Effect of Applied Cathodic Potential on Friction and Wear Behavior of CoCrMo Alloy in NaCl Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results
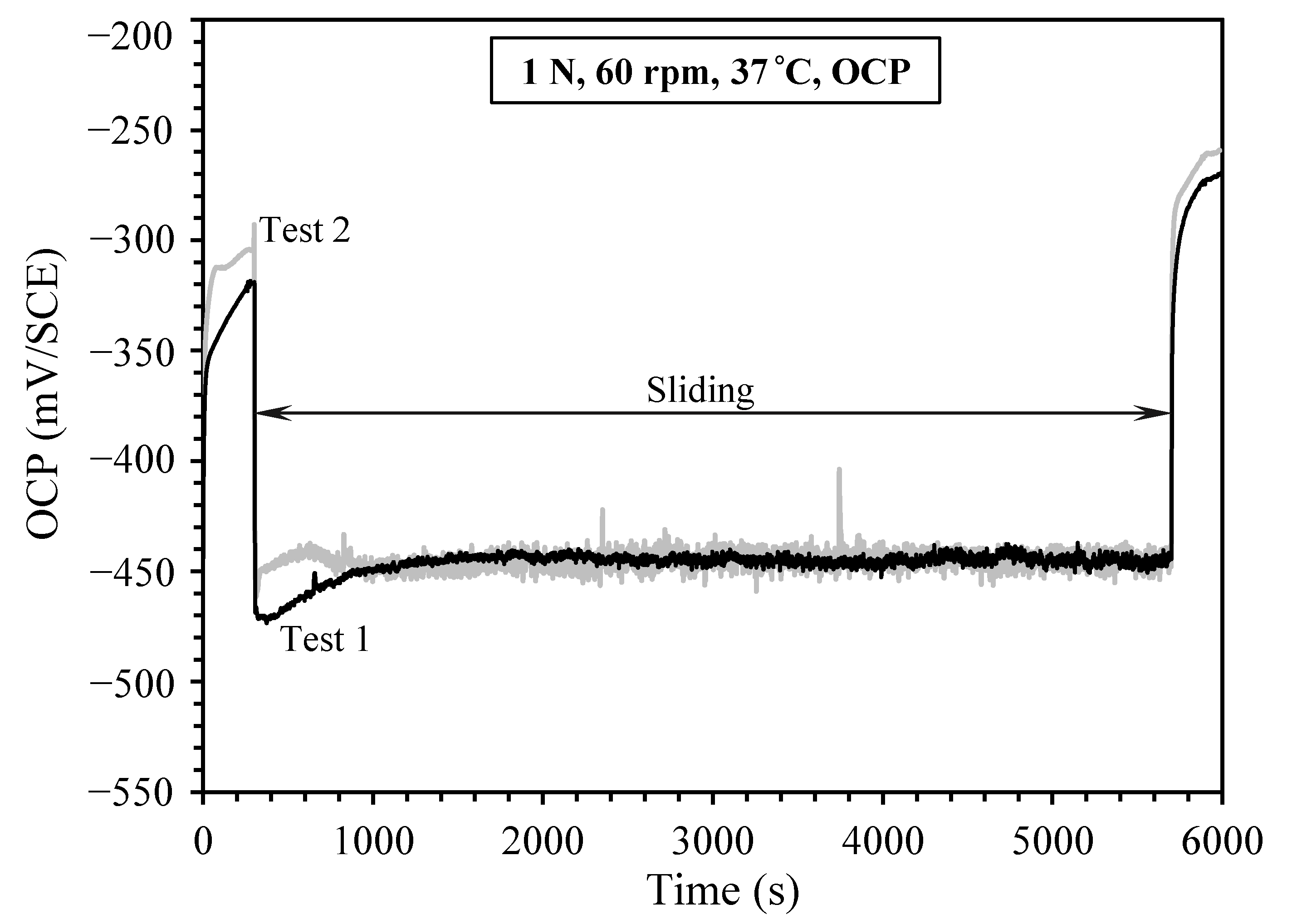
3.1. Sliding at Open Circuit under 1 N Load
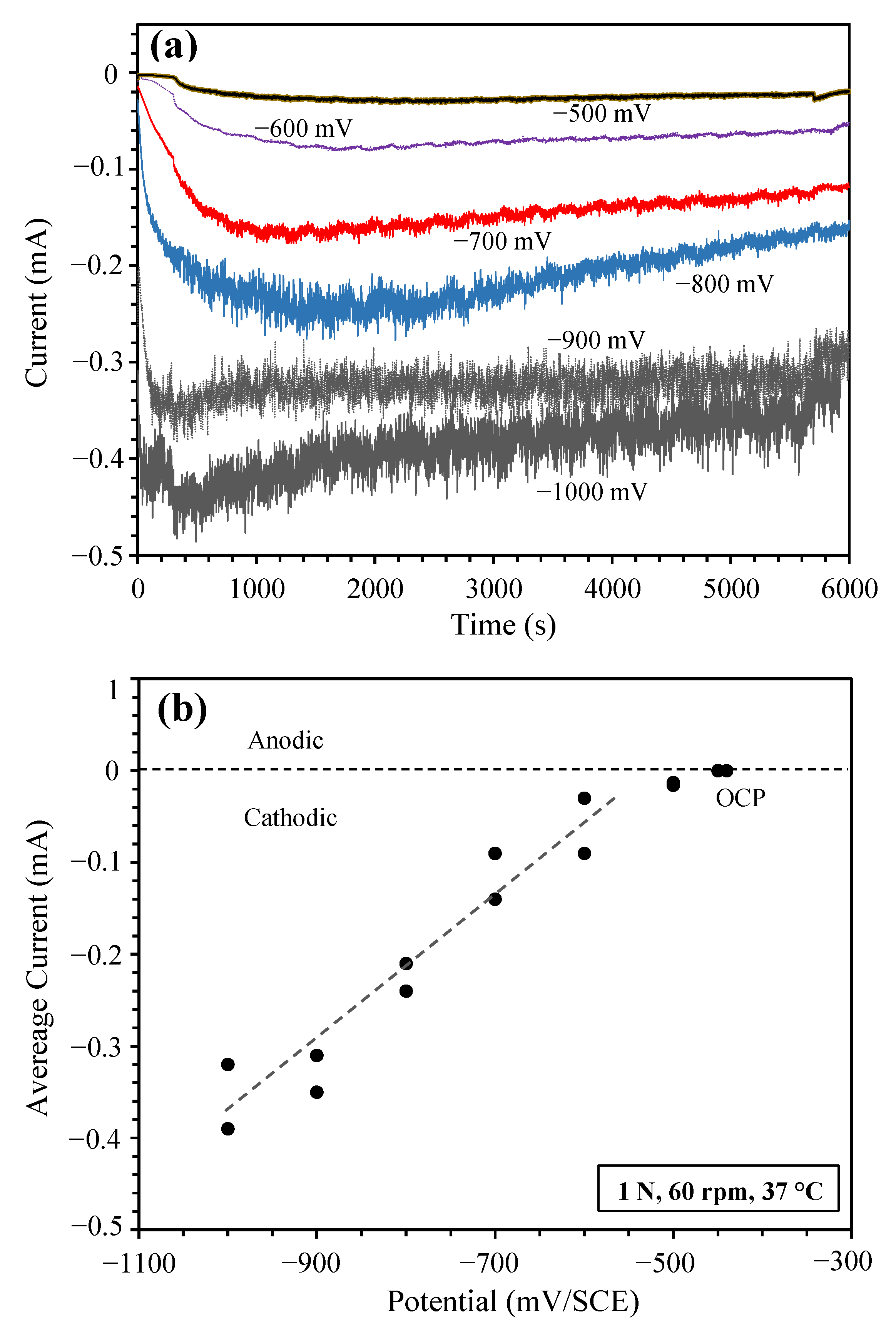
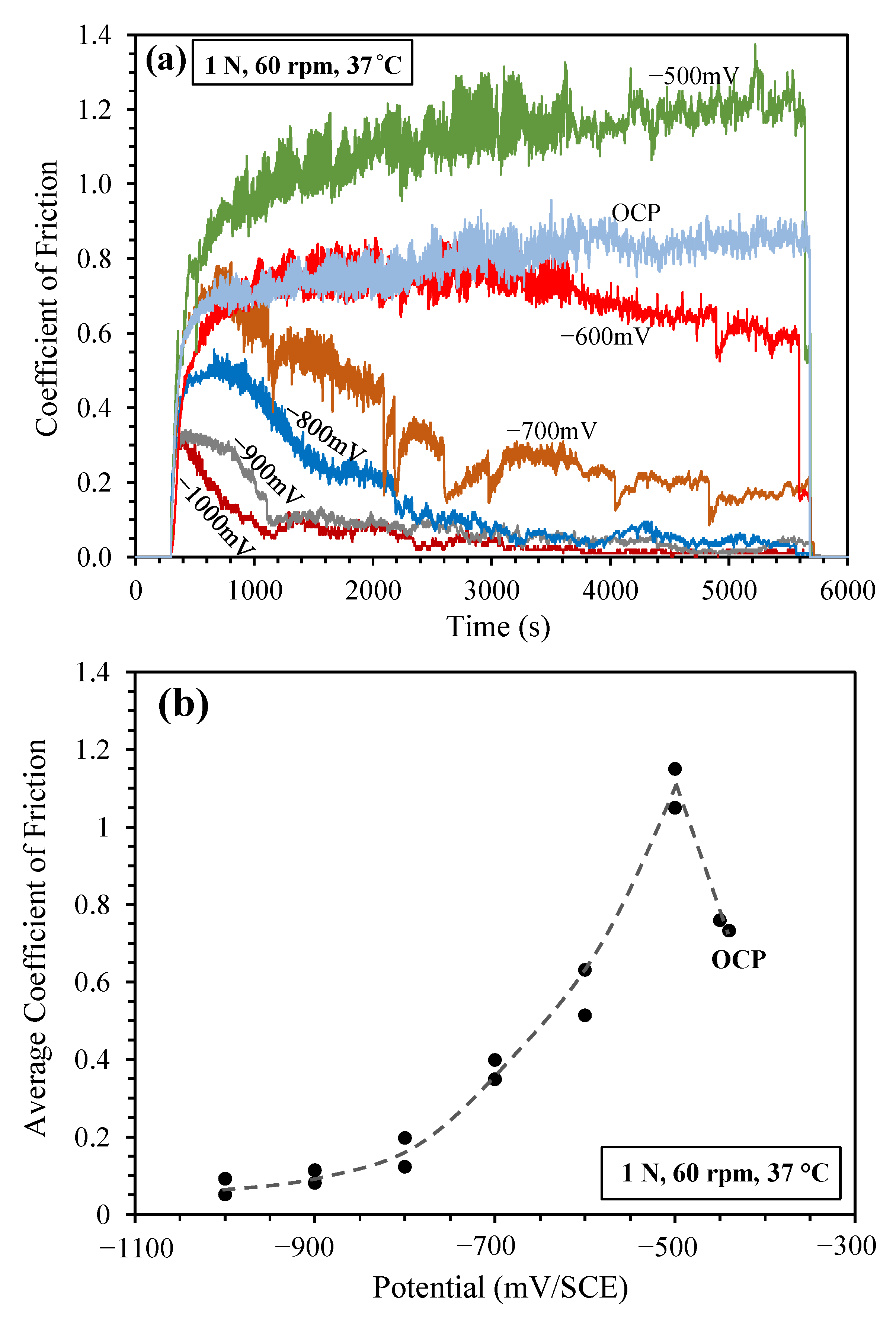
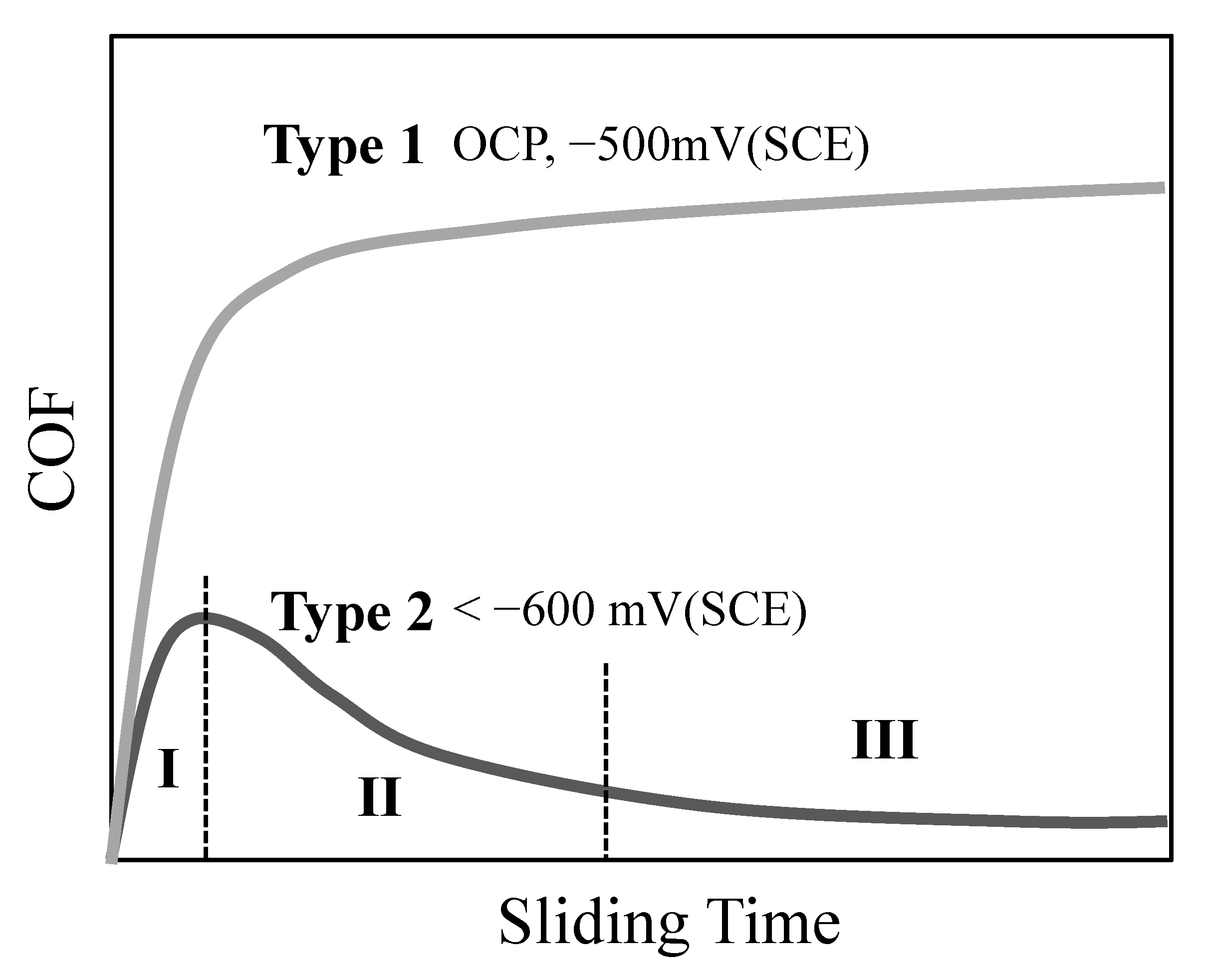
3.2. Sliding at Cathodic Potential under 1 N Load
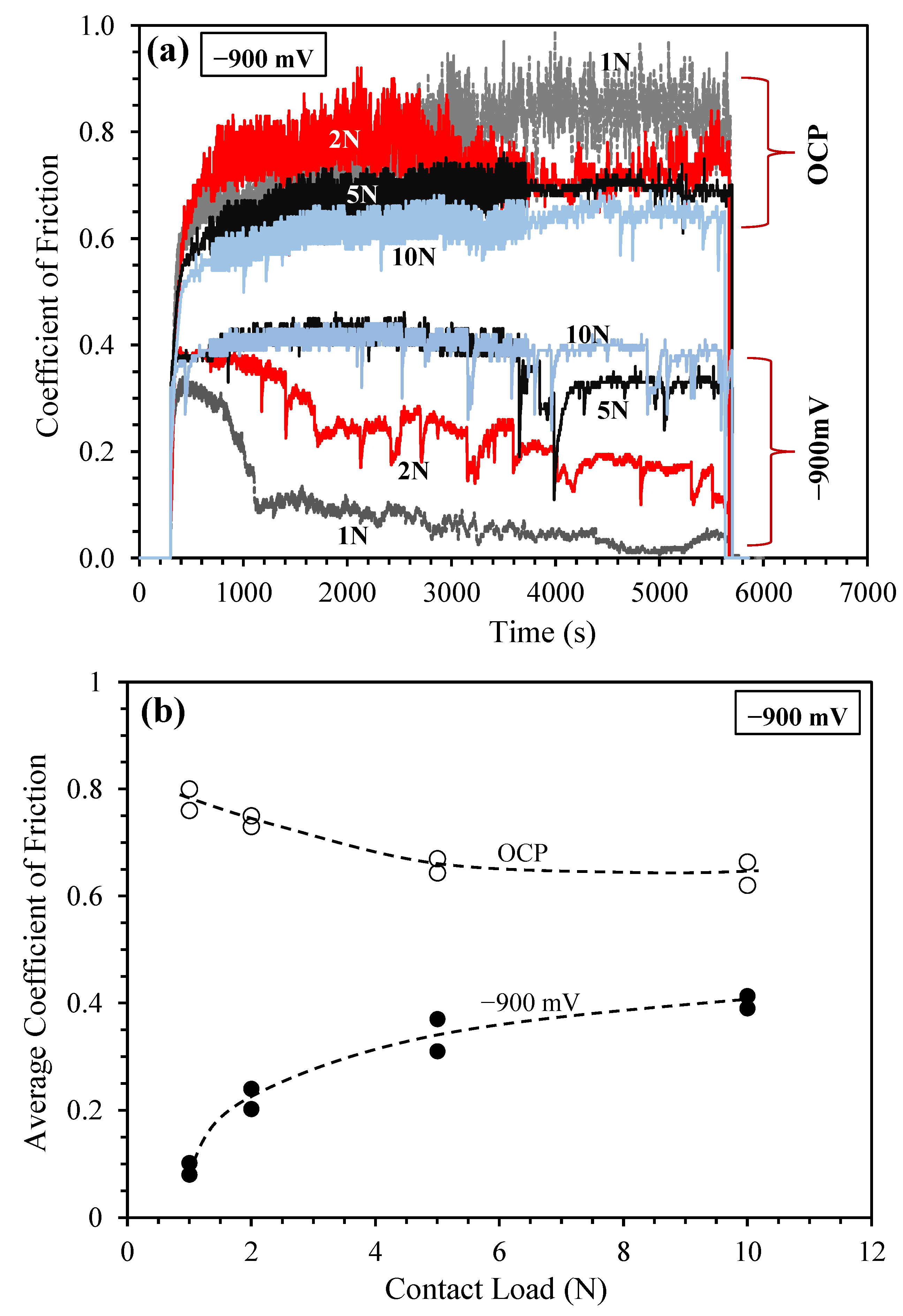
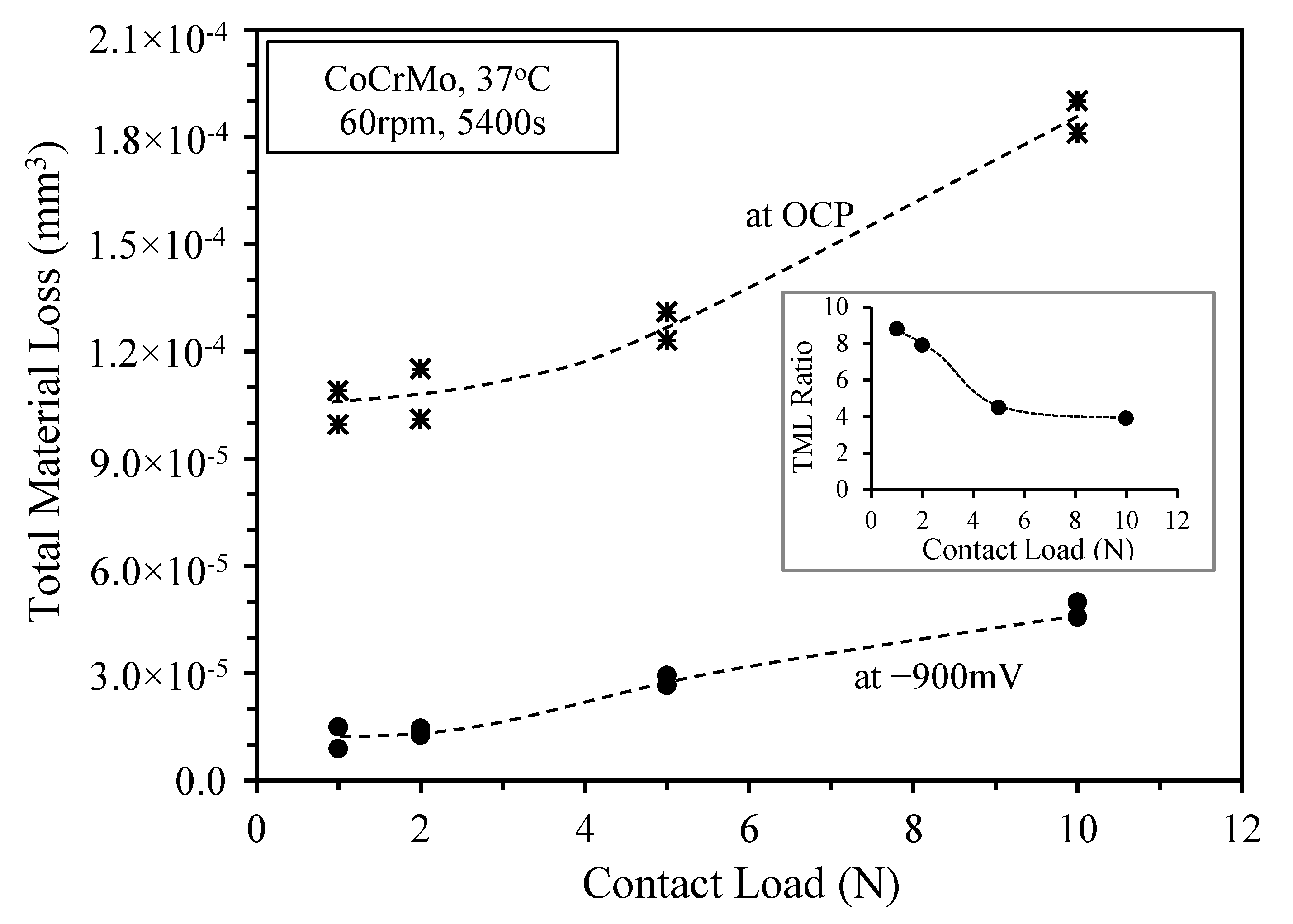
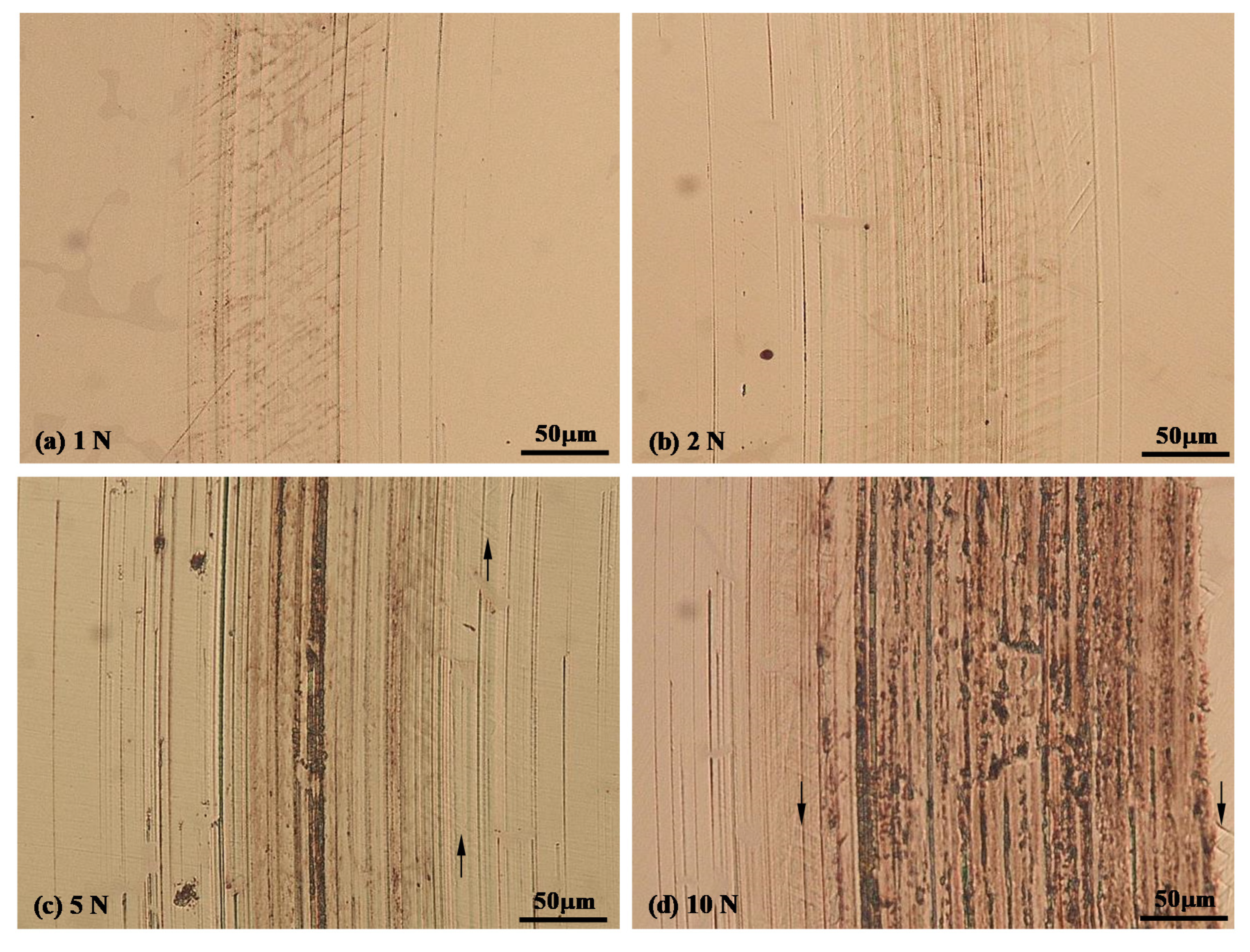
3.3. Sliding at −900 mV(SCE) under Various Contact Loads
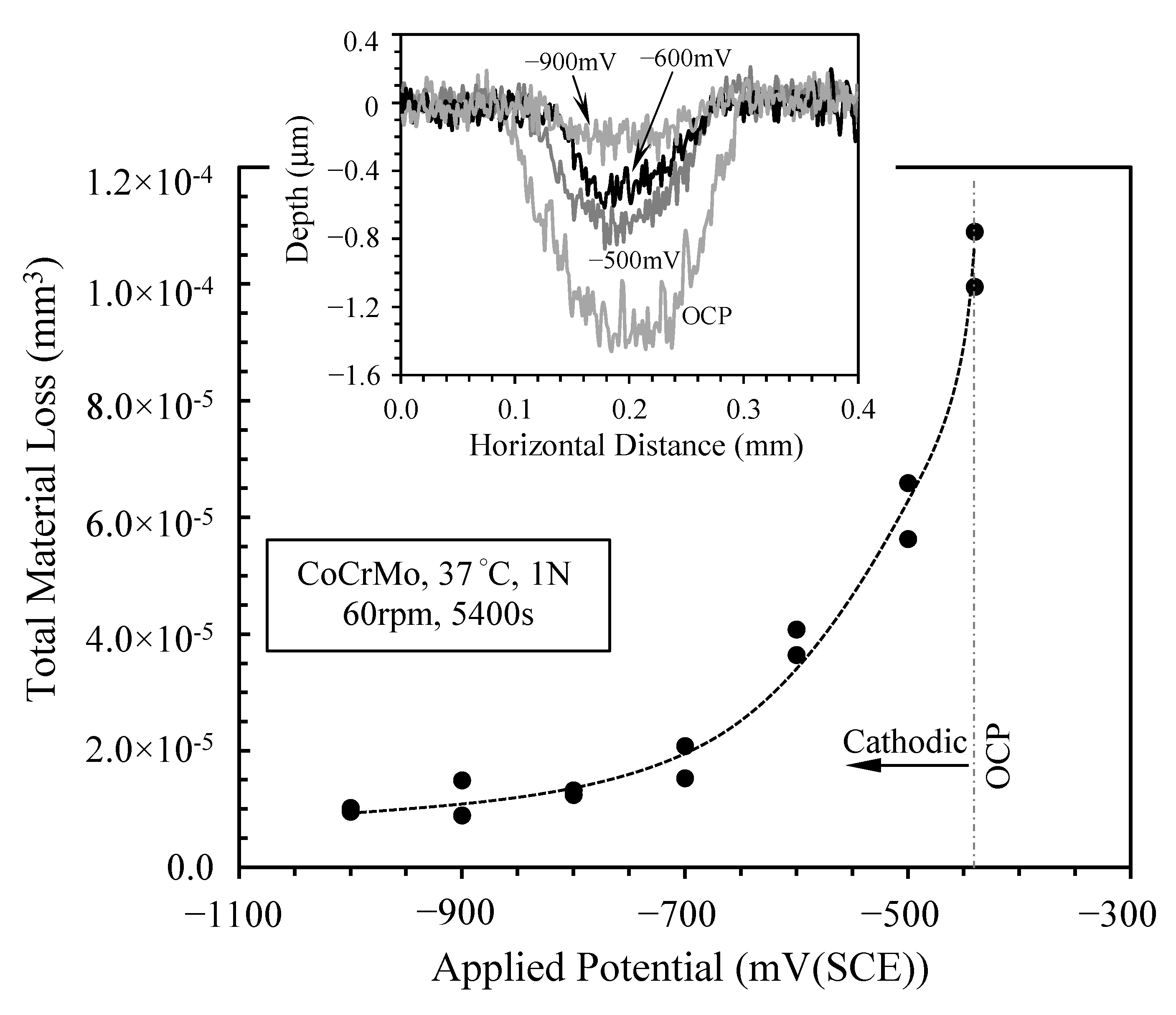
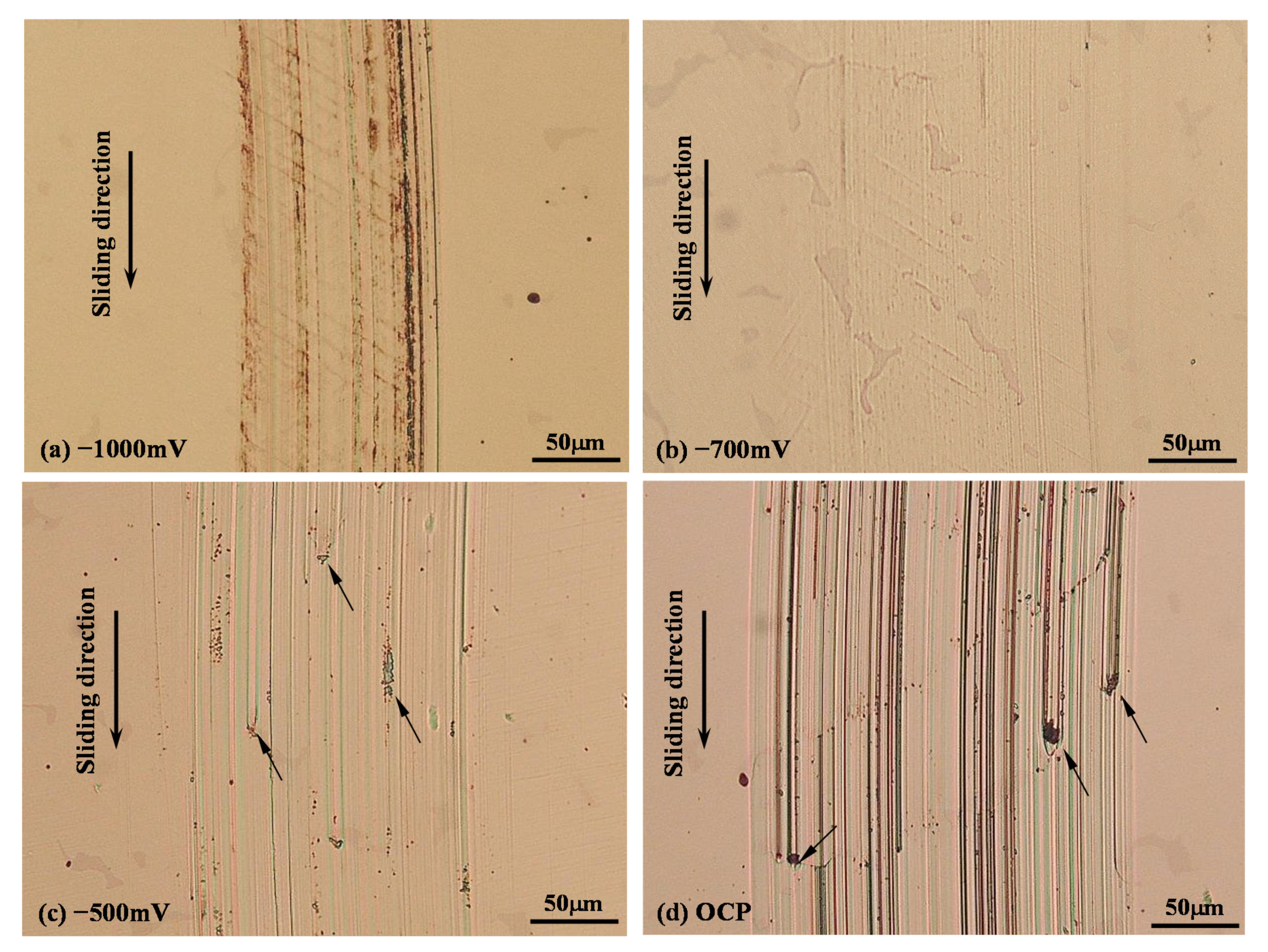
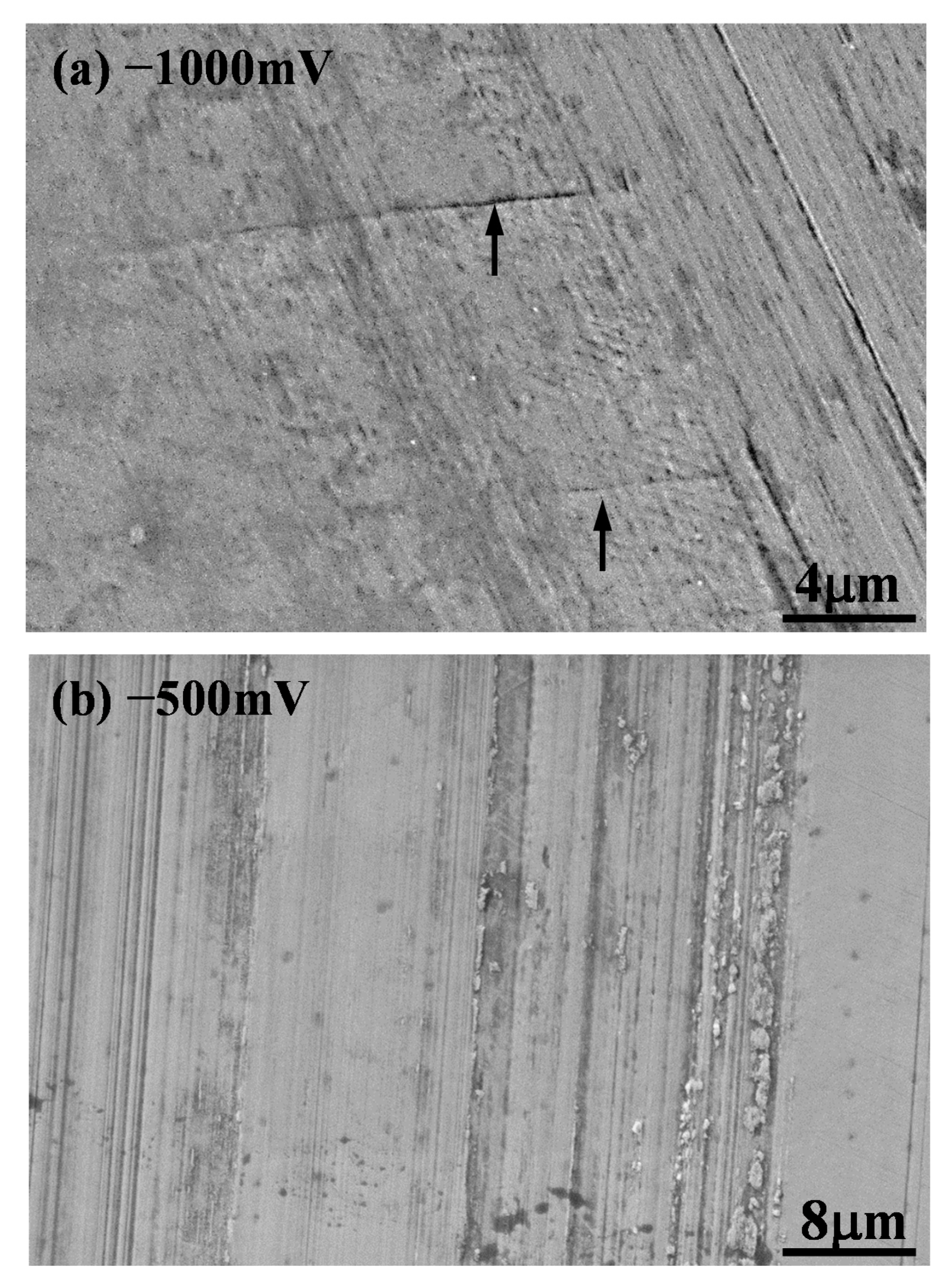
3.4. Morphology of Sliding Tracks
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lemaire, E.; le Calvar, M. Evidence of tribocorrosion wear in pressurized water reactors. Wear 2001, 249, 338–344. [Google Scholar] [CrossRef]
