Low-Temperature Rheology and Thermoanalytical Investigation of Lubricating Oils: Comparison of Phase Transition, Viscosity, and Pour Point
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polarization Microscopy
2.2. Differential Scanning Calorimetry (DSC)
2.3. Rheological Measurements
3. Results and Discussion
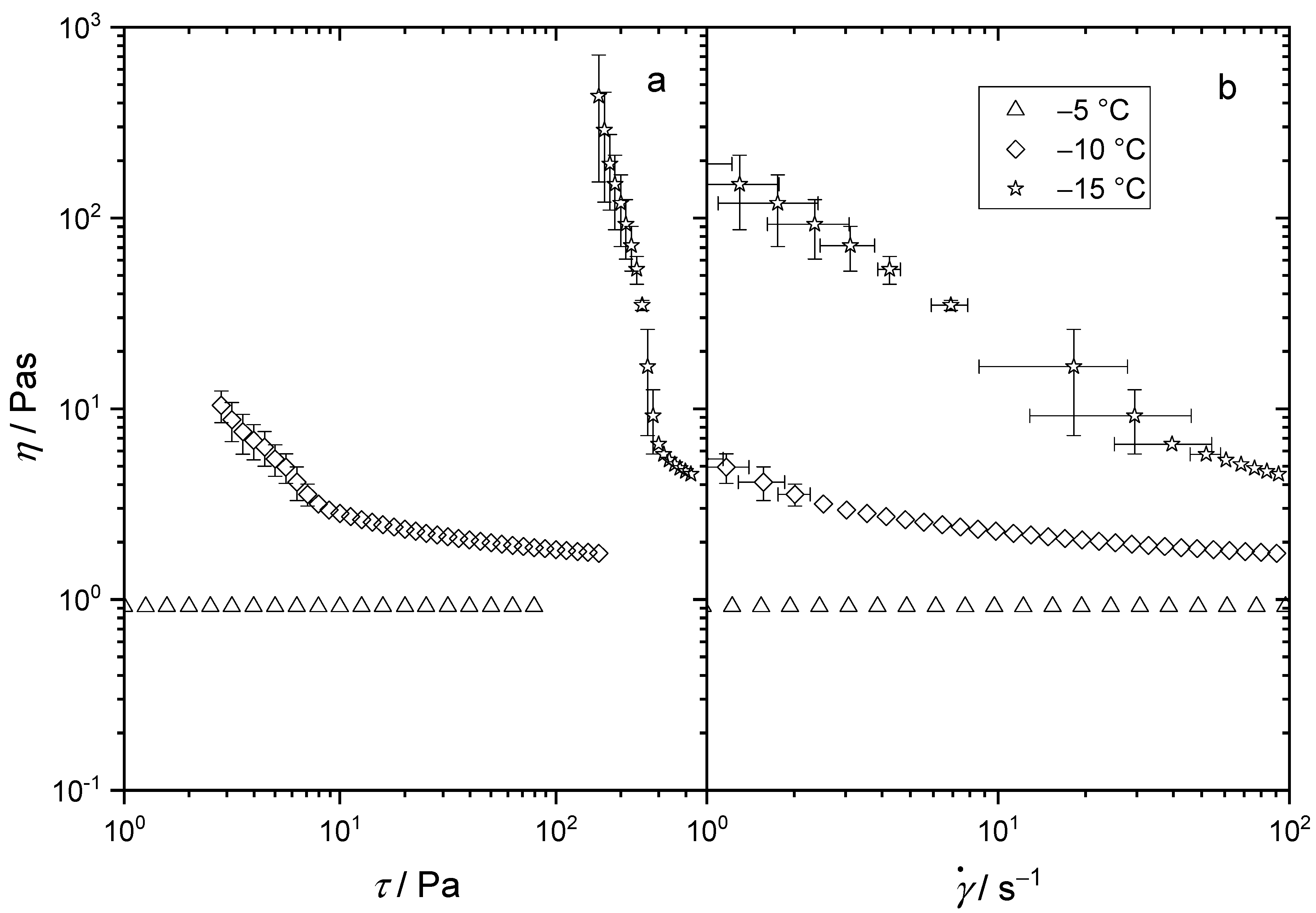
3.1. Flow Behavior of Mineral Oil (Group I) at Low Temperatures
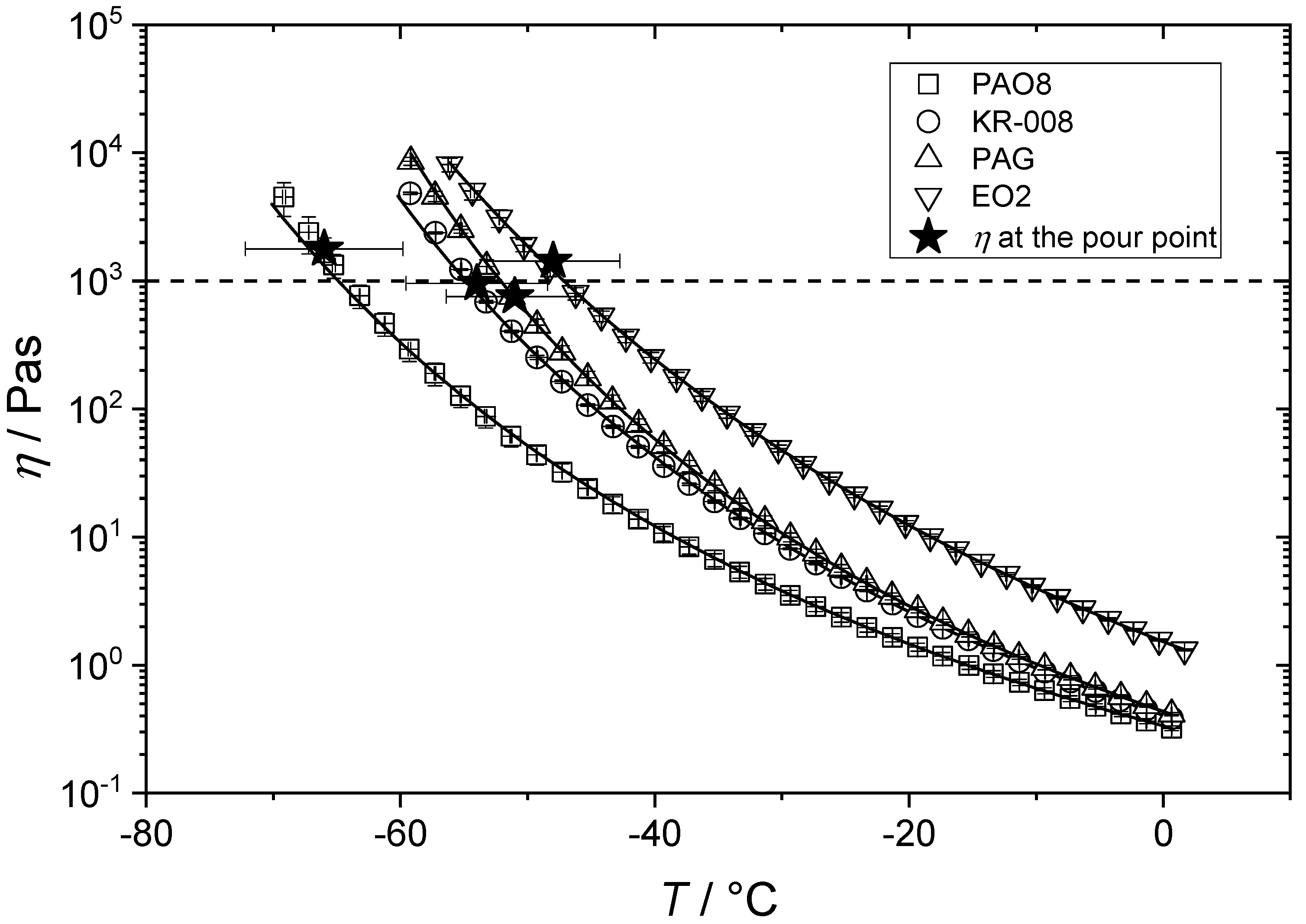
3.2. Flow Behavior of Non-Crystallizing Synthetic Lubricating Oils (Group II) at Low Temperatures
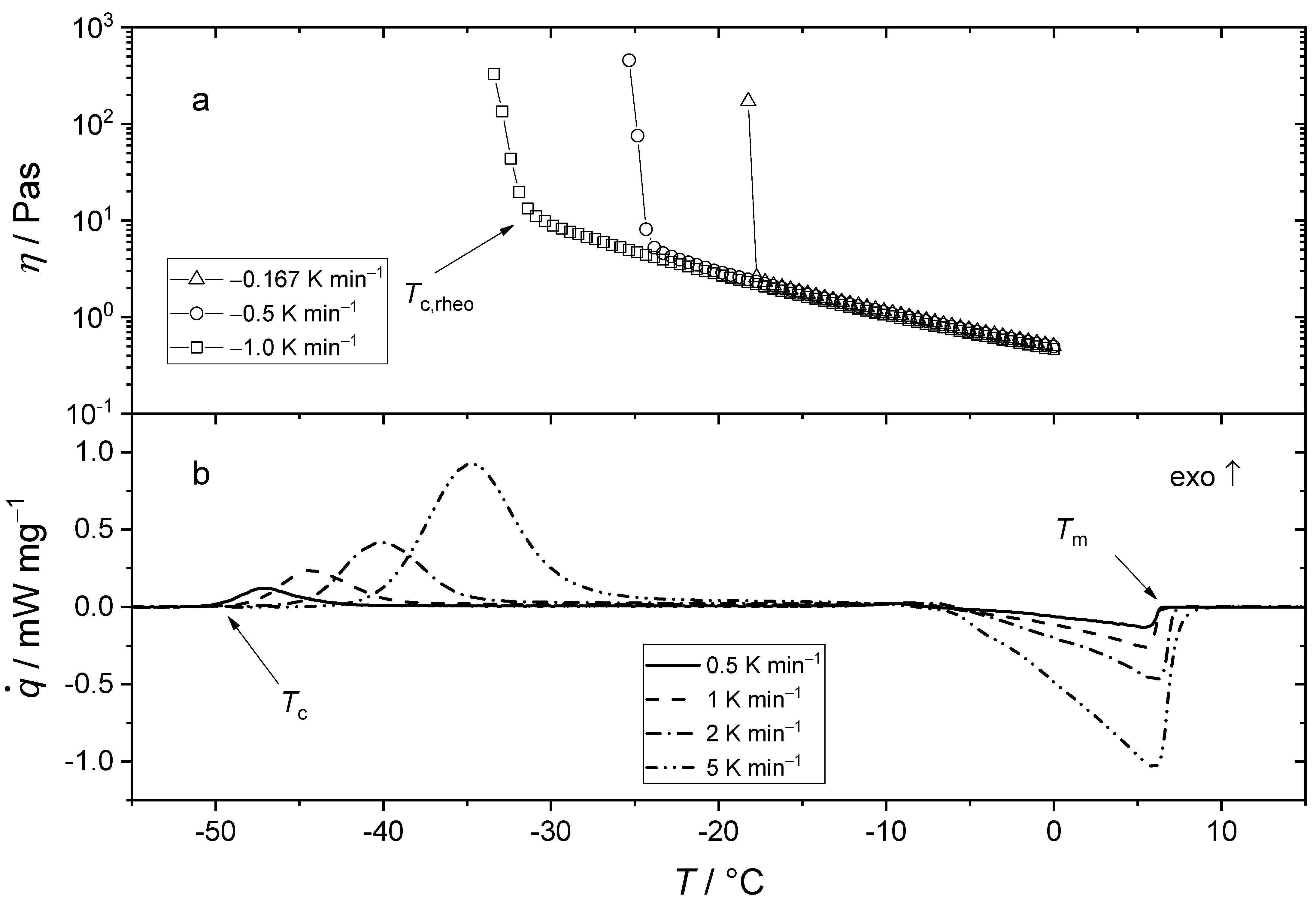
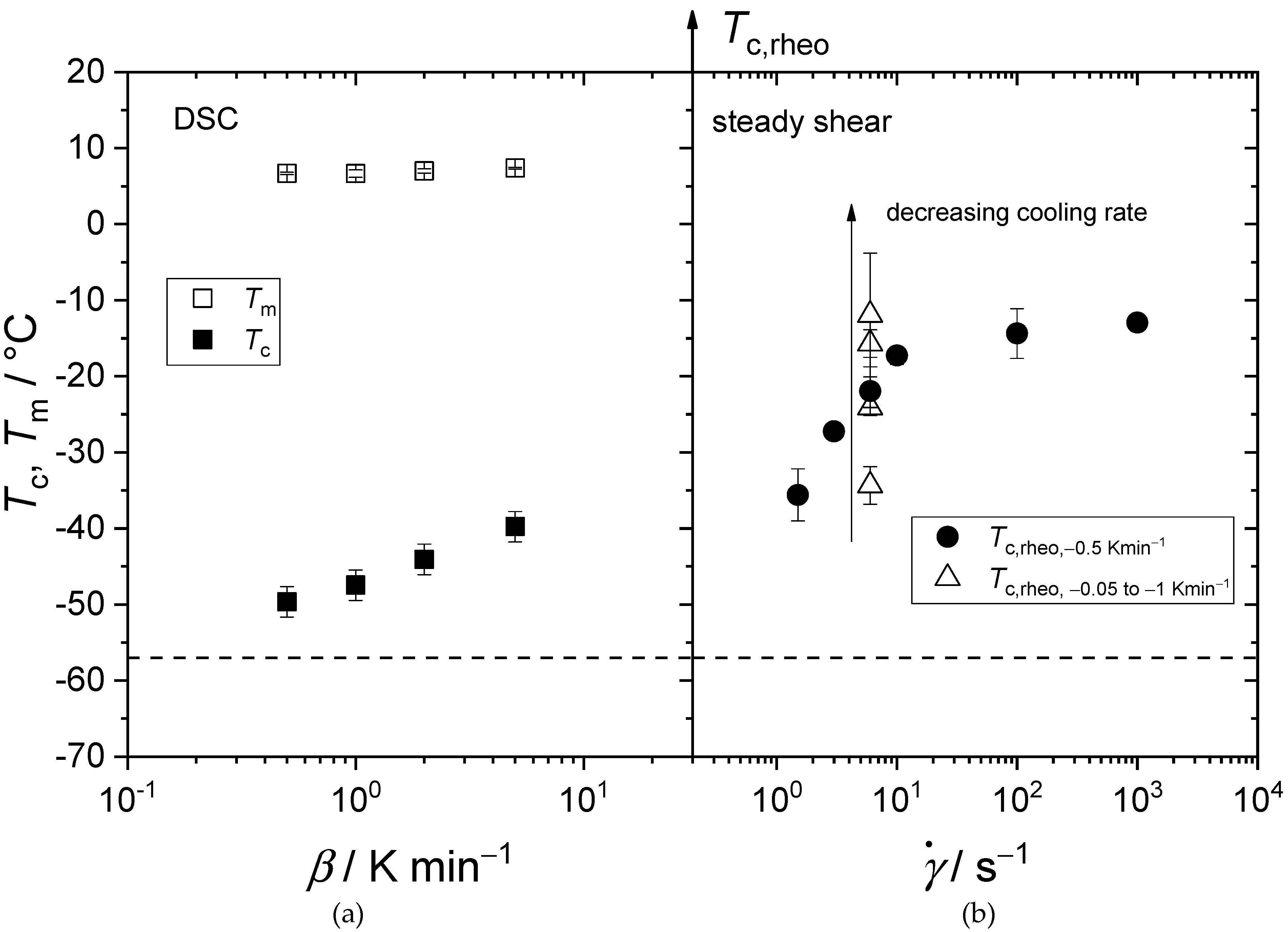
3.3. Flow Behavior of Crystallizing Synthetic Lubricating Oils (Group III) at Low Temperatures
4. Conclusions
4.1. Crystallizing Mineral Oil
4.2. Amorphously Solidifying Synthetic Lubrication Oils
4.3. Crystallizing Synthetic Lubricating Oils
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ASTM D97. Standard Test Method for Pour Point of Petroleum Products; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Webber, R.M. Low temperature rheology of lubricating mineral oils: Effects of cooling rate and wax crystallization on flow properties of base oils. J. Rheol. 1999, 43, 911–923. [Google Scholar] [CrossRef]
