Characterization of a Vitellogenin Receptor in the Bumblebee, Bombus lantschouensis (Hymenoptera, Apidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. RNA Extraction and Synthesis of cDNA
2.3. Molecular Cloning of BLVgR
2.4. Sequence Analysis
2.5. BLVgR Expression Analysis Using Quantitative RT-PCR
2.6. RNA Interference
2.7. Data Analysis
3. Results
3.1. Full-Length cDNA of BLVgR
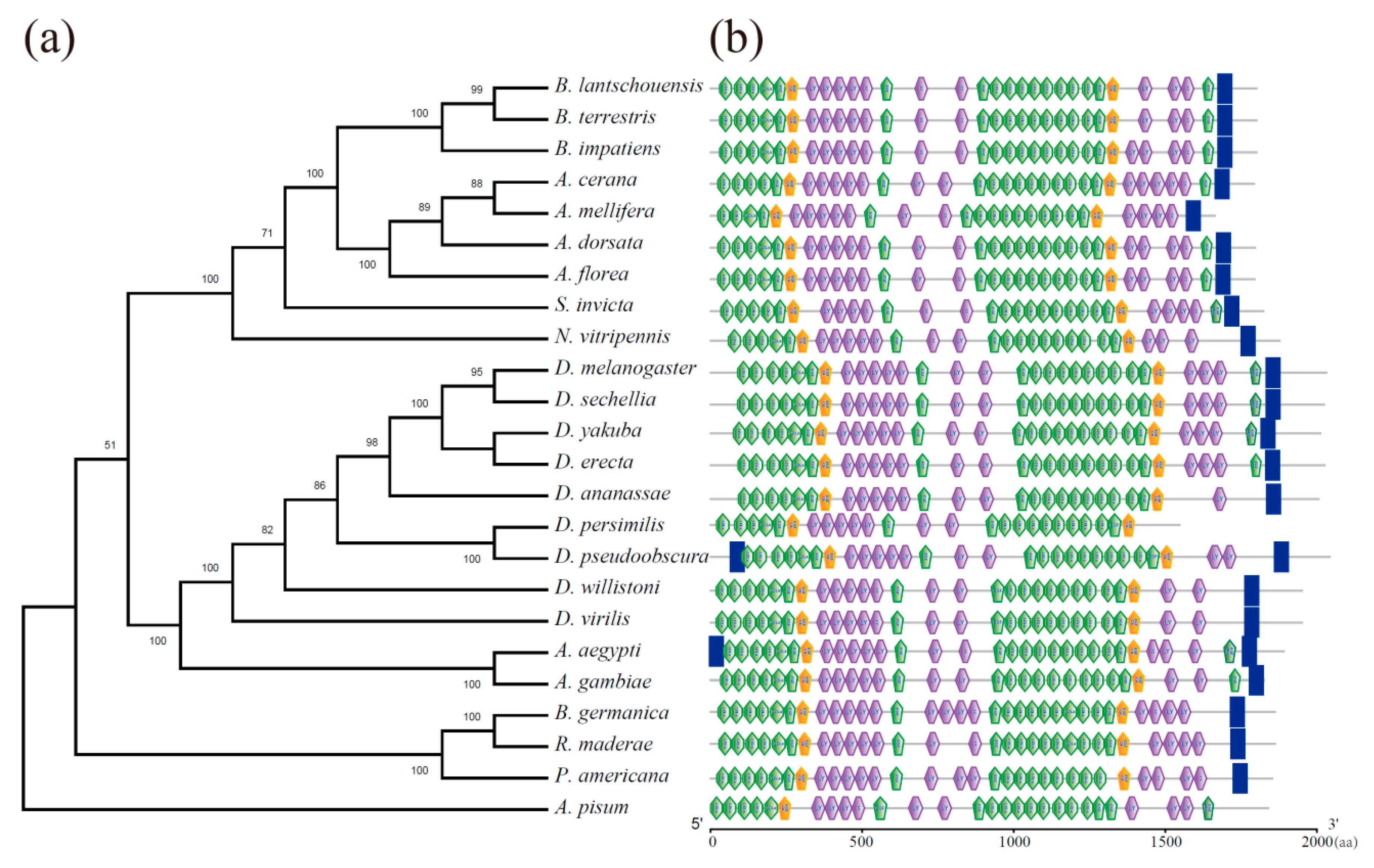
3.2. Sequence and Phylogenetic Analysis of BLVgR
3.3. Expression Patterns of BLVgRs in Different Tissues, Developmental Stages and Reproductive Status
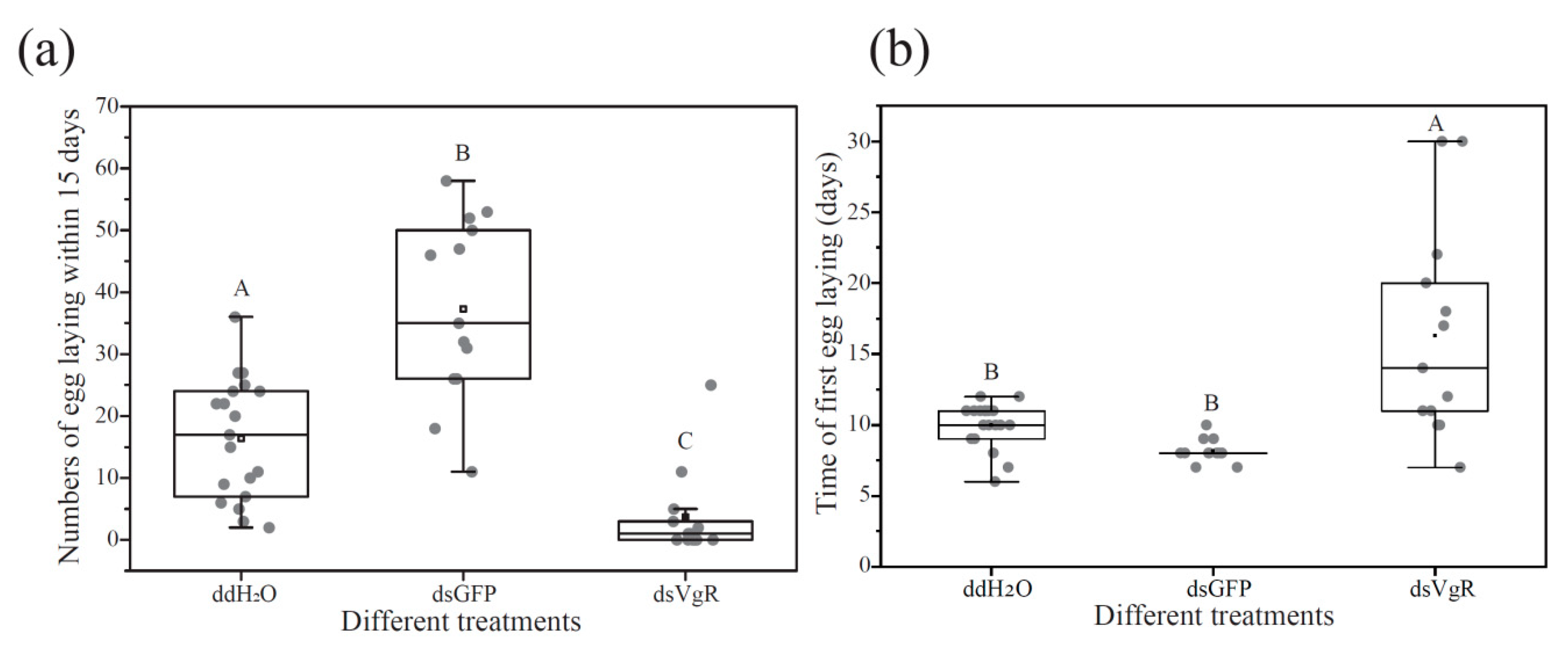
3.4. RNA Interference
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velthuis, H.; Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef] [Green Version]
- Pywell, R.F.; Warman, E.A.; Carvell, C.; Sparks, T.H.; Dicks, L.V.; Bennett, D.; Wright, A.; Critchley, C.N.R.; Sherwood, A. Providing foraging resources for bumblebees in intensively farmed landscapes. Biol. Conserv. 2005, 121, 479–494. [Google Scholar] [CrossRef]
- Roldan Serrano, A.; Guerra-Sanz, J.M. Quality fruit improvement in sweet pepper culture by bumblebee pollination. Sci. Hortic. 2006, 110, 160–166. [Google Scholar] [CrossRef]
- Morandin, L.A.; Laverty, T.M.; Kevan, P.G. Effect of bumble bee (Hymenoptera: Apidae) pollination intensity on the quality of greenhouse tomatoes. J. Econ. Entomol. 2001, 94, 172–179. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 88–104. [Google Scholar] [CrossRef]
- Raikhel, A.S.; Dhadialla, T.S. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 1992, 37, 217–251. [Google Scholar] [CrossRef]
- Engelmann, F. Insect vitellogenin: Identification, biosynthesis, and role in vitellogenesis. Adv. Insect Physiol. 1979, 14, 49–108. [Google Scholar]
- Amdam, G.V.; Norberg, K.; Fondrk, M.K.; Page, R.E. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl. Acad. Sci. USA 2004, 101, 11350–11355. [Google Scholar] [CrossRef] [Green Version]
- Corona, M.; Libbrecht, R.; Wurm, Y.; Riba-Grognuz, O.; Keller, L. Vitellogenin underwent subfunctionalization to acquire caste and behavioral specific expression in the harvester ant Pogonomyrmex barbatus. PLoS Genet. 2013, 9, e1003730. [Google Scholar] [CrossRef]
