3.1. Microscopic Investigation
As follows from the theory of self-organization, the processes that occur during friction can lead to a decrease in the wear of a rubbing body [
36]. Based on the results of the tribological tests, two alloys were selected with the lowest and highest wear rates No. 7 and No. 4, respectively, to detect differences in changes. Both alloys have the same set of alloying components and microstructure (
Figure 3 and
Figure 4). In the matrix based on a solid solution of copper, zinc, magnesium, silicon in aluminum there are solid inclusions of the θ-phase (CuAl
2) and silicon, as well as soft inclusions based on tin and lead. The latter are present in the alloy in the form of volume inclusions up to 30–50 microns. Copper-based inclusions are contouring aluminum grains providing additional strength. Zinc and magnesium are part of the aluminum matrix and soft inclusions and do not form own phases or zones of increased concentration.
The surface structure of aluminum alloys No. 7 and 4 during 40 h of testing (
Figure 5b,c) underwent significant structural changes compared to the initial surface (
Figure 5a). The direction of friction of both alloys can be traced. Alloy No. 7 is characterized by significant smearing of soft inclusions based on lead and tin in the direction of friction. Alloy No. 4 has a small amount of soft phase on the friction surface, and the direction of friction is characterized by the location of the grooves formed. On the surface, cavities are also visible, which were absent in the initial state. Compared to the state after milling, the roughness decreased from R
a = 0.783 µm to R
a = 0.128 µm for alloy No. 7 and R
a = 0.159 µm for alloy No. 4. The roughness decreased by 5–6 times during running-in due to the smoothing of all sizeable protuberances.
EDX-analysis of the friction surface, in addition to elements of an aluminum alloy, also showed the presence of lubricant components (
Table 4). The content of carbon and oxygen increased significantly. Independent inclusions of iron on the surface appeared due to the mass transfer of particles separated from the steel counterbody due to mechanical running-in. The debris are often the main cause of the formation of grooves until they are absorbed by soft inclusions. The content of tin and lead decreased in both alloys (
Table 4). Moreover, the tin content in alloy No. 4 is less than in alloy No. 7, although in the initial state alloy No. 4 contained 1.5 times more tin (
Table 1).
In contrast to the emission spectrometer, in the EDX-analysis it is important to take into account the electron penetration depth when interpreting the results, which is ~2 μm. The smearing of soft inclusions led to an increase in the distribution area of tin and lead. In this regard, the content of the latter on the friction surface of alloy No. 7 increased relative to the initial content. In alloy No. 4, there is a sharp decrease in tin and lead on the friction surface, which leads to the absence of smearing.
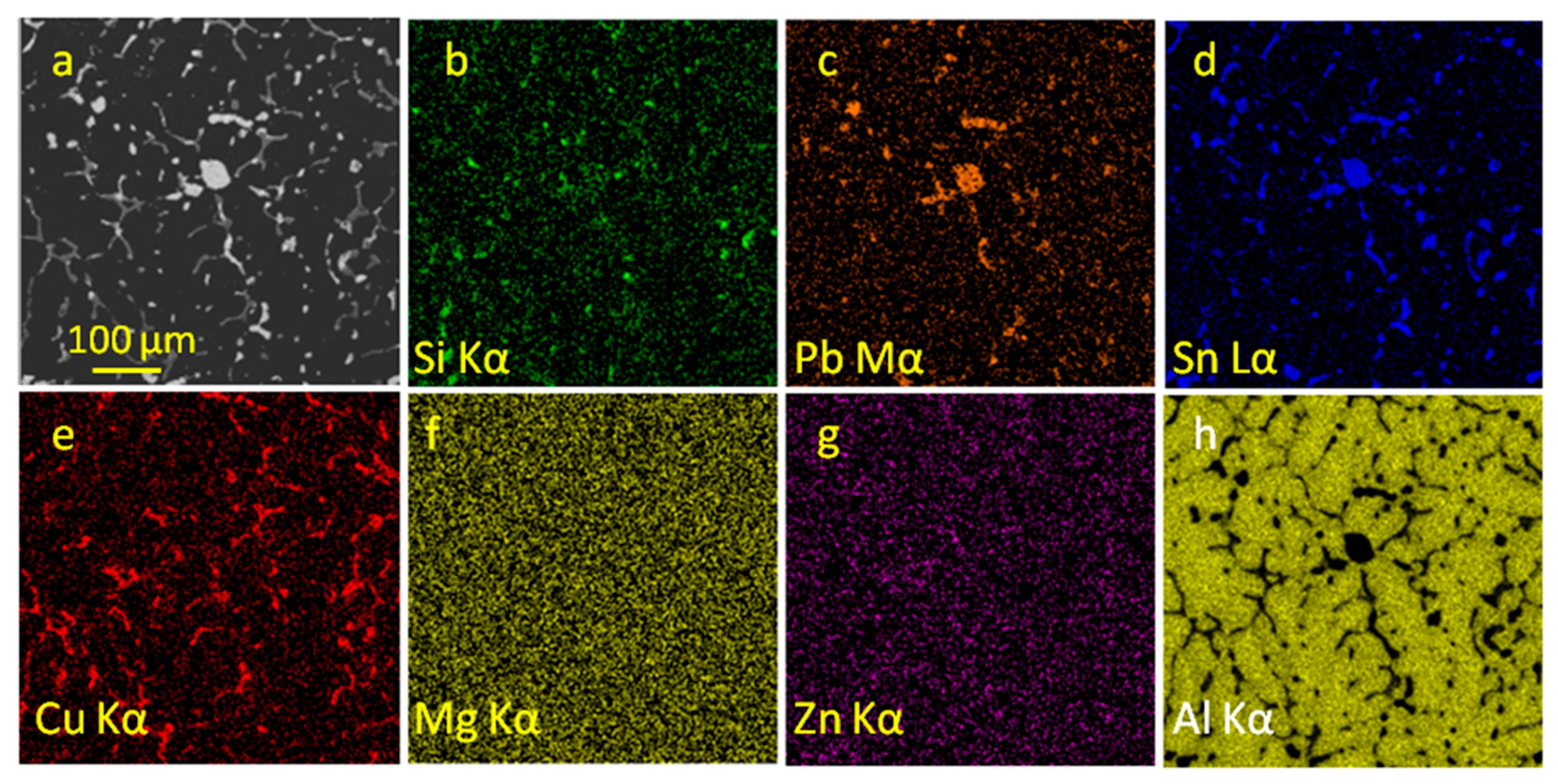
The distribution of elements on the friction surfaces of alloys is shown in
Figure 6 and
Figure 7, where it can be seen that in some cases the distribution of elements is non-random. First, for alloy No. 7, magnesium precipitates were revealed (
Figure 6e), which in the initial state had no zones of increased concentration. These zones were simultaneously enriched in carbon and oxygen. Carbon, in turn, is present on almost the entire friction surface, having different intensities in different local areas. The oxygen distribution map, for the most part, follows the location of the formed grooves. Their appearance is accompanied by constant destruction and the formation of Al