- Webber, R.M. Yield Properties of Wax Crystal Structures Formed in Lubricant Mineral Oils. Ind. Eng. Chem. Res. 2001, 40, 195–203. [Google Scholar] [CrossRef]
- Shubkin, R.L. Polyalphaolefins Meeting the Challenge for High-Performance Lubrication. Lubr. Eng. 1994, 50, 196–202. [Google Scholar]
- Tsvetkov, O.N.; Kolesova, G.E.; Bogdanov, S.K.; Toporishcheva, R.I. Rheological properties and lubricity of poly-alpha-olefin oils. Chem. Technol. Fuels Oils 1987, 23, 228–231. [Google Scholar] [CrossRef]
- Hourani, M.J.; Hessell, T.; Abramshe, R.A.; Liang, J. Alkylated Naphthalenes as High-Performance Synthetic Lubricating Fluids. Tribol. Trans. 2007, 50, 82–87. [Google Scholar] [CrossRef]
- Hessel, E.T.; Abramshe, R.A. Alkylated Naphthalenes as High-Performance Synthetic Fluids. J. Synth. Lubr. 2003, 20, 109–122. [Google Scholar] [CrossRef]
- Gul, M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Mujtaba, M.A. A Review: Role of Fatty Acids Composition in Characterizing Potential Feedstock for Sustainable Green Lubricants by Advance Transesterification Process and its Global as Well as Pakistani Prospective. Bioenerg. Res. 2020, 13, 1–22. [Google Scholar] [CrossRef]