- Cardoso Junior, C.; Oldroyd, B.; Ronai, I. Vitellogenin expression in the ovaries of adult honeybee workers provides insights into the evolution of reproductive and social traits. bioRxiv 2019, 547760. [Google Scholar] [CrossRef] [Green Version]
- Röhrkasten, A.; Ferenz, H. Properties of the vitellogenin receptor of isolated locust oocyte membranes. Int. J. Invertebr. Reprod. Dev. 1986, 10, 133–142. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, F.; Guo, J.; Yi, J.; Liu, J.; Cao, Y.; Lai, X.; Zhang, G. Molecular characterization and expression of vitellogenin and vitellogenin receptor of Thitarodes pui (Lepidoptera: Hepialidae), an insect on the Tibetan Plateau. J. Insect Sci. 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Meng, Y.; Wang, Y.X.; Luo, J.; Katsuma, S.; Yang, C.W.; Banno, Y.; Kusakabe, T.; Shimada, T.; Xia, Q.Y. Vitellogenin receptor mutation leads to the oogenesis mutant phenotype scanty vitellin of the silkworm, Bombyx Mori. J. Biol. Chem. 2013, 288, 13345–13355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.R.; Katagiri, C.; Nagao, E.; Chino, H. Purification and characterization of vitellogenin from the American cockroach, Periplaneta americana. Comp. Biochem. Physiol. B Comp. Biochem. 1992, 103, 963–967. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, L.; Xiao, H.; Xie, B.; Smagghe, G.; Guo, Y.; Liang, G. Molecular characterization and function analysis of the vitellogenin receptor from the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera, Noctuidae). PLoS ONE 2016, 11, e0155785. [Google Scholar] [CrossRef] [Green Version]
- Tufail, M.; Takeda, M. Molecular cloning, characterization and regulation of the cockroach vitellogenin receptor during oogenesis. Insect Mol. Biol. 2005, 14, 389–401. [Google Scholar] [CrossRef]
- Lu, K.; Shu, Y.; Zhou, J.; Zhang, X.; Zhang, X.; Chen, M.; Yao, Q.; Zhou, Q.; Zhang, W. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stål). J. Insect Physiol. 2015, 73, 20–29. [Google Scholar] [CrossRef]