2O
3 oxide films. The more developed the surface, the greater the amount of oxygen. Besides, oxygen is present in the lubricant in the form of various C–O compounds, which also explains its significant amount on the surface. Lead and sulfur also have almost the same distribution maps of elements (
Figure 6i,j). Each inclusion of lead from 10 microns turned out to be enriched in sulfur. Iron is present on the friction surface in small quantities in the form of small, up to 5 μm debris (
Figure 6l). Zinc, as before friction, remains an element uniformly distributed over the alloy structure (
Figure 6h).
Patterns found on the surface of alloy No. 7 did not apply to alloy No. 4. First, there is practically no release of magnesium from aluminum solid solution (
Figure 7e), despite their commensurate content in the initial state (
Table 4). As a result, carbon and oxygen have high content mainly in the formed grooves. A significant decrease in silicon content is also observed. The inclusions, compared to the alloy No. 7, are smaller in size and quantity in the studied area. Lead on the surface is represented by several small elongated inclusions. Its small amount caused the almost complete absence of sulfur on the surface. The copper content, in contrast to other alloy components, increased by 0.2%. This can be explained by the high hardness and strength of the CuAl
2 intermetallic compound, as a result of which the wear rate of such inclusions is lower than that of the other phases. Against the background of the absence of soft structures smearing that could cover copper inclusions and the intensive wear of the remaining components, the copper content on the surface increased. As a result, a large number of solid inclusions formed on the surface, which led to more intensive wear of the steel counterbody. It led to more intensive mass transfer of iron particles up to 10–20 microns and an increase in its content on the surface. In contrast to alloy No. 7, zinc is locally precipitated from aluminum solid solution No features of its interaction with lubricant products were found. Thus, it can be assumed that the formation of secondary structures for alloys No. 4 and No. 7 proceed differently.
To determine the depth of changes that occurred a beveled sample was studied.
Figure 8 clearly shows the deformation of inclusions along friction direction. While the change in elemental composition occurs mainly on the surface, the subsurface layer undergoes plastic deformation to a depth of 3 μm due to large shear stresses. Due to this, the surface of the aluminum matrix becomes hard-drawn and its hardness increases by 10–20% (
Table 5). This mechanism is common for all alloys and increases the ability of alloys to resist wear.
3.2. XPS
The possibility of the spatial detection of elements in the area is one of the main advantages of EDX-analysis. However, to confirm the occurrence of the tribochemical reaction during the friction process, it is necessary to determine the bonds established between the elements using XPS. In this case, the studied region is limited by 2–3 atomic layers.
Figure 8 shows the XPS spectra of alloys No. 4 and No. 7, as well as alloy No. 4 in the initial state. The XPS survey spectrum (
Figure 8a), in comparison with the sample that was not subjected to friction, shows the presence of several new peaks. First, the peak of iron (~708 eV), confirming the presence of mass transfer from the steel counterbody. Sodium (~1056 eV) from the lubricant is also observed. The intensity of the lead peak for alloy No. 7 increased, which is associated more with the smearing of this element over the surface.
