Abstract
Wild bees are important pollinators of wild plants and agricultural crops and they are threatened by several environmental stressors including emerging pathogens. Honey bees have been suggested as a potential source of pathogen spillover. One prevalent pathogen that has recently emerged as a honey bee disease is the microsporidian Nosema ceranae. While the impacts of N. ceranae in honey bees are well documented, virtually nothing is known about its effects in solitary wild bees. The solitary mason bee Osmia bicornis is a common pollinator in orchards and amenable to commercial management. Here, we experimentally exposed larvae of O. bicornis to food contaminated with N. ceranae and document spore presence during larval development. We measured mortality, growth parameters, and timing of pupation in a semi-field experiment. Hatched individuals were assessed for physiological state including fat body mass, wing muscle mass, and body size. We recorded higher mortality in the viable-spore-exposed group but could only detect a low number of spores among the individuals of this treatment. Viable-spore-treated individuals with higher head capsule width had a delayed pupation start. No impact on the physiological status could be detected in hatched imagines. Although we did not find overt evidence of O. bicornis infection, our findings indicate that exposure of larvae to viable N. ceranae spores could affect bee development.
1. Introduction
Pollination provided by wild and domesticated bees is essential for wild flowers and agricultural crops. Biodiversity and food security rely on pollinator communities [1,2,3]. Bee population declines have been reported globally [2,4,5,6,7,8,9,10,11,12,13]. Causes for decline include land use changes, habitat loss, and fragmentation [14], pesticide use [15,16,17], pathogens, climate change, invasive species [12,18,19,20], or the interaction of several factors [18,21,22,23,24,25,26,27]. The importance and effectiveness of wild bee pollinators has increasingly been acknowledged in recent years [28]. These studies have emphasized the importance of a diverse community for pollination provision and ecosystem stability. Social bees such as honey bees and bumble bees are regularly used in pollination management, but only one genus of solitary bees is currently used in pollination management in European agriculture, the mason bees in the genus Osmia [29]. In addition, they represent an important group of wild pollinator species and this makes Osmia an interesting species to explore the effects of honey bee pathogens on solitary wild bees.
For honey bees, a variety of pathogens are known. Those comprise viruses, fungi, and bacteria. At least 23 viruses are known from honey bees [30] with several new viruses being discovered every year [31,32,33]. The ectoparasitic mite Varroa destructor and the viruses it transmits, particularly deformed wing virus (DWV), are considered the major cause of elevated honey bee mortality [34,35,36,37,38,39,40,41]. Over the last two decades, the emerging microsporidium Nosema ceranae has also become prevalent globally and has been shown to cause honey bee mortality [42,43,44], although the extent to which it is responsible for colony loss has been debated [45,46]. More recently, the wider risk posed by N. ceranae to sympatric wild bee populations has been explored, with studies revealing transmission of the parasite to bumble bees [47,48,49] and solitary bees such as Osmia bicornis [50]. This indicates frequent transmission potential to a broad range of bee hosts [51].
Pathogen detection alone does not provide information about consequences for host fitness. Defense against pathogens is usually energetically costly because of the upregulation of the immune system of the host resulting in physiological trade-offs [52,53,54,55,56]. Studying life history effects involves assessing their impacts on physiological states like mortality rates or tradeoffs [57].
Physiological states can reveal physiological costs due to an activated immune system. However, the costs can also depend on the host life stage.
Here, we exposed O. bicornis larva experimentally to N. ceranae spores to investigate if this exposure impacts physiological states. To our knowledge, no such experiments in solitary wild bees with N. ceranae have been conducted and nothing is known about life-history effects of this pathogen on solitary bees.
We addressed the following questions: (I) Does exposure with N. ceranae cause detectable establishment of the pathogenin the larval stage of O. bicornis? (II) Does exposure with N. ceranae affect development? and (III) Does exposure with N. ceranae impact the physiological state of the imago?
2. Materials and Methods
2.1. Study Species
O. bicornis is a common solitary wild bee species in Germany with a univoltine lifestyle and a flight period from March to June. The species uses wood cavities or crevices in buildings for nesting [58,59]. O. bicornis is polylectic and favors pollen resources close to the nest site [60,61]. Polylectic bees are forage on a wide variety of unrelated plants. For our experiment we ordered cocoons of O. bicornis from a breeder (BIENENHOTEL at www.bienenhotel.de).
2.2. Ethical Statement
Permissions for the study were provided by the Senate Department for Urban Development and the Environment of Berlin and included the allowance to release Osmia bicornis bees bought from the breeder according to § 40 subsection 4 BNatSchG. Moreover, permission according to § 45 subsection sentence 1 No. 3, sentence 2 BNatSchG for scientific reasons, and permission to catch and kill the bees according to § 44 subsection 1 No. 1 BNatSchG (ibid.) was granted.
2.3. Preparation of Spore Suspension for Inoculation
For our inoculation suspension, fresh Nosema spores from Apis mellifera were required. We collected samples from several hives in and around Berlin and investigated pathogen presence. A gut of a bee was dissected and homogenized in 200 mL of NaCl (0.9%). One drop was investigated microscopically (400×). If spores were present, the solution was processed with the DNeasy Plant Mini Kit from Qiagen (Hilden, Germany) according to the manufacturer’s instructions with the following modifications: 4 µL proteinase K was added with RNAse and heated incubation was extended to 30 min. We added 3–5 metal beads to the samples and disrupted them 3 times for 30 sec at full speed during the incubation period. For the final suspension, we used 30 µL of AE buffer.
For PCR, 5 µL DNA of each sample were mixed with 5 µL RNAse free H2O, 12.5 µL KAPA 2G Fast ReadyMix with dye (Kapabiosystems Roche Diagnostics, Mannheim, Germany) and 2.5 µL of the primers designed by Gisder & Genersch [62] and ordered from Metabion International AG (Planegg, Germany) applying the described PCR program. The amplified products were analyzed in a 1.5% agarose gel stained with SYBR Gold nucleic acid stain (Thermo Fisher Scientific, Darmstadt, Germany) and run for 80 min at 80 V. In each run, a positive control with confirmed N. ceranae bands was included as well as a negative control with ddH2O. Differentiation of the samples was based on presence of a 662 bp band [62]. A subset of the positive samples was sequenced by GATC Biotech and analyzed with BLAST to confirm the identity of the pathogen. If N. ceranae was detected, we chose the honey bees from one colony as spore source for the exposure experiment.
Fresh N. ceranae spore suspension for use in exposure experiments were maintained in laboratory kept groups of A. mellifera workers. Honey bees were kept according to Williams et al. [63]. After hatching, young honey bees were maintained in sterilized cages in groups of 50–200 individuals in a breeding chamber at 28 °C. For the inoculum, we used samples from one of the previously tested hives. We killed fresh bee samples by hand and immediately processed them according to Fries et al. [64] as described before. Ten days post-exposure, up to three bees were killed and processed further by the modified protocol of Fries et al. [64]. The bee gut was homogenized in 300 µL 1 5mM 9.0 pH buffered ammonium chloride (NH4Cl), and centrifuged (Eppendorf centrifuge 5810 R) at 5000 G (relative centrifugal force) for five minutes. The supernatant was discarded and the pellet was resuspended with another 500 µL NH4Cl by vortexing and filtering again. Spores were checked under a microscope with ×400 magnification, counted with a hemocytometer (improved Neubauer chamber) [65] according to Cantwell [66], and molecularly confirmed for species identity as described before. The positive tested suspensions were diluted with a 50% sucrose solution (w/w) to feed honey bee workers for infection. The inocula was diluted with ddH2O and applied to each larvae of O. bicornis aged 2 or 3 days with a concentration of 10,000 viable spores per bee and the same amount of autoclaved spores for the controls. The spore dose was based on guidelines derived from previous infection experiments [67,68,69,70].
2.4. Study Site and Experimental Set-Up
The study site was a 14 ha orchard meadow in Brandenburg, 32 km south of Berlin within the FFH-area Nuthe-Nieplitz-Niederung (N52°21′59.6″, E13°07′53.4″). Eighteen honey bee colonies from a beekeeper were located within a 250 m distance to the nest box. The hives had been at this location for several years. A nest box measuring 1.90 × 1.00 × 0.80 m was constructed. As nests, 60 nest boards accommodating 10 nests each were used with an acrylic, high temperature resistant cover to allow experimental manipulation. The diameter of the entrance was 8 mm, suitable for Osmia bicornis (www.bienenhotel.de, 01.04.2016). Bee cocoons and nest boards were ordered on www.bienenhotel.de and kept in 5 °C until placement in the nest boxes in the field on the 29th of April 2016.
One characteristic for sex determination in O. bicornis is body size with females being generally bigger and heavier than males [71]. We estimated sex via cocoon size. In order to avoid a low return rate of released bees, the recommended 1:1 sex ratio by the breeder was increased to 1.14:1 f:m resulting in an estimated 800 female and 700 male cocoons being placed in the nest boxes.