- Chen, M.E.; Lewis, D.K.; Keeley, L.L.; Pietrantonio, P.V. cDNA cloning and transcriptional regulation of the vitellogenin receptor from the imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Insect Mol. Biol. 2004, 13, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Ciudad, L.; Piulachs, M.D.; Bellés, X. Systemic RNAi of the cockroach vitellogenin receptor results in a phenotype similar to that of the Drosophila yolkless mutant. FEBS J. 2006, 273, 325–335. [Google Scholar] [CrossRef]
- Lu, H.; Vinson, S.B.; Pietrantonio, P.V. Oocyte membrane localization of vitellogenin receptor coincides with queen flying age, and receptor silencing by RNAi disrupts egg formation in fire ant virgin queens. FEBS J. 2009, 276, 3110–3123. [Google Scholar] [CrossRef]
- Guidugli-Lazzarini, K.R.; Do Nascimento, A.M.; Tanaka, É.D.; Piulachs, M.D.; Hartfelder, K.; Bitondi, M.G.; Simões, Z.L.P. Expression analysis of putative vitellogenin and lipophorin receptors in honey bee (Apis mellifera L.) queens and workers. J. Insect Physiol. 2008, 54, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Simões, Z.L.; Guidugli, K.R.; Norberg, K.; Omholt, S.W. Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnol. 2003, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C.T.; Davy, M.W.; MacDiarmid, R.M.; Plummer, K.M.; Birch, N.P.; Newcomb, R.D. RNA interference in the light brown apple moth, Epiphyas postvittana (Walker) induced by double-stranded RNA feeding. Insect Mol. Biol. 2006, 15, 383–391. [Google Scholar] [CrossRef]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Costa, C.P.; Elias-Neto, M.; Falcon, T.; Dallacqua, R.P.; Martins, J.R.; Bitondi, M.M.G. RNAi-mediated functional analysis of bursicon genes related to adult cuticle formation and tanning in the honeybee, Apis mellifera. PLoS ONE 2016, 11, e0167421. [Google Scholar] [CrossRef]
- Marco, A.D.; Guidugli-Lazzarini, K.R.; Do, N.A.; Simoes, Z.L.; Hartfelder, K. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften 2008, 95, 953–961. [Google Scholar] [CrossRef]
- Garbian, Y.; Maori, E.; Kalev, H.; Shafir, S.; