Characterization of Hymenopteran Parasitoids of Aphis fabae in An African Smallholder Bean Farming System Through Sequencing of COI ‘Mini-barcodes’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Sampling
2.3. Parasitoid Identification
3. Results
3.1. Identification of Aphid Parasitoids and Hyperparasitoids Based on Morphological Features
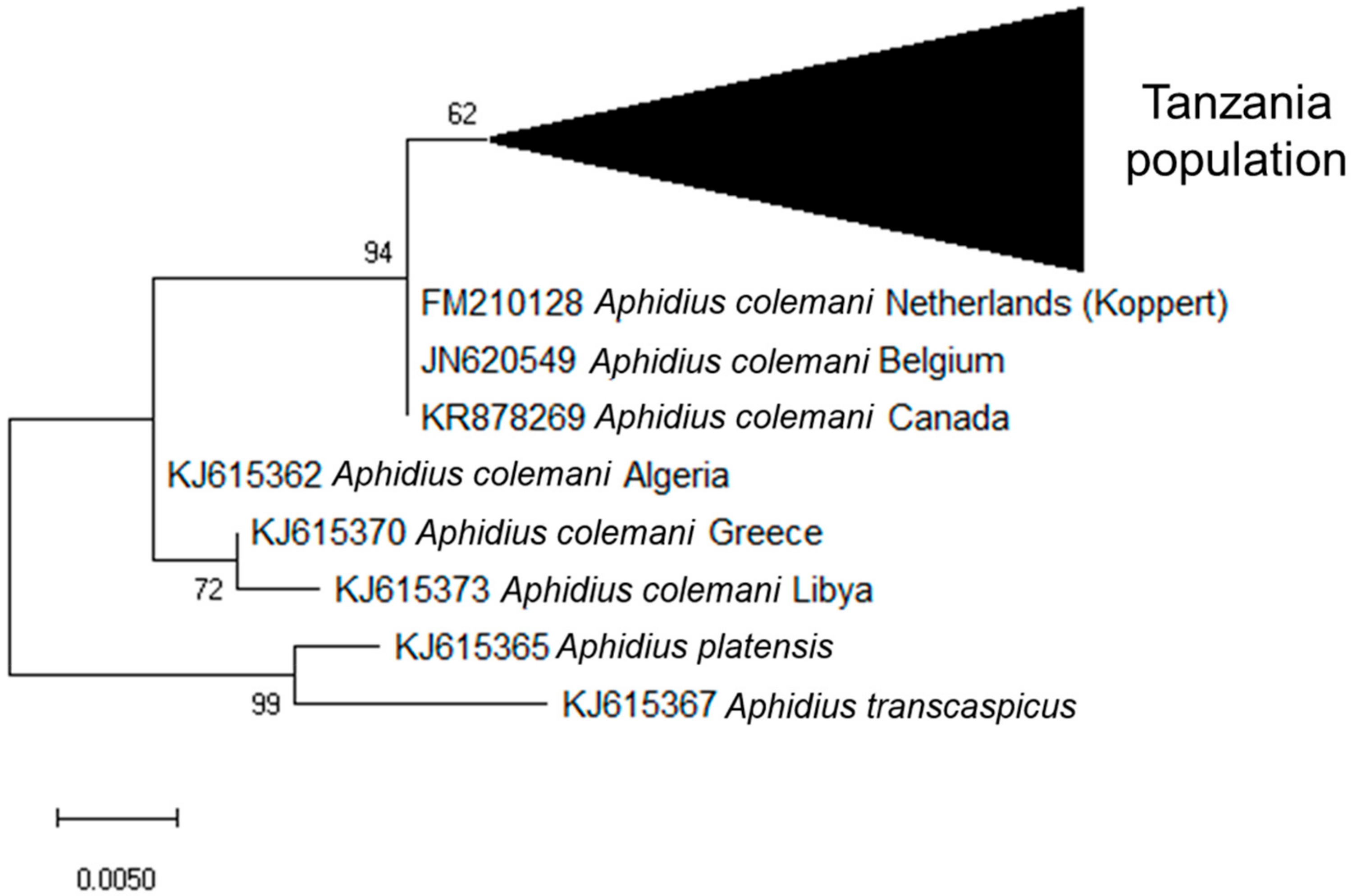
3.2. Identification of Aphid Parasitoids and Hyperparasitoids Based on Molecular Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broughton, W.J.; Hernandez, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)–model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Hillocks, R.; Madata, C.S.; Chirwa, R.; Minja, E.M.; Msolla, S. Phaseolus bean improvement in Tanzania, 1959–2005. Euphytica 2006, 150, 215–231. [Google Scholar] [CrossRef]
- Karel, A.K.; Ndunguru, B.J.; Price, M.; Semuguruka, S.H.; Singh, B.B. Bean production in Tanzania. In Potentials for Field Beans in Eastern Africa; CIAT: Cali, Colombia, 1981; pp. 122–154. [Google Scholar]
- Degri, M.; Mailafiya, D.; Wabekwa, J. Efficacy of aqueous leaf extracts and synthetic insecticide on pod-sucking bugs infestation of cowpea (Vigna unguiculata (L.) Walp) in the Guinea Savanna. Adv. Entomol. 2013, 1, 10–14. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.X.; Jander, G.; Samaniego, H.; Ramsey, J.S.; Figueroa, C.C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE 2012, 7, e36366. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friedrich, T. Improving Handling of Pesticides Application Equipment for the Safety of Applicators; Pesticide Management in West Africa Newsletter no. 2; FAO/Economic Community of West African States: Accra, Ghana, 2001; pp. 9–11. [Google Scholar]
- Matthews, G.; Wiles, T.; Baleguel, P. A survey of pesticide application in Cameroon. Crop Prot. 2003, 22, 707–714. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, F.J.F.; Rajkumar, A.; Muller, C.B.; Godfray, H.C.J. Increased reproduction by pea aphids in the presence of secondary parasitoids. Ecol. Entomol. 2001, 26, 425–429. [Google Scholar] [CrossRef]
- Van Steenis, M.J. Evaluation of four aphidiine parasitoids for biological control of Aphis gossypii. Entomol. Exp. Appl. 1995, 75, 151–157. [Google Scholar] [CrossRef]
- Sullivan, D.J. Hyperparasitism. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2009; pp. 486–488. [Google Scholar]
- Begum, M.; Gurr, G.M.; Wratten, S.D.; Hedberg, P.R.; Nicol, H.I. Using selective food plants to maximize biological control of vineyard pests. J. Appl. Ecol. 2006, 43, 547–554. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E. Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Anim. Ecol. 2008, 77, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, J.M.; Didham, R.K.; Wratten, S.D. Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 2004, 85, 658–666. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, G.; Zheng, X.; Tian, J.; Lu, Z.; Heong, K.L.; Xu, H.; Chen, G.; Yang, Y.; Gurr, G.M. Selective enhancement of parasitoids of rice Lepidoptera pests by sesame (Sesamum indicum) flowers. BioControl 2015, 60, 157–167. [Google Scholar] [CrossRef]