Bees were observed building brood cells and laying eggs. The hatching date was recorded in order to determine the age of the sampled larvae. Parasites, such as the parasitoids Anthrax anthrax (Schrank, 1781) and Monodontomerus obsoletus (Fabricius) or the parasitic fly Cacoxenus indagator (Loew), were cleared and brood cells with parasites were discarded from the experiment. Temperature and weather conditions were also recorded.
2.5. Treatment with N. ceranae
One µL of the viable N. ceranae spore-containing solution was placed either on the food source (treatment SpF) (N = 229) or directly on the larvae (treatment SpL) (N = 329). Corresponding controls were treated with solutions with autoclaved spore treatments termed CoF (N = 321) and CoL (N = 206), respectively. Food consisted of pollen in the larval brood chamber provided by the mother bee. For SpF and CoF treatments, a droplet of spore suspension was applied on the food directly in front of the larvae. For SpL and CoL treatments, the suspension was directly applied on the larval body on the first segments next to the head capsule. The randomized treatments of larvae were conducted between 13 and 29 May 2016.
2.6. Sampling of Osmia Bee Larvae
Finished brood cells were defined as closed cells with pollen provided and an attached egg and recorded daily between 14 and 29 May (except 18.5., 21.5., 26.5.) The hatching date was observed in order to determine larval age for inoculation for most of the hatched bees.
Between 5 and 16 June, 150 O. bicornis larvae were sampled on the 19th or 20th day post exposure, which corresponds to the 4th larval instar (22–24-days old). From a total of 150 larvae, 12 larvae were discarded due to presence of parasites in the brood cell or death. The sampled larvae were transferred individually to 1.5 mL microcentrifuge tubes and immediately frozen at −20 °C for measurements and dissection.
Physiological States of Osmia Bee Larvae
Head capsule width and fresh body weight of the hatched bee larvae (age 22–24 days) were taken as size measures.
The head capsule width was measured on the widest point with a binocular microscope (Olympus SZX16, ColorView, Olympus) with a caliper (1/50 mm nonius) according to Vogelweith et al. [72]. Referring to Bosch and Vicens [73], the head width constitutes the best estimators of adult weight and provision weight in both sexes besides wing lengths. No pollen for the provision weight was collected due to infestation with a parasitic moth in August, when the bees were in the pupal stage. The analyzed developmental stages mentioned in the following include the larvae, pharate referring to the individual enclosed in the cocoon, and the imago referring to the hatched individual.
2.7. Sampling of Pharates
A total of 526 pharates were sampled as fully developed bees within the cocoon (155–157 days old). The cocoon was gently cleaned by hand with a dry brush to remove feces and debris and stored immediately in a 1.5 mL microcentrifuge tube.
Physiological States of the Pharate
After recording the weight of the pupae, the cocoon was gently opened with a scalpel. The weight of the pharate (without cocoon) was recorded. The sex was determined based on the color of the clypeus [71,74]. Afterwards, the imago was frozen at −80 °C for 12 min to death, the fresh body weight was recorded, and the gut was removed from the body. Both groups, the sampled larvae and the pharates, were further dissected for the microscopic examination of the presence of N. ceranae spores.
2.8. Hatching Record of Imago
In total, 72 control and viable-spore-treated individual pupae were stored at 4 °C over winter and placed outside in two separate cages on 13 April 2017. Night and day temperatures, as well as hatching, were recorded daily. Hatching took place between 3 and 20 May 2017. Remaining cocoons were observed until 29 May when the experiment ended. The unhatched cocoons were counted. The heads of the hatched individuals were frozen at −80 °C. The remaining bee body was stored at −20 °C.
Physiological State of the Imago
We used inter-tegular span as a measure of insect size as it is well correlated with flight range for bees [75]. We also measured fat body content as indicators of immunity and longevity. The fat body stores energy and synthetizes immunoproteins [76].
Fat body content was measured using methods described by Mikolajewski et al. [77] and De Block et al. [78]. The bodies of all hatched individuals were weighed and subsequently dried in an incubator at 48 °C. Afterwards, 1.5 mL of dichlormethane was added. Samples were put on a shaker for 24 hours after which the dichlormethane was removed and the bees weighed again. Fat body mass was determined by substraction.
Body size and flight muscle ratio were measured as described by Plaistow et al. [79]. The dry fatless thorax was placed in 0.2 M potassium hydroxide at room temperature for 48 h which results in digestion of the flight muscle. Afterwards, the remaining cuticle was washed in distilled water, dried, and re-weighed. Flight muscle ratio was determined as the quotient of dry flight muscle mass and total dry abdomen and thorax mass.
2.9. Detection of N. ceranae in O. bicornis in Larvae and Pharate
Larvae (N = 138) were processed either as whole body or separated guts only (Appendix A Table A2). The guts of pharates (N = 507) were dissected as described for honey bees [64]. The guts of the two developmental stages were processed according to Fries et al. [64] as described above with the following modifications. The gut was mixed with 300 µL sterile ddH2O for homogenization and centrifugation. The supernatant was discarded, and the pellet was resuspended with 300 µL sterile ddH2O and filtrated once through cotton wool. Gut suspensions and remaining pharate bodies were frozen.
After spore preparation, presence of N. ceranae spores was checked with a phase contrast microscope (400×). If spores were present, the solution was processed with the DNeasy Plant Mini Kit from Qiagen (Hilden, Germany) as described above.
2.10. Statistical Analysis
All statistical analyses were conducted in R 3.3.4. Data were tested for normal distribution of residuals (larva, pharate) and homogeneity of variance or Shapiro-test (imago) and log-transformed if necessary. Where residuals were normally distributed, the data was tested with linear models. The gaussian family was applied for all models.
2.10.1. Statistical Analysis in Larvae and Pharates
Differences in pupation start and head capsule width were fitted against the explanatory variable “treatment” (viable-spore exposed/control). A linear multi factorial model was also fitted to the data with one-way ANOVA. The ‘pupation start’ (in days) as a response variable was tested, using treatment as a fixed factor and head capsule width as a co-variable. For the larval dataset, ANOVA was applied using head capsule width as the response variable and using treatment as a fixed factor.
Furthermore, larvae as well as the pharate dataset were tested with ANCOVA with head capsule width as response, weight difference between pupae and cocoon as fixed factor, and sex as co-variable. For the pharate dataset, weight difference as a response was tested against treatment as a fixed factor and sex as a co-variable.
Finally, linear regressions were carried out in the larval and the imago datasets by fitting head capsule width against fresh weight or pupal weight, respectively. Another regression analysis was performed to inspect the relationship between head capsule width and fresh body weight in both sexes.
2.10.2. Statistical Analysis of Imaginal Physiological State
Differences between groups were tested using general linear models. The analysis of the hatching rate included all 144 samples. For analysis of physiological states, 11 individuals were excluded from the dataset due to measurement erros resulting in N = 133.
3. Results
From a total of 1592 recorded brood cells, 1085 O. bicornis were treated with either viable or autoclaved N. ceranae spores, of which a total of 783 (138 larvae; 512 pharates; 133 imagines) Osmia bees were sampled for use in analyzing physiological state/developmental statistics, respectively (Table 1). For analyzing mortality from 1085 bees, a sample set of 915 was used. The sex ratio of sampled pharates was calculated from 506 individuals from the pharate dataset (140 female, 366 male). The overall sex ratio for all sampled pharates was 1:2.6 (female:male), including those bees which were parasitized but sex could still be determined from the remains of the head capsule. Of the imago dataset, 67 and 66 hatched imagines in control and viable spore-treated groups were analysed for differences in physiological state.. The spore application method showed no significant effect in the statistical models.

Table 1.
Overview of study sampling.
3.1. Mortality of Infected O. bicornis Larvae and Pharates
From 1085 treated bees, 176 bees died for reasons unrelated to experimental treatment, including predation or parasitism (N = 88). The remaining 88 bees that died during the semi-field experiment were derived from the following treatments; CoF (control on food): N = 22, CoL (control on larvae): N = 14, SpF (spores on food): N = 18, SpL (spores on larvae): N = 34. Nine pharates from the sampled bees were detected as dead when sampled (opened the cocoon). Of the 88 bees, 80 could be assigned to the development stages larvae (69) and pharates (11) with CoF, N = 20, CoL, N = 9, SpF, N = 18, and SpL, N = 33.
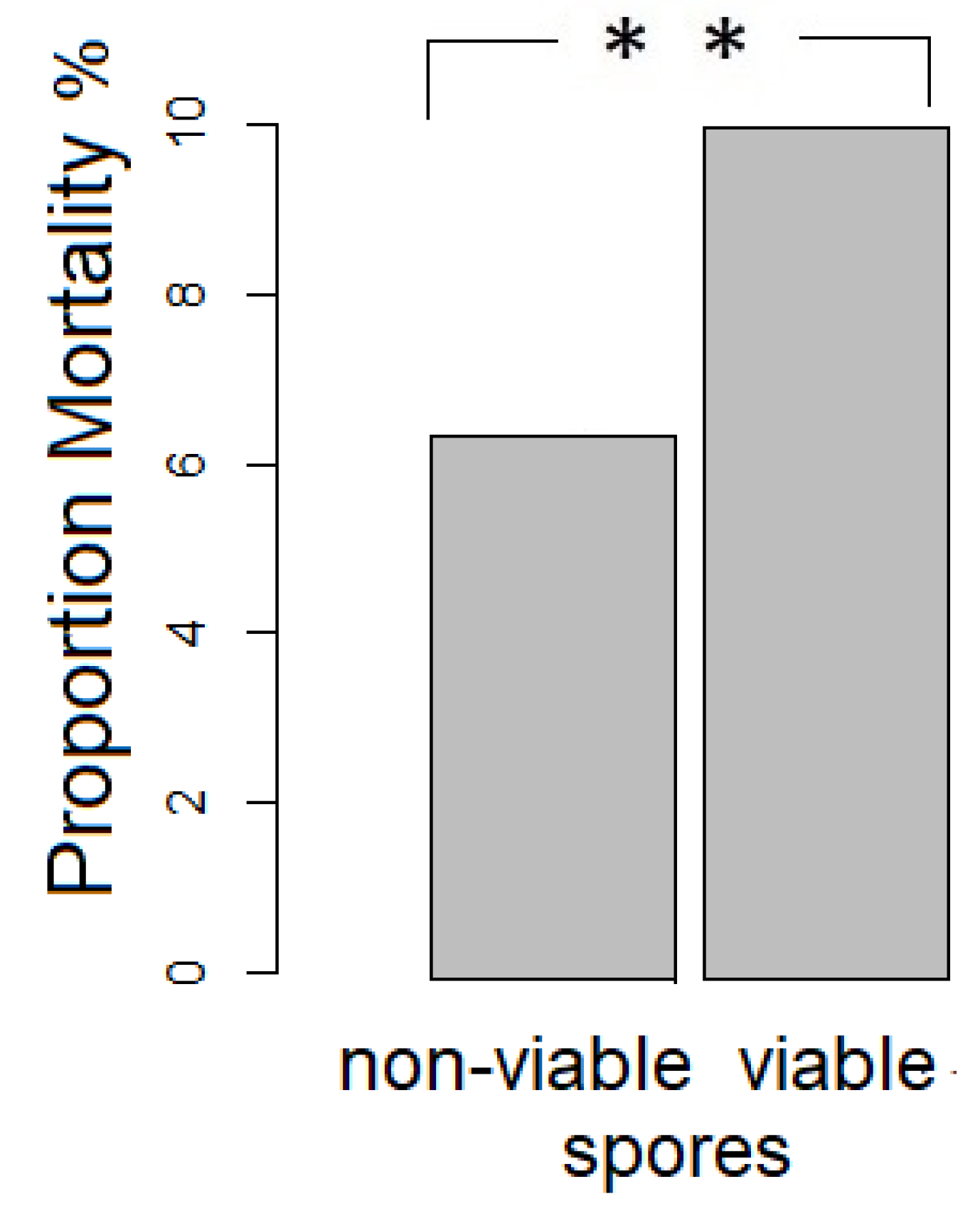
A Pearson‘s Chi-squared test with Yates‘ continuity correction revealed that significantly fewer bees from the control vs. viable spore treatment died when considering both developmental stages (ꭓ-squared = 5.2176, df = 1, p = 0.02236, Figure 1). Within the larval stage fewer bees from the control versus the viable spore treatment had died (ꭓ-squared = 4.3825, df = 1, p = 0.03136), whereas there were no significant differences in the pharate state (ꭓ-squared = 4.3825, df = 1, p = 0.5859).

Figure 1.
Significant differences in mortality between treatment groups including both larval and pharate state (N = 915, deaths non-viable spore exposure: 29, deaths viable spore exposure: 51).
3.2. Detection of N. ceranae Spores
From 545 samples (larvae and pharate), 334 were treated with viable N. ceranae spores. A total of 1.5% (5 bees) of the viable spore treated individuals yielded positive homogenates (positive detection of N. ceranae spores) with <21 spores per 10 µL gut homogenate each.
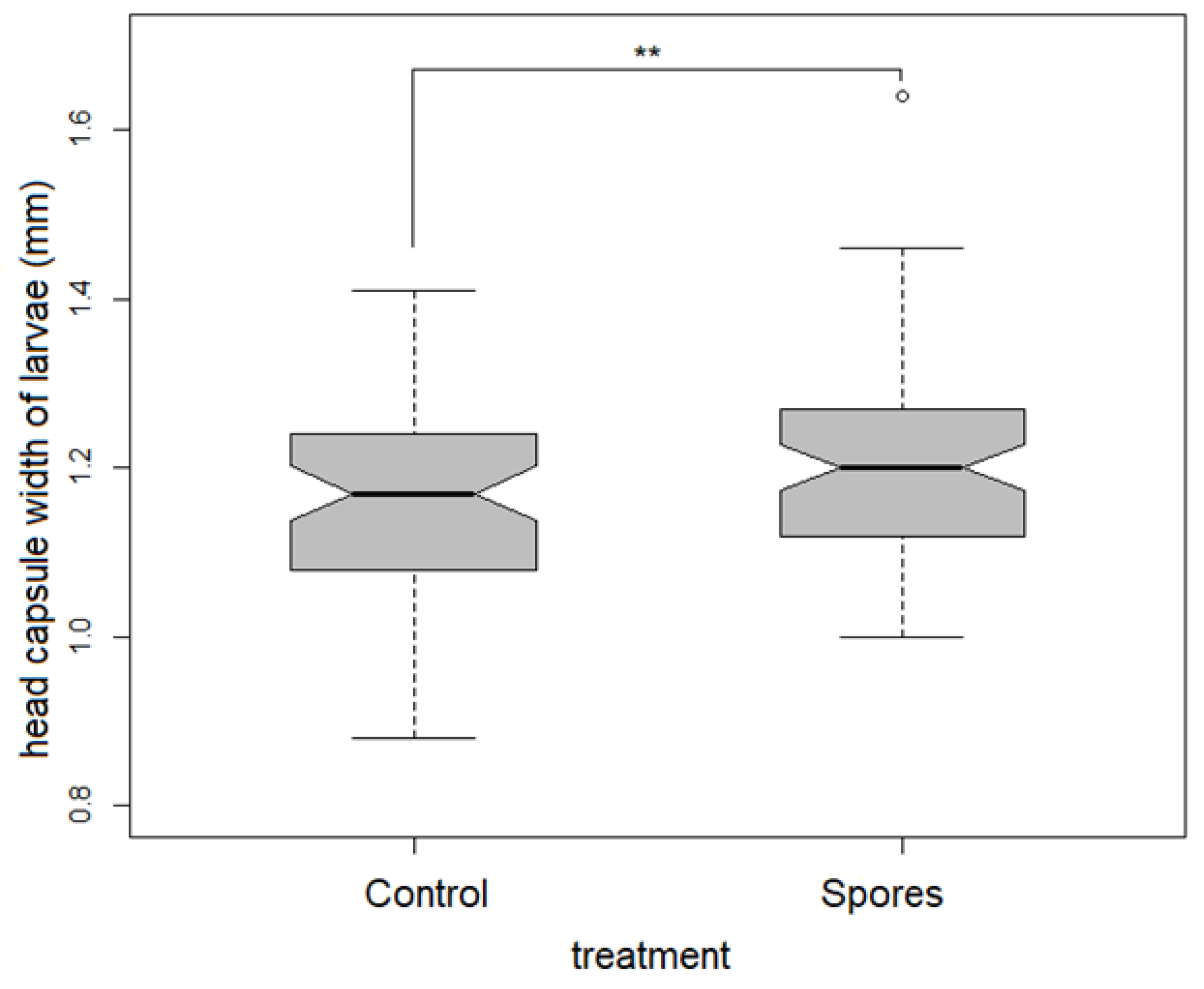
Physiological state: Head capsule width was smallest in the control treatment, both in the larval as well as the pharate dataset. ANCOVA revealed a significantly higher mean head capsule width in the viable spore treated groups in the larval dataset (F1,133 = 6.632, p = 0.011, Table 2, Figure 2).

Table 2.
Results of a linear multi-factorial model exploring the effect of treatment (viable vs. sterile spores) on the pupation start, head capsule width, and weight differences during development and results of regressions of the three datasets for two development stages. Head capsule width, bee age, or sex were used as covariates (C). lm = linear model. A table with estimates can be found in the Appendix A (Table A5).

Figure 2.
Significant differences in head capsule width in treatment groups within the larvae of both sexes.
The body size of the pharate revealed a strong correlation between head capsule width and fresh bee weight (r = 0.782). The strongest positive correlation was detected between cocoon weight and fresh weight (r = 0.995). The head capsule width, pupal weight, and fresh body weight of female pharates was 1.5 times that of males (Table A1, Table A2 and Table A3). The proportion of females in the ‘Spore’ group was 32.2% and 24% in the ‘Control’ group. The proportions differed significantly (ꭓ-squared: 4.808, df = 1, p = 00283).
