficient protection against wear; their properties are improved by adding anti-wear, anti-oxidation, and other special additives. This is economical in terms of lubricant consumption and increases the durability of the friction pairs. Under these conditions, certain analogies can be drawn with selective transfer. Such a layer has resistance to deformation in the face of the opposing pair, as usually occurs with a layer of liquid on ordinary friction surfaces.
These processes take place at a high pressure on the copper layer, where adsorption occurs, as well as a catalytic effect of the metal when the oxide layer is worn. It is assumed that, following the heating of the contact areas under higher stresses, welding of the forming layer with the basic metal material (polymer layer) takes place. As the polymer layer wears away, a new layer is formed due to the intensity of the frictional force and the increase in temperature.
In the specialized literature [53,54], it is indicated that additions of special additives soluble in lubricants, such as mixtures of metallic compounds of polybasic acids and polyamines, help form the polymer layer.
If we compare the friction of the adherent layers with the friction during selective transfer, then in the initial stage, these processes are mainly carried out, which create favorable conditions for the formation of a solid bond between the polymerization products and the metal. For this to occur, low pressures on the surfaces, comparable to the resistance of the layer, are needed, as well as free chemical compounds, which are formed in the initial stage of friction when the alloying elements of the alloy are selectively detached. These compounds are important for the interaction between the friction surfaces and the polymers that form on them. Additionally, the absence of oxide layers on the friction surfaces favors the interaction.