- Lavandero, B.; Wratten, S.D.; Didham, R.K.; Gurr, G. Increasing floral diversity for selective enhancement of biological control agents: A double-edged sword? Basic Appl. Ecol. 2006, 7, 236–243. [Google Scholar] [CrossRef]
- Araj, S.E.; Wratten, S.; Lister, A.; Buckley, H. Floral diversity, parasitoids and hyperparasitoids–A laboratory approach. Basic Appl. Ecol. 2008, 9, 588–597. [Google Scholar] [CrossRef]
- Araj, S.E.; Wratten, S.; Lister, A.; Buckley, H. Adding floral nectar resources to improve biological control: Potential pitfalls of the fourth trophic level. Basic Appl. Ecol. 2009, 10, 554–562. [Google Scholar] [CrossRef]
- Elisante, F.; Ndakidemi, P.A.; Arnold, S.E.; Belmain, S.R.; Gurr, G.M.; Darbyshire, I.; Xie, G.; Tumbo, J.; Stevenson, P.C. Enhancing knowledge among smallholders on pollinators and supporting field margins for sustainable food security. J. Rural Stud. 2019, 70, 75–86. [Google Scholar] [CrossRef]
- O’hara, R.B.; Kotze, D.J. Do not log-transform count data. Methods Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Tomanović, Ž.; Petrović, A.; Mitrović, M.; Kavallieratos, N.G.; Starý, P.; Rakhshani, E.; Rakhshanipour, M.; Popović, A.; Shukshuk, A.H.; Ivanović, A. Molecular and morphological variability within the Aphidius colemani group with redescription of Aphidius platensis Brethes (Hymenoptera: Braconidae: Aphidiinae). Bull. Entomol. Res. 2014, 104, 552–565. [Google Scholar] [CrossRef]
- Mitrović, M.; Tomanović, Ž. New internal primers targeting short fragments of the mitochondrial COI region for archival specimens from the subfamily Aphidiinae (Hymenoptera, Braconidae). J. Hymenopt. Res. 2018, 64, 191. [Google Scholar] [CrossRef]
- Smith, M.A.; Fisher, B.L.; Hebert, P.D. DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: The ants of Madagascar. Philos. Trans. R. Soc. B 2015, 360, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Rodriguez, J.J.; Whitfield, J.B.; Deans, A.R.; Janzen, D.H.; Hallwachs, W.; Hebert, P.D. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc. Natl Acad. Sci. USA 2008, 105, 12359–12364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, P.D.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; de Waard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. B 2016, 371, 20150333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaltenpoth, M.; Corneli, P.S.; Dunn, D.M.; Weiss, R.B.; Strohm, E.; Seger, J. Accelerated evolution of mitochondrial but not nuclear genomes of Hymenoptera: New evidence from crabronid wasps. PLoS ONE 2012, 7, e32826. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Brown, S.D.; Collins, R.A.; Cruickshank, R.H.; Lefort, M.C.; Malumbres-Olarte, J.; Wratten, S.D. Sliding window analyses for optimal selection of mini-barcodes, and application to 454-pyrosequencing for specimen identification from degraded DNA. PLoS ONE 2012, 7, e38215. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Agarwala, R.; Bolton, E.E.; Brister, J.R.; Canese, K.; Clark, K.; Connor, R.; Fiorini, N.; Funk, K.; Hefferon, T.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2019, 47, D23. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+ C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Pungerl, N.B. Morphometric and electrophoretic study of Aphidius species (Hymenoptera: Aphidiidae) reared from a variety of aphid hosts. Syst. Entomol. 1986, 11, 327–354. [Google Scholar] [CrossRef]