- Bouzidi, L.; Li, S.; Di Biase, S.; Rizvi, S.Q.; Dawson, P.; Narine, S.S. Lubricating and Waxy Esters II: Synthesis, Crystallization, and Melt Behavior of Branched Monoesters. Ind. Eng. Chem. Res. 2012, 51, 14892–14902. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Engineering Green Lubricants I: Optimizing Thermal and Flow Properties of Linear Diesters Derived from Vegetable Oils. ACS Sustain. Chem. Eng. 2016, 4, 686–692. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Branched Biobased Diesters with Exceptional Low Temperature and Flow Properties for Use in Lubricant Formulations. ACS Sustain. Chem. Eng. 2016, 4, 2542–2549. [Google Scholar] [CrossRef]
- Moura Ramos, J.J.; Diogo, H.P. Are Crystallization and Melting the Reverse Transformation of Each Other? J. Chem. Educ. 2006, 83, 1389–1392. [Google Scholar] [CrossRef]
- March, N.H.; Street, R.A.; Tosi, M.P. Amorphous Solids and the Liquid State, 1st ed.; Springer: Boston, MA, USA, 1985; pp. 401–406. [Google Scholar] [CrossRef]
- ASTMD7346. Standard Test Method for No Flow Point and Pour Point of Petroleum Products and Liquid Fuels; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar] [CrossRef]
- Oliver, M.J.; Calvert, P.D. Homogenous Nucleation of n-Alkanes measured by Differential scanning calorimetry. J. Cryst. Growth 1975, 30, 343–351. [Google Scholar] [CrossRef]
- Webber, R.M.; George, H.F.; Covitch, M.J. The Relation between Low-Temperature Rheology of Lubricating Mineral Oils and Gelatin Index. In Oil Flow Studies at Low Temperatures in Modern Engines; ASTM International: West Conshohocken, PA, USA, 2000; pp. 113–151. [Google Scholar]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Boyde, S. Low-temperature characteristics of synthetic fluids. J. Synth. Lubr. 2001, 18, 99–114. [Google Scholar] [CrossRef]
- Wellen, R.M.R.; Canedo, E.; Rabello, M.S. Nonisothermal cold crystallization of poly(ethylene terephthalate). J. Mater. Res. 2011, 26, 1107–1115. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Fischer, L.J.; Worlitschek, J.; Stamatiou, A. Assessment of the Thermal Properties of Aromatic Esters as Novel Phase Change Materials. Crystals 2020, 10, 919. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Fischer, L.J.; Worlitschek, J.; Stamatiou, A. Investigation of the Thermal Properties of Diesters from Methanol, 1-Pentanol, and 1-Decanol as Sustainable Phase Change Materials. Materials 2020, 13, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, T.W.; Maxwell, B. Effects of shear stress on the crystallization of linear polyethylene and polybutene-1. Polym. Eng. Sci. 1969, 9, 225–241. [Google Scholar] [CrossRef]
- Vleeshouwers, S.; Meijer, H.E. A rheological study of shear induced crystallization. Rheol. Acta 1996, 35, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Lellinger, D.; Floudas, G.; Alig, I. Shear induced crystallization in poly(e-caprolactone): Effect of shear rate. Polymer 2003, 44, 5759–5769. [Google Scholar] [CrossRef]



| Lubricating Oil | Chemical Nature | Group | ν@40 °C/cSt. | ν@100 °C/cSt. | VI | Pour Point/°C |
|---|---|---|---|---|---|---|
| MO | mineral oil (SN100/SN500) | I | 48 | 5.3 | 105 | −12 |
| PAO8 | polyalphaolefin | II | 47 | 8 | 139 | −66 |
| KR-008 | alkylated naphthalene | II | 36 | 7 | 68 | −54 |
| PAG | polypropylenglycole | II | 57 | 10.4 | 188 | −51 |
| EO2 | tris(2-ethylhexyl)trimellitate | II | 87 | 9.6 | 80 | −48 |
| EO1 | Trimellitate * | III | 52 | 8.1 | 128 | −57 |
| PAO8 | KR-008 | EO2 | PAG | |
|---|---|---|---|---|
| TG,DSC/°C | −84.5 ± 0.4 | −75.7 ± 0.4 | −78.5 ± 0.5 | −79.3 ± 0.9 |
| c1 | 8.3 | 9.4 | 11.7 | 8.4 |
| c2/K | 133.9 | 121.5 | 135.1 | 109.1 |
| PPASTM D7347/°C * | −66 ± 6 | −54 ± 6 | −48 ± 6 | −51 ± 6 |
| T1000Pas/°C | −64 ± 1 | −55 ± 0.6 | −47 ± 0.6 | −52 ± 0.6 |