The chemical analysis and mass spectroscopy showed [16] that, following the mechanochemical processes on the friction surfaces between brass or bronze and steel lubricated with glycerin, a series of products derived from glycerin (aldehyde, glycerin acid, acrolein, formic acid aldehyde, etc.) is formed as a result of oxidation, but through triboactivation, they can also directly polymerize the hydrocarbons.
The polymers that form help the other processes of friction and wear, creating new areas of sliding surfaces. Apart from this, the polymerized products have a so-called poly-liquid consistency, as was also observed in practice [3,14].
The character of adhesion–deformation in friction is one of the bases for increasing the reliability of friction pairs. In the present case, the forces were taken over by the polymer layer, and we speak of its so-called servowitte properties.
In practice, there are different possibilities for achieving selective transfer in friction pairs. For example, selective transfer can also be achieved in the friction pairs of the steel/steel type, cast iron/Teflon with a copper insert, steel/glass, etc. Among the possibilities, we can mention brass coating and the use of bronze additions [2,3]. The advantage of using such methods and processes to reduce friction and wear is significant.
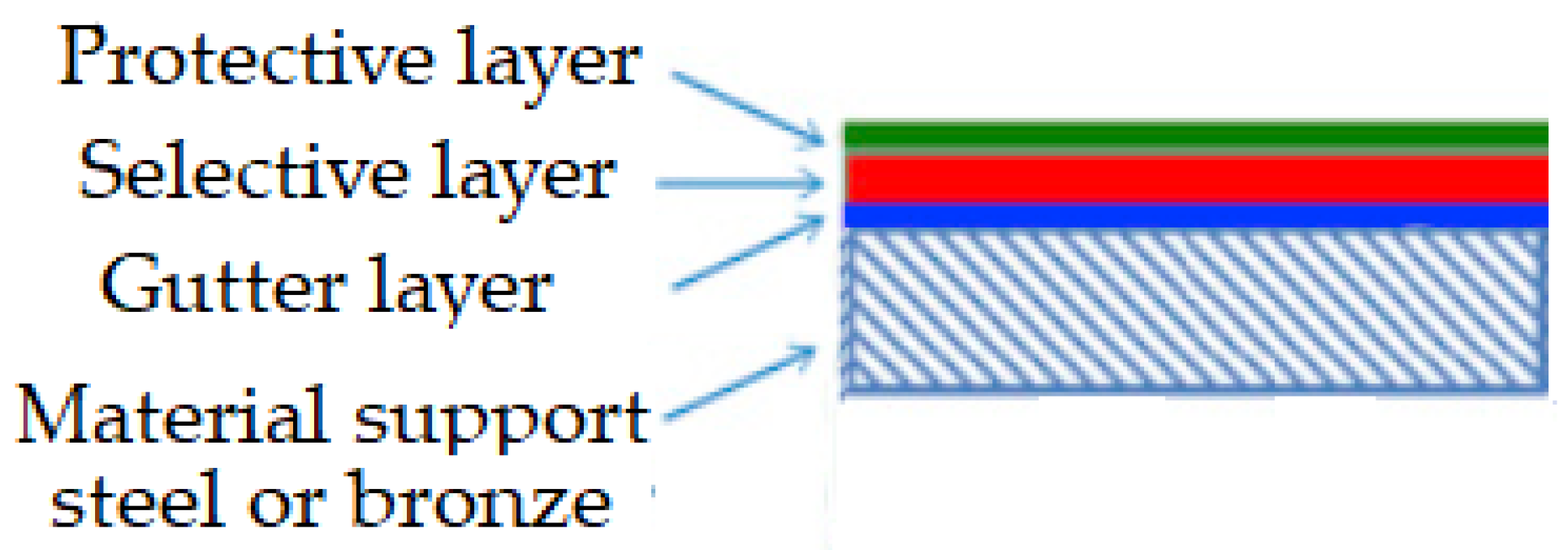
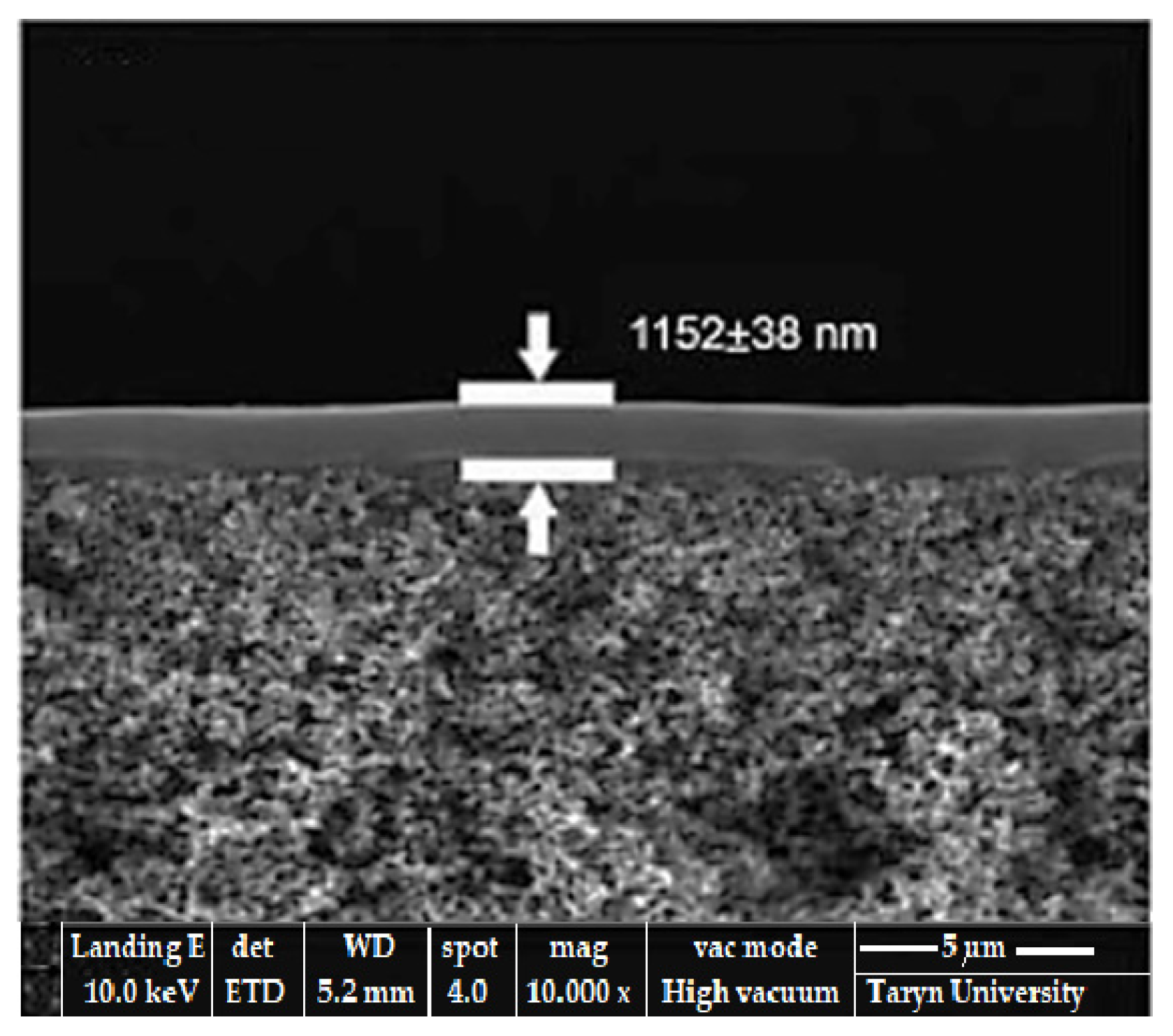
Therefore, in the friction process, the lubricant material (glycerin) polymerizes and creates an additional layer of friction surface protection and prevents direct contact [29,55]. From the free radicals of the organic substances, which appear in the process of tribodestruction of the lubricating material (glycerin, see Figure 5), a polymerization film is formed [26,27,28,29,55]. Figure 12 presents a typical selective-layer composite structure based on SEM images.
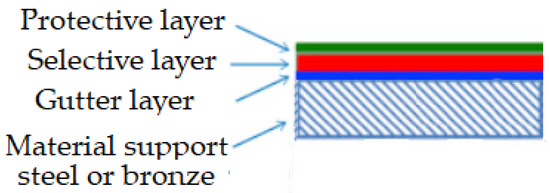
Figure 12.
Typical structure of a selective-layer composite.
The composite structure of a selective layer contains at least a porous support and the selective layer, and in many cases, a gutter layer between the porous support and the selective layer, as well as a protective layer above the selective layer.
It is mentioned that polymerization in the case of the bronze/steel pair considered and lubricated with glycerin is an interfacial polymerization [56,57], the SEM image of which is presented in Figure 13.
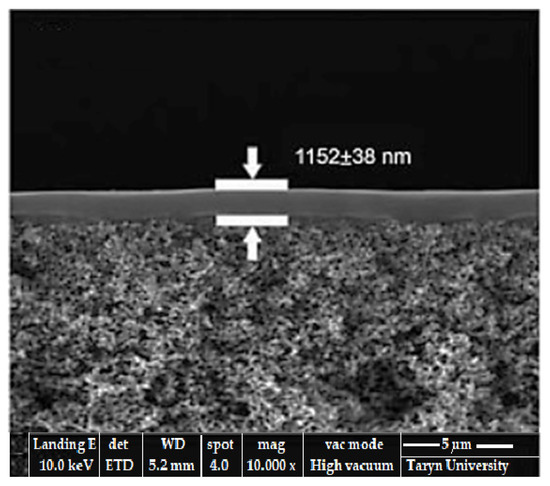
Figure 13.
SEM image of a composite selective layer obtained by interfacial polymerization in the friction process.
4. Conclusions
At the core of selective transfer are complex physiochemical processes that ensure the lubricant’s thermodynamic instability and allow the contact materials to transfer some particles from the component elements. This transfer is carried out in the presence of relative movement presence and under conditions of local energy favoring the process, serving as a useful application that affects the friction and wear processes.
The experimental research proved that the physiochemical processes at the interface of two solid materials and the intermolecular phenomena are complex and take place in the real contact area as long as the relative movement lasts, but also afterward.
Under selective transfer conditions, reducing friction and wear is the effect of the self-regulating phenomena that influence the equilibrium processes, which are disturbed during the friction process. The self-regulating character of the protective layer formed by selective transfer is conditioned by the existence of specific local energetic conditions, relative motion, and the special properties of one or more chemical elements within the materials.
These properties are specific to contact between rough surfaces and are particularly focused on the reduced resistance to sliding in a certain direction and the ability to transfer within a very short time. During sliding, secondary processes also take place, which favor the dynamics of the transfer, in addition to oxidation, as a determining phenomenon of the process, namely:
- -
- reduction of the real pressure in the contact area;
- -
- double electric layer formation on the real surface;
- -
- concentration of superficial dislocations and reduction of tangential stresses;
- -
- depolarization and destruction of oxide layers leading to the acceleration of corrosion processes;
- -
- electron emission in areas without oxides, causing variation in sliding speed direction;
- -
- formation of metalloorganic compounds, colloids, and other active substances, which transport metal particles to the contact area.
Therefore, the complex physiochemical processes that occur in the contact areas of friction pairs lead an increase in their safety and bearing capacity. It can be stated that when operating under selective transfer conditions, the stresses on the components of a machine can be increased considerably without an increase in their mass and dimensions.