- Benelli, G.; Messing, R.H.; Wright, M.G.; Giunti, G.; Kavallieratos, N.G.; Canale, A. Cues triggering mating and host-seeking behavior in the aphid parasitoid Aphidius colemani (Hymenoptera: Braconidae: Aphidiinae): Implications for biological control. J. Econ. Entomol. 2014, 107, 2005–2022. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, G.M.; Orr, D.B.; Baker, J.R. Efficacy assessment of Aphidius colemani (Hymenoptera: Braconidae) for suppression of Aphis gossypii (Homoptera: Aphididae) in greenhouse-grown chrysanthemum. J. Econ. Entomol. 2006, 99, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.; Jandricic, S.; Frank, S. Ecological interactions affecting the efficacy of Aphidius colemani in greenhouse crops. Insects 2015, 6, 538–575. [Google Scholar] [CrossRef] [PubMed]
- Garantonakis, N.; Perdikis, D.; Lykouressis, D.; Kourti, A.; Gkouvitsas, T. Studies on the identity of the parasitoids Aphidius colemani and Aphidius transcaspicus (Hymenoptera: Braconidae). Eur. J. Entomol. 2009, 106, 491–498. [Google Scholar] [CrossRef]
- Stary, P. Aphidius colemani Viereck: Its taxonomy, distribution and host range (Hymenoptera, Aphidiidae). Acta Entomol. Bohemoslov. 1975, 72, 156–163. [Google Scholar]
- Adisu, B.; Starý, P.; Freier, B.; Büttner, C. Aphidius colemani Vier. (Hymenoptera, Braconidae, Aphidiinae) detected in cereal fields in Germany. J. Pest Sci. 2002, 75, 89–94. [Google Scholar] [CrossRef]
- Starý, P. Field establishment of Aphidius colemani Vier. (Hym., Braconidae, Aphidiinae) in the Czech Republic. J. Appl. Entomol. 2002, 126, 405–408. [Google Scholar] [CrossRef]
- Messing, R.H.; Rabasse, J.M. Oviposition behaviour of the polyphagous aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Aphidiidae). Agric. Ecosyst. Environ. 1995, 52, 13–17. [Google Scholar] [CrossRef]
- Heinz, K.M. Dispersal and dispersion of aphids (Homoptera: Aphididae) and selected natural enemies in spatially subdivided greenhouse environments. Environ. Entomol. 1998, 27, 1029–1038. [Google Scholar] [CrossRef]
- Höller, C.; Borgemeister, C.; Haardt, H.; Powell, W. The relationship between primary parasitoids and hyperparasitoids of cereal aphids: An analysis of field data. J. Anim. Ecol. 1993, 62, 12–21. [Google Scholar] [CrossRef]
- Acebes, A.L.; Messing, R.H. Comparative susceptibility to hyperparasitism of Binodoxys communis and Aphidius colemani, primary aphid parasitoids introduced to Hawaii. Biol. Control 2013, 65, 286–292. [Google Scholar] [CrossRef]
- Budenberg, W.J. Honeydew as a contact kairomone for aphid parasitoids. Entomol. Exp. Appl. 1990, 55, 139–148. [Google Scholar] [CrossRef]