An analysis of covariance of head capsule width and fresh weight (F2,499 = 950.1, p < 0.001, Table 2) as well as head capsule width and pupal weight (F2,499 = 956.4, p < 0.001, Table 2) with sex as co-variate revealed significant differences between sexes. Regressions of head capsule width to fresh body weight for both sexes are shown in Figure A1 and Figure A2.
3.3. Development Time of the Larvae
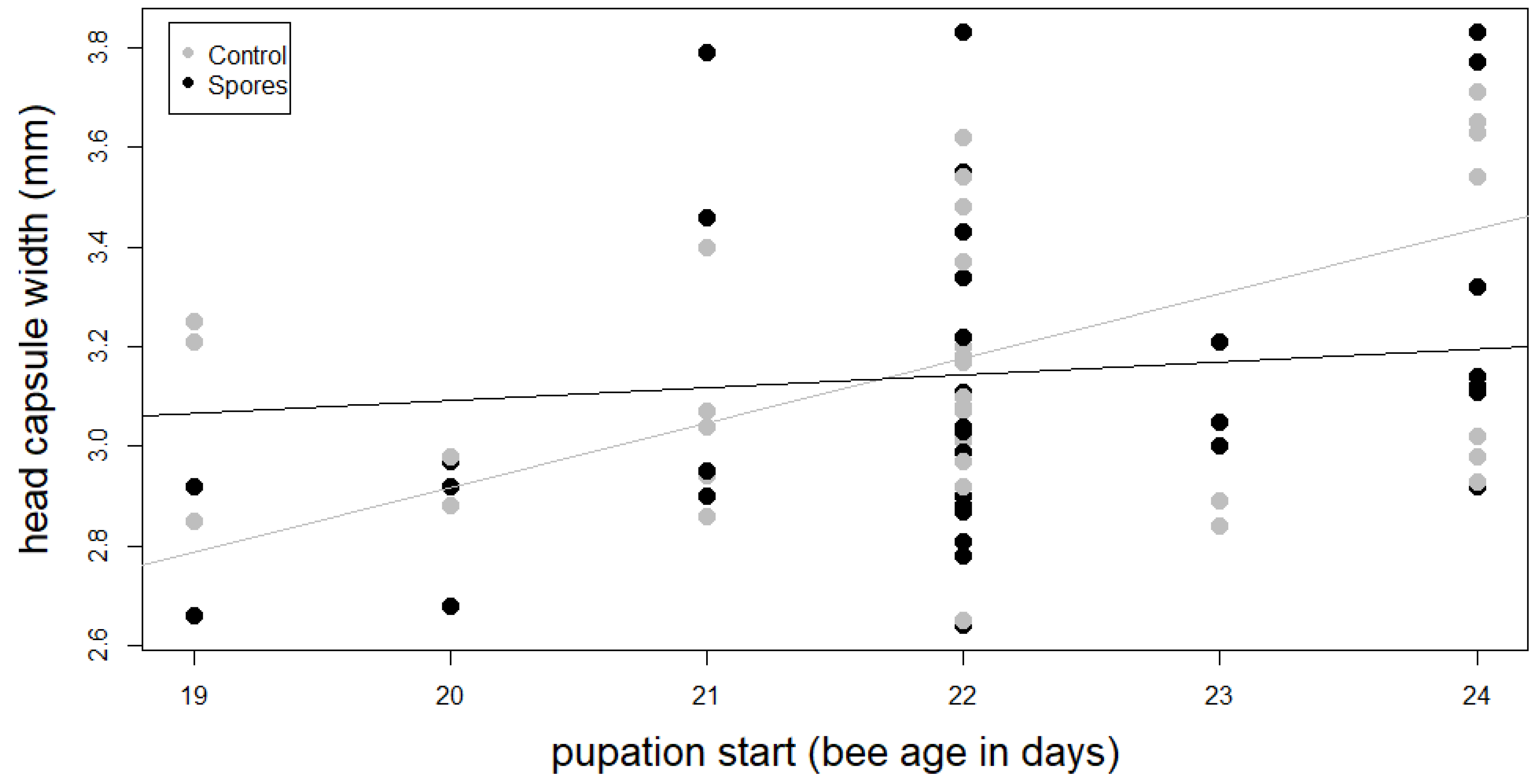
The ANOVA of the transformed linear model revealed a significant interaction between treatment and head capsule width on the onset of pupation (F1,74 = 4.61, p = 0.035, Table 2, Figure 3, Table A4).

Figure 3.
Interaction between head capsule width and pupation start of main treatment groups ‘Control’ and ‘Spores’.
3.4. Physiological State Imago
Adult emergence rate was 93% (sterile spore) and 91.6% (viable spore), N=144. Sterile and viable spore treated groups for the subsequent physiological state analysis contained 26 females and 41 males, and 26 females and 40 males, respectively as individuals with measurement errors were excluded.
Fat content was compared between treated and control males and females. As the data was not normally distributed (males: Shapiro-Test p = 0.03255, females: (Shapiro-Test p = 0.03404).) a general linear model was performed that showed no significant differences in fat content between the treatments in males (F79,81 = 0.257, p = 0.798) or females (F36,52 = 1.72, p = 0.0941). For tegulae size, the data were normally distributed in males (Shapiro-Test p = 0.3696) and females (Shapiro-Test p = 0.5313), but as above with fat body, a linear model indicated no significant difference between the groups (males: F79,81 = 0.4379; females: F36,52 = −1558, p = 0.1281). The wing muscle weight analysis showed no significant difference in males (Shapiro-Test p = 0.01899, glm: F76,78 = 1360, p = 0.178) or females (Shapiro-Test p = 1.03 × 10−4, glm F37,39 = 0.994, p = 0.32667, Table 3).

Table 3.
Physiological state tests of hatched imagines (males/females) from spore-treated and control groups.
4. Discussion
In our study on a solitary bee exposed to a honeybee pathogen during development, we found treatment-dependent life history responses in different physiological states. We detected (1) significantly higher mortality in viable spore-exposed individuals and low spore detection as well as (2) a delayed onset of pupation related to an interactive effect of increased head capsule width and spore-exposure. However, (3) no impact on physiological states of hatched individuals were found.
(1) The higher mortality in the viable spore-treated larvae and pharates could be explained by the high effort of synthesis of storage proteins during larval development. As has been demonstrated in honey bees, triggering a stronger larval immune response can result in lower spore load in adults but can impose a cost in the form of reduced life-span [70]. Moreover, in A. mellifera, N. ceranae impairs its host by damaging intestinal tissue and preventing renewal by inhibiting defense genes [80], using host-ATP for its own growth and reproduction [81]. It is also thought to suppress the host’s immune response [82,83,84,85]. The deprived hosts consequently lack functional tissue, energy necessary for its own metabolic maintenance and can invest less in immune defense. A combination of these processes may explain our findings. For example, in starved bumble bee workers, immune challenge resulted in decreased survival [54]. Furthermore, it has been shown that short-term survival is traded off in order to ensure reproductive success in an ant queen [86].
We found only very few individuals with spores in the cohorts treated with viable spores. The results contrast with infection experiments with N. ceranae on honey bee larvae [70] and Nosema bombi in larvae of Bombus spp. [87]. Differences to other studies might be attributed to initial spore doses, bee species, strains of Nosema, the bee immune responses at different developmental stages, or combinations of these factors [88,89]. We used the same dose of the same species as in one cohort in Eiri et al. [70] where it resulted in spore elevations in larvae in A. mellifera. Our low spore detection in the larvae taken out of the brood cells suggests no establishment of the pathogen. It was demonstrated in honey bees that some individuals exhibited resistance to the pathogen by countering its manipulation of apoptosis and defecating the infected cells [90].
(2) Immune activation and maintenance in infected insect hosts require high nutritional and energetic resources which then cannot be shunted into growth and development [52,91,92,93,94,95]. Thus, we assessed the impact of a treatment with viable spores on the physiological state of growth and development times. The literature results [52,91,92,93,94,95] contrast with our finding of an increased head capsule width in viable-spore-treated individuals. However, an infection with N. ceranae increases hunger level, thus food intake, and could present an explanation [96]. The delayed start of pupation we observed in the viable-spore-cohort with an increased head capsule width could be attributed to a trade-off between investments in defense versus development. Increased pupation periods after immune challenge were reported in a variety of studies such as in the beet armyworm [97], the tobacco caterpillar [98], and cabbage loopers [99].
(3) We found no difference in fat content between viable-spore-treated and control individuals in the hatched imagines. Apart from energy storage, the fat body has an essential role in the immunity of insects. In response to an immune challenge, lipids are mobilized to the haemolyph [100,101]. Lipids are suggested to be used as an energy resource in combating the infection or in membrane biogenesis in haemocytes [102]. Consequently, variations in fat body mass might impact effectiveness of immune response. The present study suggests that exposure with viable Nosema spores does not result in impacts on the investigated physiological state parameters of hatched individuals. Body size, measured through tegulae distance, did not differ either. Body size is determined by conditions during larval development [103]. All bees were provided with an adequate amount of pollen. Our results suggest that the cost of immune defense was paid earlier during development, consistent with the higher mortality in spore-treated larvae.
5. Conclusions
Treatment with viable spores of N. ceranae resulted in higher mortality during the larval stage but spores were only detected in low numbers in a few of the exposed individuals indicating that the pathogen cannot easily establish itself in this species. An interaction between treatment with viable spores and increased head capsule width resulted in delayed pupation start which might be due to immune effects during development, although no impacts on physiological states could be detected in the hatched imagines. Even in the absence of successful infection, exposure to N. ceranae could still compromise the development and life history of O. bicornis.
Author Contributions
Conceptualization K.B., U.M., J.R.; methodology, K.B., U.M.; formal analysis K.B., U.M.; investigation, K.B. (larvae, pharate) and U.M. (adults); resources J.R., D.P.M.; data curation, K.B.; writing—original draft preparation, K.B.; U.M. writing—review and editing, K.B., U.M.; D.P.M.; J.R.; visualization, K.B.; supervision, J.R.; D.P.M.; project administration, K.B.; U.M.; funding acquisition, U.M., J.R.
Funding
This research was funded by the DEUTSCHE BUNDESSTIFTUNG UMWELT (DBU).
Acknowledgments
We would like to thank Erhard Strohm for his sharing his expertise on working with O. bicornis. Many thanks goes to the beekeepers Erwin Biller, Peter Knoll, Marika Harz, Francois Gayte and Benedikt Polaczek for getting honey bees to test them on Nosema ceranae.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Figure A1.
Female head capsule width and fresh body weight (pharate dataset), N = 139.
Figure A1.
Female head capsule width and fresh body weight (pharate dataset), N = 139.
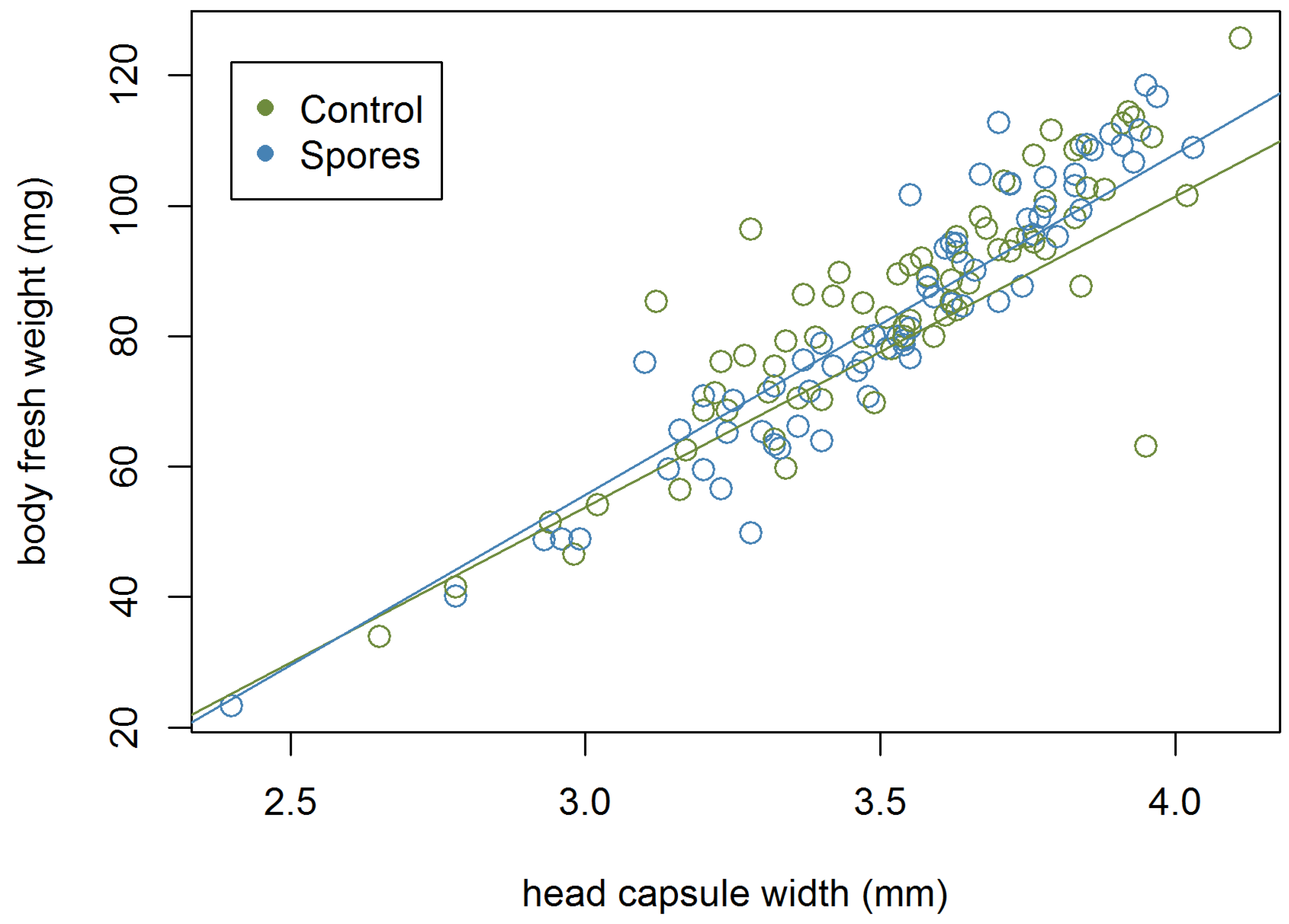

Figure A2.
Male head capsule width and fresh body weight (pharate dataset), N = 363.
Figure A2.
Male head capsule width and fresh body weight (pharate dataset), N = 363.
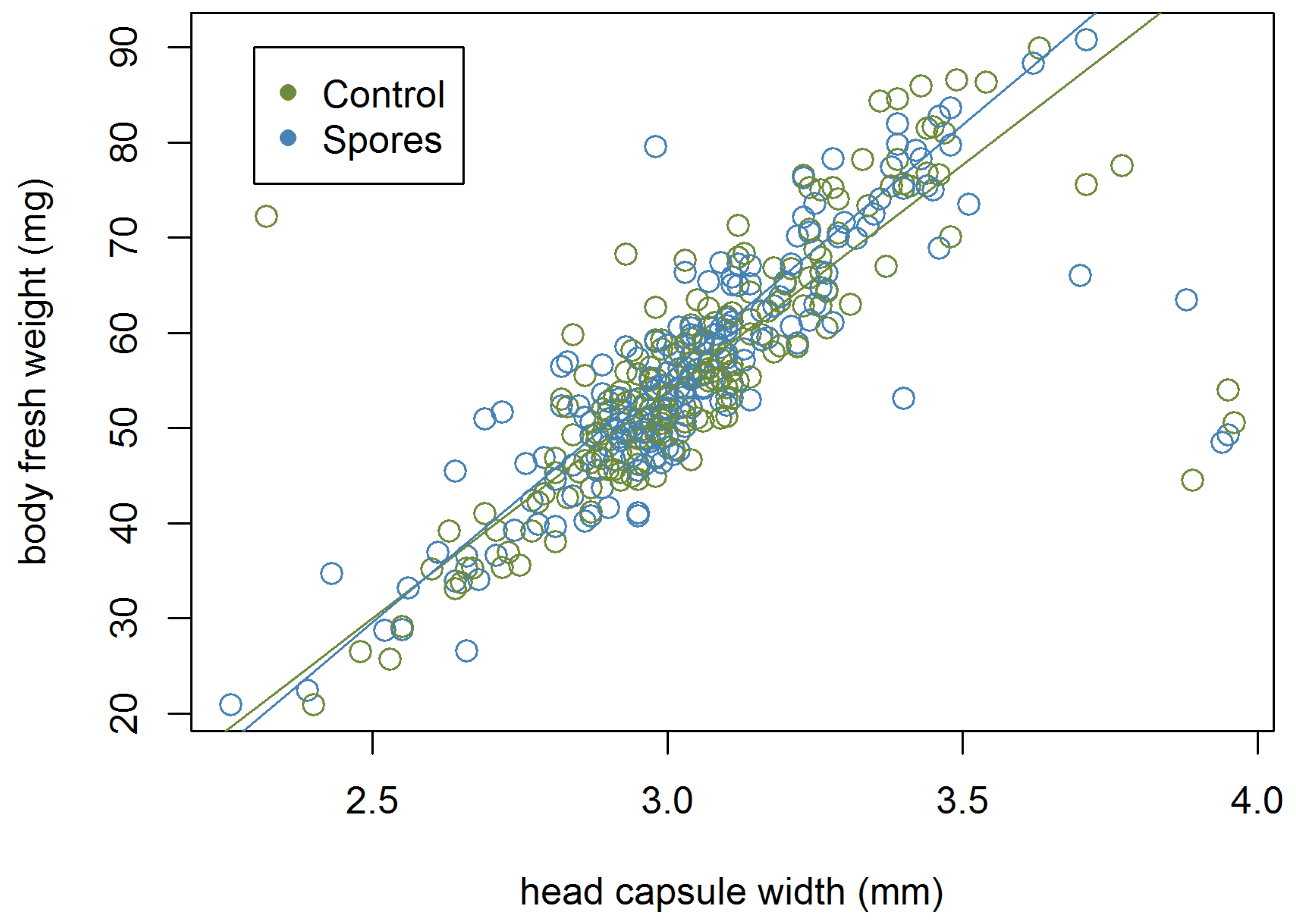

Table A1.
Overview for processed larvae and pharates during sampling.
Table A1.
Overview for processed larvae and pharates during sampling.
| Sample size | Treatment | Count of Processed Part of the Larva for Spore Check | |
|---|---|---|---|
| Sum of larvae | whole larvae | separated larvae (body and gut) | |
| 61 | Control on food | 15 | 18 |
| Control on larva | 20 | 8 | |
| 77 | Spores on food | 35 | 2 |
| Spores on larva | 21 | 19 | |
| Sum of pharate | separated pharate (gut separated for spore check, spore check with n = 407) | ||
| 255 | Control on food | 127 | |
| Control on larva | 128 | ||
| 257 | Spores on food | 129 | |
| Spores on larva | 128 | ||

Table A2.
Measurements dependent on sex within the pharate dataset.
Table A2.
Measurements dependent on sex within the pharate dataset.
| Sex | Kind of Measurement | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| females | head capsule width | 139 | 3.52 | 0.30 | 2.40 | 4.11 |
| pupal weight (mg) | 97.33 | 23.16 | 25.98 | 148.10 | ||
| fresh body weight | 84.22 | 19.31 | 23.29 | 125.8 | ||
| males | head capsule width | 363 | 3.05 | 0.26 | 2.26 | 3.96 |
| pupal weight (mg) | 66.21 | 15.44 | 23.03 | 108.75 | ||
| fresh body weight | 56.28 | 21.155 | 20.89 | 90.83 |

Table A3.
Overview of measurements dependent on sex within the pharate dataset.
Table A3.
Overview of measurements dependent on sex within the pharate dataset.
| Sex | Kind of Measurement | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| females | head capsule width ‘Co’ | 59 | 3.54 | 0.28 | 2.40 | 4.03 |
| head capsule width ‘Sp’ | 80 | 3.51 | 0.32 | 2.65 | 4.11 | |
| pupal weight ‘Co’ | 59 | 97.59 | 21.91 | 25.98 | 131.12 | |
| pupal weight ‘Sp’ | 80 | 97.13 | 24.18 | 37.83 | 148.10 | |
| fresh body weight ‘Co’ | 59 | 84.23 | 18.38 | 23.29 | 112.81 | |
| fresh body weight ‘Sp’ | 80 | 84.22 | 20.08 | 34.01 | 125.80 | |
| males | head capsule width ‘Co’ | 193 | 3.06 | 0.27 | 2.26 | 3.96 |
| head capsule width ‘Sp’ | 170 | 3.05 | 0.24 | 2.32 | 3.89 | |
| pupal weight ‘Co’ | 193 | 64.78 | 15.10 | 23.03 | 108.75 | |
| pupal weight ‘Sp’ | 170 | 67.83 | 15.70 | 25.89 | 102.14 | |
| fresh body weight ‘Co’ | 193 | 55.18 | 12.32 | 20.89 | 90.83 | |
| fresh body weight ‘Sp’ | 170 | 57.53 | 12.78 | 22.43 | 85.92 |

Table A4.
Dispersion of dataset history for the set of pupation start.
Table A4.
Dispersion of dataset history for the set of pupation start.
| Treatment | Dataset/Treatment | N | Mean | SD | Min | Max |
| Complete dataset | 87 | 22.0 | 1.4 | 19 | 24 | |
| Control | Control on food | 24 | 22.1 | 1.4 | 19 | 24 |
| Control on larva | 23 | 21.5 | 1.3 | 19 | 24 | |
| Control sum | 47 | 21.8 | 1.4 | 19 | 24 | |
| Spores | Spores on food | 28 | 22.5 | 1.5 | 19 | 24 |
| Spores on larva | 12 | 21.8 | 0.5 | 21 | 22 | |
| Spores sum | 40 | 22.3 | 1.3 | 19 | 24 |

Table A5.
Results of a linear multi factorial model exploring the effect of treatment (Viable vs Sterile spores) on the pupation start, head capsule width and weight differences during development and results of regressions of the three datasets for two development stages. Head capsule width, bee age or sex were used as covariates (C). lm = lineaer model, Estimate abbreviations: c.weight = cocoon weight, head = head capsule width, m = male Sp = Spores.
Table A5.
Results of a linear multi factorial model exploring the effect of treatment (Viable vs Sterile spores) on the pupation start, head capsule width and weight differences during development and results of regressions of the three datasets for two development stages. Head capsule width, bee age or sex were used as covariates (C). lm = lineaer model, Estimate abbreviations: c.weight = cocoon weight, head = head capsule width, m = male Sp = Spores.
| Samples | Depen-Dent Variable | Independent Variable | Co-variate (C)/Interaction (I) | Num. d. f. | F-Value | p-Value | Estimate |
|---|---|---|---|---|---|---|---|
| pharate (history) | pupation start | treatment, inoculation type | C: head Capsule width | 73 | 4.304 | 0.0035 | Head 1.44769 |
| pharate (history) | pupation start | treatment | I: head Capsule width | 74 | 6.521 | 0.0005 | treatSp 6.8983 Head 2.5875 treatSp:head −2.0463 |
| larvae | head capsule width | treatment | 133 | 6.632 | 0.011 | treatSp 0.05150 | |
| pharate | head capsule width | fresh weight | C: sex | 499 | 950.1 | < 0.001 | Weight 0.0149293 Sexm −0.0552998 |
| pharate | head capsule width | cocoon weight | C: sex | 499 | 956.4 | < 0.001 | c.weight 0.0123092 sexm −0.0894422 |
| pharate | Fresh weight | cocoon weight | C: sex | 499 | 6.292e+04 | < 0.001 | c.weight 0.82060 sexm −2.40835 |
| pharate | weight difference | treatment | C: sex | 500 | 3.934 | 0.0479 | teatmentSp 0.6557 |
References
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services IPBES. Full Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Imperatriz Fonseca, V.L., Potts, G.S., Baste, I.A., Apau Oteng Yeboah, A., Eds.; IPBES: Bonn, Germany, 2016. [Google Scholar]
- Stokstad, E. The Case of the Empty Hives. Science 2007, 316, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, B.P. What’s Killing American Honey Bees? PLoS Biol. 2007, 5, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; Criado, M.G.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Neumann, P.; Carreck, P. Honey Bee Colony Losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L.; Robinson, G.E. Patterns of Widespread Decline in North American Bumble Bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Fitzpatrick, Ú.; Murray, T.E.; Paxton, R.J.; Breen, J.; Cotton, D.; Santorum, V.; Brown, M.J.F. Rarity and Decline in Bumblebees—A Test of Causes and Correlates in the Irish Fauna. Biol. Conserv. 2007, 136, 185–194. [Google Scholar] [CrossRef]
- Bartomeus, I.; Ascher, J.S.; Gibbs, J.; Danforth, B.N.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical Changes in Northeastern US Bee Pollinators Related to Shared Ecological Traits. Proc. Natl. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Garratt, M.P. Threats to an Ecosystem Service: Pressures on Pollinators. Front. Ecol. Environ. 2013, 251–259. [Google Scholar] [CrossRef]
- Brown, M.J.F.F.; Paxton, R.J. The Conservation of Bees: A Global Perspective. Apidologie 2009, 40, 410–416. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schafers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherland. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Kremen, C. Are Ecosystem Services Stabilized by Differences among Species? A Test Using Crop Pollination. Proc. R. Soc. B 2009, 276, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed Coating with a Neonicotinoid Insecticide Negatively Affects Wild Bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Isaac, N.J.B.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of Neonicotinoid Use on Long-Term Population Changes in Wild Bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Dicks, L.V.; Paxton, R.J.; Baldock, K.C.R.; Barron, A.B.; Chauzat, M.-P.; Freitas, B.M.; Goulson, D.; Jepsen, S.; Kremen, C.; et al. A Horizon Scan of Future Threats and Opportunities for Pollinators and Pollination. PeerJ 2016, 4, e2249. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Sci. Express 2015, 347, 1–16. [Google Scholar] [CrossRef]
- Sánchez-bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are Bee Diseases Linked to Pesticides? A Brief Review. 2016, 90, 7–11. [Google Scholar]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, 1–13. [Google Scholar] [CrossRef]