| ηat the PP/Pas | 1780 ± 380 | 910 ± 15 | 1240 ± 170 | 754 ± 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conrad, A.; Hodapp, A.; Hochstein, B.; Willenbacher, N.; Jacob, K.-H. Low-Temperature Rheology and Thermoanalytical Investigation of Lubricating Oils: Comparison of Phase Transition, Viscosity, and Pour Point. Lubricants 2021, 9, 99. https://doi.org/10.3390/lubricants9100099
Conrad A, Hodapp A, Hochstein B, Willenbacher N, Jacob K-H. Low-Temperature Rheology and Thermoanalytical Investigation of Lubricating Oils: Comparison of Phase Transition, Viscosity, and Pour Point. Lubricants. 2021; 9(10):99. https://doi.org/10.3390/lubricants9100099
Chicago/Turabian StyleConrad, Andreas, Annika Hodapp, Bernhard Hochstein, Norbert Willenbacher, and Karl-Heinz Jacob. 2021. "Low-Temperature Rheology and Thermoanalytical Investigation of Lubricating Oils: Comparison of Phase Transition, Viscosity, and Pour Point" Lubricants 9, no. 10: 99. https://doi.org/10.3390/lubricants9100099
APA StyleConrad, A., Hodapp, A., Hochstein, B., Willenbacher, N., & Jacob, K.-H. (2021). Low-Temperature Rheology and Thermoanalytical Investigation of Lubricating Oils: Comparison of Phase Transition, Viscosity, and Pour Point. Lubricants, 9(10), 99. https://doi.org/10.3390/lubricants9100099