- Watson, S.W.; Friedersdorf, F.J.; Madsen, B.W.; Cramer, S.D. Methods of measuring wear-corrosion synergism. Wear 1995, 181, 476–484. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Ji, C.; Zhang, Y.; Wang, H. Tribocorrosion of Fe-based amorphous coating in simulated body fluids. Lubricants 2018, 6, 37. [Google Scholar] [CrossRef]
- Jiang, J.; Stack, M.M.; Neville, A. Modelling the tribo-corrosion interaction in aqueous sliding conditions. Tribol. Int. 2002, 35, 669–679. [Google Scholar] [CrossRef]
- Li, X.; Dou, W.; Tian, L.; Dong, H. Combating the tribo-corrosion of LDX2404 lean duplex stainless steel by low temperature plasma nitriding. Lubricants 2018, 6, 93. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Ponthiaux, P.; Wenger, F.; Drees, D.; Celis, J.P. Electrochemical techniques for studying tribocorrosion processes. Wear 2004, 256, 459–468. [Google Scholar] [CrossRef]
- Tao, S.; Li, D.Y. Investigation of corrosion-wear synergistic attach on nanocrystalline Cu deposits. Wear 2007, 263, 363–370. [Google Scholar] [CrossRef]
- Benea, L.; Ponthiaux, P.; Wenger, F.; Galland, J.; Hertz, D.; Malo, J.Y. Tribocorrosion of stellite 6 in sulphuric acid medium: Electrochemical behavior and wear. Wear 2004, 256, 948. [Google Scholar] [CrossRef]
- Sun, Y.; Rana, V. Tribocorrosion behaviour of AISI 304 stainless steel in 0.5M NaCl solution. Mater. Chem. Phys. 2011, 129, 138–147. [Google Scholar] [CrossRef]
- Akonko, S.; Li, D.Y.; Ziomek-Moroz, M. Effect of cathodic protection on corrosive wear of 304 stainless steel. Trib. Lett. 2005, 18, 405–410. [Google Scholar] [CrossRef]
- Munoz, A.I.; Julian, L.C. Influence of electrochemical potential on the tribocorrosion behaviour of high carbon CoCrMo biomedical alloy in simulated body fluids by electrochemical impedance spectroscopy. Electrochim. Acta 2010, 55, 5428–5439. [Google Scholar] [CrossRef]
- Favero, M.; Stadelmann, P.; Mischler, S. Effect of applied potential on the near surface microstructure of a 316L steel submitted to tribocorrosion in sulfuric acid. J. Phys. D Appl. Phys. 2006, 39, 3175. [Google Scholar] [CrossRef]