- Brunner, F.S.; Schmid-Hempel, P.; Barribeau, S.M. Protein-Poor Diet Reduces Host-Specific Immune Gene Expression in Bombus Terrestris. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140128. [Google Scholar] [CrossRef] [PubMed]
- Bryden, J.; Gill, R.J.; Mitton, R.A.A.; Raine, N.E.; Jansen, V.A.A. Chronic Sublethal Stress Causes Bee Colony Failure. Ecol. Lett. 2013, 16, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid Clothianidin Adversely Affects Insect Immunity and Promotes Replication of a Viral Pathogen in Honey Bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Doublet, V.; Labarussias, M.; De Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under Stress: Sublethal Doses of a Neonicotinoid Pesticide and Pathogens Interact to Elevate Honey Bee Mortality across the Life Cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef]
- Manley, R.; Boots, M.; Wilfert, L. Condition-dependent Virulence of Slow Bee Paralysis Virus in Bombus Terrestris: Are the Impacts of Honeybee Viruses in Wild Pollinators Underestimated? Oecologia 2017, 184, 305–315. [Google Scholar] [CrossRef]
- Garibaldi, L.; Steffen-Dewenter, I.; Winfree, R.; Al, E. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Bosch, J.; Bosch, J.; Kemp, W.P. Developing and Establishing Bee Species as Crop Pollinators: The Example of Osmia Spp. (Hymenoptera: Megachilidae) and Fruit Trees. Bull. Entomol. Res. 2002, 92, 3–16. [Google Scholar]
- McMenamin, A.J.; Genersch, E. Honey Bee Colony Losses and Associated Viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a Honey Bee Pathogen: First Report of a Third Master Variant of the Deformed Wing Virus Quasispecies. ISME J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef]
- Remnant, E.J.; Shi, M.; Buchmann, G.; Blacquière, T.; Holmes, E.C.; Beekman, M.; Ashe, A. A Diverse Range of Novel RNA Viruses in Geographically Distinct Honey Bee Populations. J. Virol. 2017, 91, e00158-17. [Google Scholar] [CrossRef]
- Galbraith, D.A.; Fuller, Z.L.; Ray, A.M.; Brockmann, A.; Frazier, M.; Gikungu, M.W.; Martinez, J.F.I.; Kapheim, K.M.; Kerby, J.T.; Kocher, S.D.; et al. Investigating the Viral Ecology of Global Bee Communities with High-Throughput Metagenomics. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Budge, G.E.; Pietravalle, S.; Brown, M.; Laurenson, L.; Jones, B.; Tomkies, V.; Delaplane, K.S. Pathogens as Predictors of Honey Bee Colony Strength in England and Wales. PLoS ONE 2015, 10, e0133228. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A Mutualistic Symbiosis between a Parasitic Mite and a Pathogenic Virus Undermines Honey Bee Immunity and Health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-Virus Interaction in Collapsing Honey Bee Colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E. Honey Bee Pathology: Current Threats to Honey Bees and Beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Highfield, A.C.; El Nagar, A.; Mackinder, L.C.M.; Noël, L.M.L.J.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed Wing Virus Implicated in Overwintering Honeybee Colony Losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Vedova, G.D.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Nguyen, B.K.; Ribière, M.; vanEngelsdorp, D.; Snoeck, C.; Saegerman, C.; Kalkstein, A.L.; Schurr, F.; Brostaux, Y.; Faucon, J.-P.; Haubruge, E. Effects of Honey Bee Virus Prevalence, Varroa Destructor Load and Queen Condition on Honey Bee Colony Survival over the Winter in Belgium. J. Apic. Res. 2011, 50, 195–202. [Google Scholar] [CrossRef]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema Ceranae Has Infected Apis Mellifera in Europe since at Least 1998 and May Be More Virulent than Nosema Apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Natsopoulou, M.E.; Doublet, V.; Paxton, R.J. European Isolates of the Microsporidia Nosema Apis and Nosema Ceranae Have Similar Virulence in Laboratory Tests on European Worker Honey Bees. Apidologie 2016, 47, 57–65. [Google Scholar] [CrossRef][Green Version]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; García-Palencia, P.; Meana, A. Detection of Infective Nosema Ceranae (Microsporidia) Spores in Corbicular Pollen of Forager Honey bees. J. Invertebr. Pathol. 2008, 97, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Hedtke, K.; Mockel, N.; Frielitz, M.C.; Linde, A.; Genersch, E. Five-Year Cohort Study of Nosema Spp. in Germany: Does Climate Shape Virulence and Assertiveness of Nosema Ceranae? Appl. Environ. Microbiol. 2010, 76, 3032–3038. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.M.; Puerta, F.; Cousinou, M.; Dios-Palomares, R.; Campano, F.; Redondo, L. Asymptomatic Presence of Nosema Spp. in Spanish Commercial Apiaries. J. Invertebr. Pathol. 2012, 111, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease Associations between Hone ybees and Bumblebees as a Threat to Wild Pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef]
- Graystock, P.; Yates, K.; Darvill, B.; Goulson, D.; Hughes, W.O.H. Emerging Dangers: Deadly Effects of an Emergent Parasite in a New Pollinator Host. J. Invertebr. Pathol. 2013, 114, 114–119. [Google Scholar] [CrossRef]
- Graystock, P.; Yates, K.; Evison, S.E.F.; Darvill, B.; Goulson, D.; Hughes, W.O.H. The Trojan Hives: Pollinator Pathogens, Imported and Distributed in Bumblebee Colonies. J. Appl. Ecol. 2013, 50, 1207–1215. [Google Scholar] [CrossRef]
- Ravoet, J.; De Smet, L.; Meeus, I.; Smagghe, G.; Wenseleers, T.; de Graaf, D.C. Widespread Occurrence of Honey Bee Pathogens in Solitary Bees. J. Invertebr. Pathol. 2014, 122, 55–58. [Google Scholar] [CrossRef]
- Shafer, A.B.A.; Williams, G.R.; Shutler, D.; Rogers, R.E.L.; Stewart, D.T.; Journal, T.; Shafer, B.A.; Williams, R.; Shutler, D.; Stewart, T. Cophylogeny of Nosema (Microsporidia: Nosematidae) and Bees (Hymenoptera: Apidae) Suggests Both Cospeciation and a Host-Switch Reviewed Work (s): Published by: The American Society of Parasitologists Content in a Trusted Digital Archive. We Use. J. Parasitol. 2009, 95, 198–203. [Google Scholar] [CrossRef]
- Sheldon, B.C.; Verhulst, S. Ecological Immunology: Costly Parasiet Defences and Trade-Offs in Evolutionary Ecology. Tree 1996, 5347, 317–321. [Google Scholar] [CrossRef]
- Armitage, S.A.O.; Thompson, J.J.W.; Rolff, J. Examining Costs of Induced and Constitutive Immune Investment in Tenebrio Molitor. J. Evol. Biol. 2003, 16, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Moret, Y.; Schmid-Hempel, P. Survival for Immunity: The Price of Immune System Activation for Bumblebee Workers. Science 2000, 290, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Variation in Immune Defence as a Question of Evolutionary Ecology. Proc. R. Soc. B Biol. Sci. 2003, 270, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kirschman, L.J.; Crespi, E.J.; Warne, R.W. Critical Disease Windows Shaped by Stress Exposure Alter Allocation Trade-Offs between Development and Immunity. J. Anim. Ecol. 2018, 87, 235–246. [Google Scholar] [CrossRef]
- Stearns, S.C. Life History Evolution: Successes, Limitations, and Prospects. Naturwissenschaften 2000, 87, 476–486. [Google Scholar] [CrossRef]
- Strohm, E. How Can Cleptoparasitic Drosophilid Flies Emerge from the Closed Brood Cells of the Red Mason Bee? Physiol. Entomol. 2011, 36, 77–83. [Google Scholar] [CrossRef]
- Kornmilch, J.-C. Einsatz von Mauerbienen Zur Bestäubung von Obstkulturen—Handbuch Zur Nutzung Der Roten Mauerbiene in Obstplantagen Und Kleingärten. Available online: http://www.bund-lemgo.de/download/Handbuch_der_Mauerbienenzucht.pdf (accessed on 16 September 2019).