Table 6 shows the atomic percentages of the observed elements.
The high-resolution spectra of the elements by which a match was found during EDX-analysis are shown in
Figure 8b–g. In alloy No. 7, magnesium, which before friction was present in the alloy only in solid solutions, was released after friction, as indicated by the peak of elemental magnesium with a binding energy of 1303.12 ± 0.5 eV. Most of the magnesium is oxidized to magnesium oxide MgO, which has a binding energy of 1304.68 ± 0.5 eV. Under friction conditions and intensive carbon deposition, magnesium carbonate MgCO
3 is formed (1305.74 ± 0.5 eV). Discovered compounds correlate with distribution maps of elements (
Figure 5). In alloy No. 4, the intensity of elemental magnesium and MgCO
3 is rather low compared to alloy No. 7, and magnesium oxide is dominant (
Figure 9b,e). The most likely cause is a lower amount of magnesium released from the solid solution. Given the low electronegativity of magnesium, it can be concluded that oxide formation is a priority [
53], which then reacts with carbon during friction to form magnesium carbonate. One of the reasons for its formation is the mechanical activation of magnesium oxide particles, due to the constant impact of rubbing objects on it. The process is accompanied by an increase in free energy that is spent on initiating reactions, instead of the physical wear of the body.
The solubility of magnesium in aluminum, according to the equilibrium state diagram at room temperature, is about 5%. In addition, aluminum has a higher affinity for oxygen than that of magnesium. Therefore, the release of magnesium from a solid solution is not a spontaneous process and is accompanied by a negative production of entropy. This may be a mechanism of dissipative structures formation [
47]. Alloy No. 7 has two times more magnesium on its friction surface than alloy No. 4. Given the same time of the friction test, the intensity of magnesium release from the solid solution of alloy No. 7 is two times higher than that of alloy No. 4. Accordingly, the negative entropy production in alloy No. 7 is approximately two times greater (in absolute value) than in alloy No. 4. The increased wear rate of Alloy No. 4 is probably related to the ability of magnesium to precipitate from a solid solution.
Lead in both alloys after friction is present in a small amount in the elemental state, as indicated by the peak at a binding energy of 136.8 ± 0.5 eV (
Figure 8c,f). The main part of lead in alloy No. 4 is in the form of oxides Pb
3O
4 (138.4 ± 0.5 eV), PbO
2 (137.2 ± 0.5 eV) and PbO (137.9 ± 0.5 eV), of which the latter is the dominant compound. On the other hand, alloy No. 7 is characterized by a significant amount of lead sulfide compound PbS (137.4 ± 0.5). Various lead oxides are also present on the friction surface of alloy No. 7 (
Figure 9c). The beneficial effect of lead sulfide upon friction lies in the possibility of the formation of long films on the surface, which are easily transferred to the steel counterbody. The crystal lattice of this compound is symmetrical and can be cleaved along the {100} plane. It is noted that sulfur is capable of being transported and reacting with iron at the point of contact with the formation of FeS compound, which is also characterized by a lamellar structure with low shear resistance [
33,
54]. Thus, the friction in the area is reduced to the contact of two solid lubricants, which can easily wear out under the action of shear stresses.
No significant differences in the interaction with carbon in alloys No. 4 and No. 7 were observed. A greater amount of it occurs in adventitious hydrocarbon contamination C–C/C–H with the binding energy of 285 ± 0.5 eV, the source of which is not only lubricant, but also the environment. The formation of C–O–C/C–OH (286.3 ± 0.5 eV) compounds on the friction surface is seen, which indicates the decomposition of the lubricant and its subsequent polymerization. The O–C=O peak (289.2 eV) represents the presence of carbonates on the surface.
3.3. XRD
To detect changes in the phase composition, an X-ray phase analysis of the samples was carried out before and after friction, as well as a sample of steel 38HN3MA in its initial state (
Figure 10). It can be seen that the samples after friction underwent some changes, as indicated by the presence of new peaks compared to the initial state (
Table 6). Moreover, there are no steel-related peaks, which would indicate the presence of mass transfer. The number of transferred counterbody particles is small and is below the sensitivity limit of the device.
The friction surface structure of alloy No. 7 differs from alloy No. 4 by the presence of peaks 1 and 2 (
Table 7), which were identified as lead sulfide PbS (96-301-3404), which confirms the XPS-analysis. The newly formed peaks 3–6 (
Table 7) have low intensity and, based on interplanar spacings, were identified as aluminum carboxide in various stoichiometry, which is also the result of the interaction of the matrix with the lubricant.
The formation of the compound occurs on the surface over the entire area of the aluminum matrix, as a result of which such compounds are the basis of the secondary structures formed during friction. The presence of aluminum carboxides after friction is common for both alloys. Compounds Al2OC and Al4OC4 are the main components of ceramics and have high hardness and, as a result, higher wear resistance than the original aluminum matrix. That mechanism of secondary structures formation is a response of the system to the destruction caused by friction and aimed at reducing wear.