Author Contributions
Conceptualization, F.I.; methodology, F.I., C.-D.C., and A.J.; software, C.-D.C.; validation, F.I., C.-D.C., and A.J.; formal analysis, C.-D.C. and A.J.; investigation, F.I., C.-D.C., and A.J.; resources, C.-D.C. and A.J.; data curation, F.I., C.-D.C., and A.J.; writing—original draft preparation, F.I.; writing—review and editing, F.I.; visualization, C.-D.C. and A.J.; supervision, F.I., C.-D.C., and A.J.; project administration, F.I.; funding acquisition, F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garkunov, D.N. Erhöhung der Verschleissfestigkeit auf der Selektiven Übertragung; VEB Technik: Berlin, Germany, 1981. [Google Scholar]
- Ilie, F. Tribological Study of the Thin Superficial Layers Formed in the Friction Couples by Selective Transfer; Technical Publishing House: Bucharest, Romania, 2002. [Google Scholar]
- Garkunov, D.N. Triboengineering. Design, Production and Operation of Machines; Izd. MSKhA: Moskau, Russia, 2002. (In Russian) [Google Scholar]
- Rybakova, L.M.; Kuksenova, L.I. Structure and Wear Resistance of Metal; Mashinostroenie: Miskau, Russia, 1982; 212p. (In Russian) [Google Scholar]
- Polyakov, A.A.; Ruzanov, F.I. Friction Based on Self-Organization; Nauka: Moskau, Russia, 1992; 132p. (In Russian) [Google Scholar]
- Kuzharov, A.S. The concept of wearlessness in modern tribology. Izv. Vuzov. Sev.-Kavk. Region. Tekhn. Nauk. (News High. Educ. Inst. North Cauc. Region. Ser. Eng. Sci.) 2014, 2, 23–31. (In Russian) [Google Scholar]
- Kuksenova, L.I.; Savenko, V.I. Effect of Surface-Active Media on Contact Elastoplastic Deformation of Surface Layers of Metals and Their Tribological Characteristics. Russ. J. Phys. Chem. A 2024, 98, 1411–1424. [Google Scholar] [CrossRef]
- Kuksenova, L.I.; Savenko, V.I. Structural Changes and Diffusion in the Zone of Contact Deformation of Copper Alloys Under Friction. Met. Sci. Heat Treat. 2024, 65, 790–800. [Google Scholar] [CrossRef]
- Kargul, M.; Konieczny, M. Copper matrix composites reinforced with steel particles. AIMS Mater. Sci. 2021, 8, 321–342. [Google Scholar] [CrossRef]
- Bouchoucha, A.; Kadiri, E.; Robert, F.; Zaidi, H.; Paulmier, D. Metals transfer and oxidation of copper—Steel surfaces in electrical sliding contact. Surf. Coatings Technol. 1995, 76–77, 521–527. [Google Scholar] [CrossRef]
- Mousavi, A.; Sperk, T.; Gietzelt, T.; Kunze, T.; Lasagni, A.F.; Brosius, A. Effect of Contact Area on Friction Force in Sheet Metal Forming Operations. Key Eng. Mater. 2018, 767, 77–84. [Google Scholar] [CrossRef]
- Aniołek, K.; Barylski, A.; Kowalewski, P.; Kaptacz, S. Investigation of Dry Sliding Friction, Wear and Mechanical Behavior of the Ti-6Al-7Nb Alloy after Thermal Oxidation. Materials 2022, 15, 3168. [Google Scholar] [CrossRef]
- Popov, V.L.; Li, Q.; Lyashenko, I.A.; Pohrt, R. Adhesion and friction in hard and soft contacts: Theory and experiment. Friction 2021, 9, 1688–1706. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Wang, H.S.; Truong, N.P.; Anastasaki, A. Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update. Chem 2020, 6, 1575–1588. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Ilie, F. Tribological behaviour of the steel/bronze friction pair (journal bearing type) functioning with selective mass transfer. Int. J. Heat Mass Transf. 2018, 124, 655–662. [Google Scholar] [CrossRef]