- Westrich, P. Die Wildbienen Baden-Würtembergs, 1. Teil: Lebensräume, Verhalten, Ökologie und Schutz, 2. Spezieller Teil: Die Gattungen und Arten; Eugen Ulmer Verlag: Stuttgart, Germany, 1989; p. 972. [Google Scholar]
- Radmacher, S.; Strohm, E. Effects of Constant and Fluctuating Temperatures on the Development of the Solitary Bee Osmia Bicornis (Hymenoptera: Megachilidae). Apidologie 2011, 42, 711–720. [Google Scholar] [CrossRef]
- Gisder, S.; Genersch, E. Molecular Differentiation of Nosema Apis and Nosema Ceranae Based on Species–Specific Sequence Differences in a Protein Coding Gene. J. Invertebr. Pathol. 2013, 113, 1–6. [Google Scholar] [CrossRef]
- Williams, G.R.; Alaux, C.; Costa, C.; Csáki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P.; et al. Standard Methods for Maintaining Adult Apis Mellifera in Cages under in vitro Laboratory Conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard Methods for Nosema Research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Human, H.; Brodschneider, R.; Dietemann, V.; Dively, G.; Ellis, J.D.; Forsgren, E.; Fries, I.; Hatjina, F.; Hu, F.L.; Jaffé, R.; et al. Miscellaneous Standard Methods for Apis Mellifera Research. J. Apic. Res. 2013, 52, 1–56. [Google Scholar] [CrossRef]
- Cantwell, G.E. Standard Methods for Counting Nosema Spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Fries, I. Nosema Ceranae in European Honey Bees (Apis Mellifera). J. Invertebr. Pathol. 2010, 103 (Suppl. 1), S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E.; Fries, I. Comparative Virulence of Nosema Ceranae and Nosema Apis in Individual European Honey Bees. Vet. Parasitol. 2010, 170, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of Pollen Dilution on Infection of Nosema Ceranae in Honey Bees. J. Insect Physiol. 2016, 87, 12–19. [Google Scholar] [CrossRef]
- Eiri, D.M.; Suwannapong, G.; Endler, M.; Nieh, J.C. Nosema Ceranae Can Infect Honey Bee Larvae and Reduces Subsequent Adult Longevity. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Raw, A. The Biology of the Solitary Bee Osmia Rufa (L.) Megachilidae. Trans. R. Entemological Soc. Lond. 1972, 124, 213–229. [Google Scholar] [CrossRef]
- Vogelweith, F.; Thiery, D.; Moret, Y.; Moreau, J. Immunocompetence Increases with Larval Body Size in a Phytophagous Moth. Physiol. Entomol. 2013, 38, 219–225. [Google Scholar] [CrossRef]
- Bosch, J.; Vicens, N. Body Size as an Estimator of Production Costs in a Solitary Bee. Ecol. Entomol. 2002, 27, 129–137. [Google Scholar] [CrossRef]
- Amiet, F.; Krebs, A. Bienen Mitteleuropas. Gattungen, Lebensweise, Beobachtung. 2.korrigierte Auflage; Haupt Verlag: Bern, Switzerland, 2014; p. 424. [Google Scholar]
- Cane, J.H. Estimation of Bee Size Using Intertegular Span ( Apoidea ). J. Kansas Entomol. Soc. 1987, 60, 145–147. [Google Scholar]
- Amdam, G.V.; Omholt, S.W. The Regulatory Anatomy of Honeybee Lifespan. J. Theor. Biol. 2002, 216, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewski, D.J.; De Block, M.; Stoks, R. The Interplay of Adult and Larval Time Constraints Shapes Species Differences in Larval Life History. Ecology 2015, 96, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- De Block, M.; Slos, S.; Johansson, F.; Stoks, R. Integrating Life History and Physiology to Understand Latitudinal Size Variation in a Damselfly. Ecography (Cop.) 2008, 31, 115–123. [Google Scholar] [CrossRef]
- Plaistow, S.; Siva-Jothy, M.T. Energetic Constraints and Male Mate-Securing Tactics in the Damselfly Calopteryx Splendens Xanthostoma (Charpentier). Proc. R. Soc. B Biol. Sci. 1996, 263, 1233–1239. [Google Scholar]
- Dussaubat, C.; Sagastume, S.; Gómez-Moracho, T.; Botías, C.; García-Palencia, P.; Martín-Hernández, R.; Le Conte, Y.; Higes, M. Comparative Study of Nosema Ceranae (Microsporidia) Isolates from Two Different Geographic Origins. Vet. Microbiol. 2013, 162, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Hacker, C.; Howell, M.; Bhella, D.; Lucocq, J. Strategies for Maximizing ATP Supply in the Microsporidian Encephalitozoon Cuniculi: Direct Binding of Mitochondria to the Parasitophorous Vacuole and Clustering of the Mitochondrial Porin VDAC. Cell. Microbiol. 2014, 16, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune Suppression in the Honey Bee (Apis Mellifera) Following Infection by Nosema Ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Huang, Q.; Kryger, P.; Le Conte, Y.; Moritz, R.F.A. Survival and Immune Response of Drones of a Nosemosis Tolerant Honey Bee Strain towards N. Ceranae Infections. J. Invertebr. Pathol. 2012, 109, 297–302. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome Analyses of the Honeybee Response to Nosema Ceranae and Insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-Sequence Analysis of Gene Expression from Honey bees (Apis Mellifera) Infected with Nosema Ceranae. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef]
- Baer, B.; Armitage, S.A.O.; Boomsma, J.J. Sperm Storage Induces an Immunity Cost in Ants. Nature 2006, 441, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P.; Loosli, R. A Contribution to the Knowledge of Nosema Infections in Bumble Bees, Bombus Spp. Apidologie 1998, 29, 525–535. [Google Scholar] [CrossRef][Green Version]
- Higes, M.; Juarranz, Á.; Dias-Almeida, J.; Lucena, S.; Botías, C.; Meana, A.; García-Palencia, P.; Martín-Hernández, R. Apoptosis in the Pathogenesis of Nosema Ceranae (Microsporidia: Nosematidae) in Honey Bees (Apis Mellifera). Environ. Microbiol. Rep. 2013, 5, 530–536. [Google Scholar] [CrossRef]
- Fontbonne, R.; Garnery, L.; Vidau, C.; Aufauvre, J.; Texier, C.; Tchamitchian, S.; El Alaoui, H.; Brunet, J.L.; Delbac, F.; Biron, D.G. Comparative Susceptibility of Three Western Honeybee Taxa to the Microsporidian Parasite Nosema Ceranae. Infect. Genet. Evol. 2013, 17, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kurze, C.; Routtu, J.; Moritz, R.F.A. Parasite Resistance and Tolerance in Honey bees at the Individual and Social Level. Zoology 2016, 119, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lochmiller, R.L.; Deerenberg, C. Trade-Offs in Evolutionary Immunology: Just what is the Cost of Immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Moret, Y.; Sciences, P.; Sheffield, U.; Bank, W.; Tn, S.U.K. Explaining Ariable Costs of the Immune Response: Selection for Specific 7 Ersus Non—Specific Immunity and Facultati 7 e Life History Change. Oikos 2003, 102, 213–216. [Google Scholar] [CrossRef]
- Rowley, A.F.; Powell, A.; Rowley, A.F.; Powell, A. Invertebrate Immune Systems—Specific, Quasi-Specific, or Nonspecific? J. Immunol. 2007, 179, 7209–7214. [Google Scholar] [CrossRef]
- Zanchi, C.; Troussard, J.; Martinaud, G. Differential Expression and Costs between Maternally and Paternally Derived Immune Priming for Offspring in an Insect. J. Anim. Ecol. 2011, 2010, 1174–1183. [Google Scholar] [CrossRef]
- Laughton, A.M.; Boots, M.; Siva-Jothy, M.T. The Ontogeny of Immunity in the Honey Bee, Apis Mellifera L. Following an Immune Challenge. J. Insect Physiol. 2011, 57, 1023–1032. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic Stress in the Honeybee Apis Mellifera from Nosema Ceranae Infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Cabodevilla, O.; Villar, E.; Virto, C.; Murillo, R.; Williams, T.; Caballero, P. Intra- and Intergenerational Persistence of an Insect Nucleopolyhedrovirus: Adverse Effects of Sublethal Disease on Host Development, Reproduction, and Susceptibility to Superinfection. Appl. Environ. Microbiol. 2011, 77, 2954–2960. [Google Scholar] [CrossRef]
- Monobrullah, M.; Shankar, U. Sub-Lethal Effects of Splt MNPV Infection on Developmental Stages of Spodoptera Litura (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 2008, 18, 431–437. [Google Scholar] [CrossRef]
- Milks, M.L.; Burnstyn, I.; Myers, J.H. Influence of Larval Age on the Lethal and Sublethal Effects of the Nucleopolyhedrovirus of Trichoplusia Ni in the Cabbage Looper. Biol. Control 1998, 126, 119–126. [Google Scholar] [CrossRef]
- Mullen, L.; Goldsworthy, G. Birkbeck EPrints: An Open Access Repository of the Research Output of Birkbeck College Mullen, Lisa and Goldsworthy, Graham (2003). Changes in Lipophorins Are Related to the Activation of Phenoloxidase in the Haemolymph of Locusta Migratoria in Respo. Insect Biochem. Mol. Biol. 2003, 33, 661–670. [Google Scholar] [CrossRef]
- Cheon, H.; Shin, S.W.; Bian, G.; Park, J.; Raikhel, A.S. Regulation of Lipid Metabolism Genes, Lipid Carrier Protein Lipophorin, and Its Receptor during Immune Challenge in the Mosquito Aedes Aegypti. J. Biol. Chem. 2006, 281, 8426–8435. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B. Thermoregulation in Bumblebees. J. Comp. Physiol. 1974, 88, 129–140. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).