- Sun, Y.; Dearnley, P.A. Tribocorrosion behaviour of duplex S/Cr(N) and S/CrC coatings on CoCrMo alloy in 0.89% NaCl solution. J. Bio Tribo-Corros. 2015, 1, 1–13. [Google Scholar] [CrossRef][Green Version]
- Song, J.; Curtin, W.A. Mechanisms of hydrogen-enhanced localized plasticity: An atomistic study using α-Fe as a model system. Acta Mater. 2014, 68, 61–69. [Google Scholar] [CrossRef]
- Liang, X.; Gao, X.; Yang, H.; Yu, L. Effect of static hydrogen charging on corrosion and hydrogen embrittlement of high speed steel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012049. [Google Scholar]
- Murakami, T.; Mano, H.; Kaneda, K.; Hata, M.; Sasaki, S.; Sugimura, J. Friction and wear properties of zirconium and niobium in a hydrogen environment. Wear 2010, 268, 721–729. [Google Scholar] [CrossRef]
- Georgiou, E.P.; Cevallos, V.P.; van der Donck, T.; Drees, D.; Meersschaut, J.; Panagopoulos, C.N.; Celis, J.-P. Effect of cathodic hydrogen charging on the wear behaviour of 5754 Al alloy. Wear 2017, 390, 295–301. [Google Scholar] [CrossRef]
- Amoush, A.S.E. Investigation of wear properties of hydrogenated tin brass heat exchanger. J. Alloys Compd. 2008, 448, 257–262. [Google Scholar] [CrossRef]
- Zhang, T.C.; Jiang, X.X.; Li, S.Z. Hydrogen-induced embrittlement wear of a high-strength low alloy steel in an acidic environment. Corrosion 1997, 53, 200–205. [Google Scholar] [CrossRef]
- Pokhmurskii, V.I.; Vynar, V.A.; Vasyliv, C.h.B.; Ratska, N.B. Effects of hydrogen exposure on the mechanical and tribological properties of α-titanium surfaces. Wear 2013, 306, 47–50. [Google Scholar] [CrossRef]
- Cassar, J.; Mallia, B.; Mazzonello, A.; Karl, A.; Buhagiar, J. Improved tribocorrosion resistance of a CoCrMo implant material by carburizing. Lubricants 2018, 6, 76. [Google Scholar] [CrossRef]
- Mischler, S.; Munoz, A.I. Wear of CoCrMo alloys used in metal-on-metal hip joints: A tribocorrosion appraisal. Wear 2013, 297, 1081–1094. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Su, Y.; Qiao, L. Effect of electrochemical corrosion on the subsurface microstructure evolution of a CoCrMo alloy in albumin containing environment. Appl. Surf. Sci. 2017, 406, 319–329. [Google Scholar] [CrossRef]