- Chen, X.; Han, Z.; Li, X.; Lu, K. Lowering coefficient of friction in Cu alloys with stable gradient nanostructures. Sci. Adv. 2016, 2, e1601942. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhou, X.; Li, X.; Lu, K. Friction mechanism in the running-in stage of copper: From plastic deformation to delamination and oxidation. Tribol. Int. 2017, 115, 3–7. [Google Scholar] [CrossRef]
- Nunez, E.E.; Polycarpou, A.A. The effect of surface roughness on the transfer of polymer films under unlubricated testing conditions. Wear 2015, 326–327, 74–83. [Google Scholar] [CrossRef]
- Ilie, F. Diffusion and mass transfer mechanisms during frictional selective transfer. Int. J. Heat Mass Transf. 2018, 116, 1260–1265. [Google Scholar] [CrossRef]
- Kohara, M.; Kawamura, T.; Egami, M. Study on Mechanism of Hydrogen Generation from Lubricants. Tribol. Trans. 2006, 49, 53–60. [Google Scholar] [CrossRef]
- Winkel, L.; Wochele, J.; Ludwig, C.; Alxneit, I.; Sturzenegger, M. Decomposition of copper concentrates at high-temperatures: An efficient method to remove volatile impurities. Miner. Eng. 2008, 21, 731–742. [Google Scholar] [CrossRef]
- Pascher, T.F.; Ončák, M.; van der Linde, C.; Beyer, M.K. Decomposition of Copper Formate Clusters: Insight into Elementary Steps of Calcination and Carbon Dioxide Activation. ChemistryOpen 2019, 8, 1453–1459. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yu, K.; Qian, M. A two-dimensional thin film structure with spectral selective emission capability suitable for high-temperature environments. Case Stud. Therm. Eng. 2024, 63, 105261. [Google Scholar] [CrossRef]
- Zayed, E.M.; Shazly, M.; El-Sabbagh, A.; El-Mahallawy, N.A. Deformation behavior and properties of severe plastic deformation techniques for bulk materials: A review. Heliyon 2023, 9, e16700. [Google Scholar] [CrossRef]
- Chen, Z.; Khajeh, A.; Martini, A.; Kim, S.H. Chemical and physical origins of friction on surfaces with atomic steps. Sci. Adv. 2019, 5, eaaw0513. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E.; Karfidov, E.; Kazakovtseva, N. Anodic selective dissolution of copper alloys in chloride and carbonate melts. J. Alloys Compd. 2020, 845, 156238. [Google Scholar] [CrossRef]
- Radionenko, O.; Kindrachuk, M.; Tisov, O.; Kryzhanovskyi, A. Features of transition modes of friction surfaces with partially regular microrelief. Aviation 2018, 22, 86–92. [Google Scholar] [CrossRef]
- Zhao, P.; Klein, J. Lubricating Polymer Gels/Coatings: Syntheses and Measurement Strategies. Gels 2024, 10, 407. [Google Scholar] [CrossRef]
- Snyder, D.T.; Pulliam, C.J.; Ouyang, Z.; Cooks, R.G. Miniature and Fieldable Mass Spectrometers: Recent Advances. Anal. Chem. 2016, 88, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Tudor, A. Real Contact of Friction Surfaces; Romanian Academy Publishing House: Bucharest, Romania, 1990. (In Romanian) [Google Scholar]
- Kraghelski, I.V. Reibung und Versleiss; VEB Tehnik: Berlin, Germany, 1971. [Google Scholar]
- Tudor, A.; Pavelescu, D.; Ilie, F.; Ranea, C.; Lazar, C.; Tanjala, L. Tribology—Calculation Guide; Bucharest Polytechnic Institute: Bucharest, Romania, 1985. (In Romanian) [Google Scholar]
- Kuzharov, A.S.; Marchak, R. Peculiarities of the evolutionary transition of the brass-glycerin-steel tribologi-cal system to the wear-free friction mode. Dokl. RAN [Rep. Russ. Acad. Sci.] 1997, 354, 642–644. (In Russian) [Google Scholar]
- Ilie, F. Study of Superficial Layers Obtained by Selective Transfer in the Friction Couples. Eur. J. Eng. Technol. Res. 2017, 2, 54–58. [Google Scholar] [CrossRef][Green Version]
- EN 13835: 2002; EN European Steel and Cast Iron Standards. BSI: London, UK, 2002.