- Boenisch, A.; Petersen, G.; Wyss, U. Influence of the hyperparasitoid Dendrocerus carpenteri on the reproduction of the grain aphid Sitobion avenae. Ecol. Entomol. 1997, 22, 1–6. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Messing, R.H. Development and use of molecular diagnostic tools to determine trophic links and interspecific interactions in aphid–parasitoid communities in Hawaii. Biol. Control 2012, 60, 26–38. [Google Scholar] [CrossRef]
- Plećaš, M.; Gagić, V.; Janković, M.; Petrović-Obradović, O.; Kavallieratos, N.G.; Tomanović, Ž.; Thies, C.; Tscharntke, T.; Ćetković, A. Landscape composition and configuration influence cereal aphid–parasitoid–hyperparasitoid interactions and biological control differentially across years. Agric. Ecosyst. Environ. 2014, 183, 1–10. [Google Scholar] [CrossRef]
- Gagic, V.; Hänke, S.; Thies, C.; Scherber, C.; Tomanović, Ž.; Tscharntke, T. Agricultural intensification and cereal aphid–parasitoid–hyperparasitoid food webs: Network complexity, temporal variability and parasitism rates. Oecologia 2012, 170, 1099–1109. [Google Scholar] [CrossRef]
- Beddington, J.R.; Hammond, P.S. On the dynamics of host-parasite–hyperparasite interactions. J. Anim. Ecol. 1977, 46, 811–821. [Google Scholar] [CrossRef]
- Charles, J.J.; Paine, T.D. Fitness effects of food resources on the polyphagous aphid parasitoid, Aphidius colemani Viereck (Hymenoptera: Braconidae: Aphidiinae). PLoS ONE 2016, 11, e0147551. [Google Scholar] [CrossRef]
- Jado, R.H.; Araj, S.E.; Abu-Irmaileh, B.; Shields, M.W.; Wratten, S.D. Floral resources to enhance the potential of the parasitoid Aphidius colemani for biological control of the aphid Myzus persicae. J. Appl. Entomol. 2019, 143, 34–42. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkenda, P.A.; Ndakidemi, P.A.; Stevenson, P.C.; Arnold, S.E.J.; Belmain, S.R.; Chidege, M.; Gurr, G.M.; Woolley, V.C. Characterization of Hymenopteran Parasitoids of Aphis fabae in An African Smallholder Bean Farming System Through Sequencing of COI ‘Mini-barcodes’. Insects 2019, 10, 331. https://doi.org/10.3390/insects10100331
Mkenda PA, Ndakidemi PA, Stevenson PC, Arnold SEJ, Belmain SR, Chidege M, Gurr GM, Woolley VC. Characterization of Hymenopteran Parasitoids of Aphis fabae in An African Smallholder Bean Farming System Through Sequencing of COI ‘Mini-barcodes’. Insects. 2019; 10(10):331. https://doi.org/10.3390/insects10100331
Chicago/Turabian StyleMkenda, Prisila A., Patrick A. Ndakidemi, Philip C. Stevenson, Sarah E. J. Arnold, Steven R. Belmain, Maneno Chidege, Geoff M. Gurr, and Victoria C. Woolley. 2019. "Characterization of Hymenopteran Parasitoids of Aphis fabae in An African Smallholder Bean Farming System Through Sequencing of COI ‘Mini-barcodes’" Insects 10, no. 10: 331. https://doi.org/10.3390/insects10100331
APA StyleMkenda, P. A., Ndakidemi, P. A., Stevenson, P. C., Arnold, S. E. J., Belmain, S. R., Chidege, M., Gurr, G. M., & Woolley, V. C. (2019). Characterization of Hymenopteran Parasitoids of Aphis fabae in An African Smallholder Bean Farming System Through Sequencing of COI ‘Mini-barcodes’. Insects, 10(10), 331. https://doi.org/10.3390/insects10100331