- Quiram, G.; Gindri, I.M.; Kerwell, S.; Shull, K.; Mathew, M.T. Nanoscale mechanical evaluation of electrochemically generated tribolayer on CoCrMo alloy for hip joint application. J. Bio Tribo-Corros. 2016, 2, 15. [Google Scholar] [CrossRef][Green Version]
- Ji, X.; Luo, C.; Sun, Y.; Zhao, J. Corrosive wear of multi-layer Fe-based coatings laser cladded from amorphous powders. Wear 2019, 438, 203113. [Google Scholar] [CrossRef]
- Munoz, A.I.; Mischler, S. Electrochemical quartz crystal microbalance and X-ray photoelectron spectroscopy study of cathodic reactions in bovine serum albumin containing solutions on a physical vapour deposition CoCrMo biomedical alloy. Electrochem. Acta 2015, 180, 96–103. [Google Scholar] [CrossRef]
- Sinnett-Jones, P.E.; Wharton, J.A.; Wood, R.J.K. Micro-abrasion-corrosion of CoCrMo alloy in simulated artificial hip joint environments. Wear 2005, 259, 898–909. [Google Scholar] [CrossRef]
- Rosenak, P. Defects producing formation of micro-cracks in aluminum during electrochemical charging with hydrogen. J. Alloys Comp. 2005, 400, 106–111. [Google Scholar] [CrossRef]
- Escobar, D.P.; Minambre, C.; Duprez, L.; Verbeken, K.; Verhaege, M. Internal and surface damage of multiphase steels and pure iron after electrochemical hydrogen charging. Corros. Sci. 2011, 53, 3166–3176. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A Review. Int. J. Hydrogen Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Hardie, D.; Liu, S. The effect of stress concentration on hydrogen embrittlement of a low alloy steel. Corros. Sci. 1996, 38, 721–733. [Google Scholar] [CrossRef]
- Su-Il, P.; Jong-Sang, K.; Frisch, B.; Messerschmidt, C. Steady state hydrogen evolution enhanced during the abrasive wear from mild steel. Wear 1998, 124, 331–336. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Kurosu, S.; Yamanaka, K.; Tang, N.; Koizumi, Y.; Chiba, A. Effects of sigma phase and carbide on the wear behavior of CoCrMo alloys in Hanks’ solution. Wear 2014, 310, 51–62. [Google Scholar] [CrossRef]
- Buscher, R.; Fischer, A. The pathways of dynamic recrystallization in all-metal hip joints. Wear 2005, 259, 887–897. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Bailey, R. Effect of Applied Cathodic Potential on Friction and Wear Behavior of CoCrMo Alloy in NaCl Solution. Lubricants 2020, 8, 101. https://doi.org/10.3390/lubricants8110101
Sun Y, Bailey R. Effect of Applied Cathodic Potential on Friction and Wear Behavior of CoCrMo Alloy in NaCl Solution. Lubricants. 2020; 8(11):101. https://doi.org/10.3390/lubricants8110101
Chicago/Turabian StyleSun, Yong, and Richard Bailey. 2020. "Effect of Applied Cathodic Potential on Friction and Wear Behavior of CoCrMo Alloy in NaCl Solution" Lubricants 8, no. 11: 101. https://doi.org/10.3390/lubricants8110101
APA StyleSun, Y., & Bailey, R. (2020). Effect of Applied Cathodic Potential on Friction and Wear Behavior of CoCrMo Alloy in NaCl Solution. Lubricants, 8(11), 101. https://doi.org/10.3390/lubricants8110101