- Ilie, F. Tribological aspects of reducing friction and wear under conditions of selective transfer. Constr. Mach. 1990, 42, 294–297. (In Romanian) [Google Scholar]
- Poliakov, A.A. Dislocation-vacancy mechanism of selective transfer. Wear-Free. Eff. Tribotechology 1992, 3–4, 5–10. (In Russian) [Google Scholar]
- Ilie, F.; Ipate, G. A Modelling Study of the Correlation between the Layer Obtained by Selective Transfer and the Dislocations Movement at the Friction Surfaces Limit. Metals 2022, 12, 180. [Google Scholar] [CrossRef]
- Garkunov, D.N.; Simakov, Y.S. Selektive Übertragung bei der Reibung, unter Redaktion von Garkunov D.N., Simakov Yu.S.; Nauka: Moskau, Russia, 1975. [Google Scholar]
- Tison, R.P.; Synder, D.D. A novel, two-phase medium for electrochemically induced conversion coatings. J. Appl. Electrochem. 1990, 20, 457–462. [Google Scholar] [CrossRef]
- Tung, S.C.; Wang, S.S. Friction Reduction From Electrochemically Deposited Films. Tribol. Trans. 2008, 34, 23–34. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H. Recent advances in electrochemical techniques for characterizing surface properties of minerals. Adv. Colloid Interface Sci. 2021, 288, 102346. [Google Scholar] [CrossRef]
- Jiang, Z.; Fang, J.; Chen, F.; Wang, X.; Chen, B.; Gu, K.; Wu, J.; Wang, J. Research Progress on Tribo-Electrophysical and Tribo-Electrochemical Mechanisms. Tribology 2017, 37, 695–706. [Google Scholar] [CrossRef]
- Padgurskas, J.; Snitka, V.; Jankauskas, V.; Andriušis, A. Selective transfer phenomenon in lubricated sliding surfaces with copper and its alloy coatings made by electro-pulse spraying. Wear 2006, 260, 652–661. [Google Scholar] [CrossRef]
- Rezaeian, I.; Zahedi, P.; Rezaeian, A. Rubber Adhesion to Different Substrates and Its Importance in Industrial Applications: A Review. J. Adhes. Sci. Technol. 2012, 26, 721–744. [Google Scholar] [CrossRef]
- Ajayi, O.; Akanni, M.; Lambi, J.; Jeynes, C.; Watts, J. Compositional studies of various metal oxide coatings on glass. Thin Solid Films 1990, 185, 123–136. [Google Scholar] [CrossRef]
- Elnashar, E.A. Bearing Design Science in Technologically of Different Materials for Textiles Industry. Eng. Technol. Open Access J. 2023, 4, 555647. [Google Scholar] [CrossRef]
- Lim, S.C.; Ashby, M.F. Recent Developments in Wear-Mechanism Maps. Tribol. Int. 1998, 31, 87–97. [Google Scholar] [CrossRef]
- Mishra, A. Influence of Oxidation on the Wear of Alloys. Int. J. Mech. Eng. Rob. Res. 2014, 3, 583. [Google Scholar]
- Yin, C.; Li, N.; Wu, Y.; Liang, Y.; Yang, C.; Wu, J. Frictional shear-induced nanolamellar oxidation and transformation to oxide nanoparticles during pearlitic steel sliding. Mater. Des. 2023, 233, 112299. [Google Scholar] [CrossRef]
- Chandross, M.; Curry, J.F.; Babuska, T.F.; Lu, P.; Furnish, T.A.; Kustas, A.B.; Nation, B.L.; Staats, W.L.; Argibay, N. Shear-induced softening of nanocrystalline metal interfaces at cryogenic temperatures. Scr. Mater. 2018, 143, 54–58. [Google Scholar] [CrossRef]
- Stott, F. High-temperature sliding wear of metals. Tribol. Int. 2002, 35, 489–495. [Google Scholar] [CrossRef]
- Ye, J.; Burris, D.L.; Xie, T. A Review of Transfer Films and Their Role in Ultra-Low-Wear Sliding of Polymers. Lubricants 2016, 4, 4. [Google Scholar] [CrossRef]
- Eman, A.; Nabhan, A.; Nouby, M.; Abdel Jaber, G. Influence of adding contaminants particles to lithium grease on the frictional coefficient. J. Egypt. Soc. Tribol. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Liu, G.; Feng, Y.; Zhao, N.; Chen, Z.; Shi, J.; Zhou, F. Polymer-based lubricating materials for functional hydration lubrication. Chem. Eng. J. 2022, 429, 132324. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.; Wang, S. Interfacial Polymerization: From Chemistry to Functional Materials. Angew. Chem. Int. Ed. Engl. 2020, 59, 21840–21856. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).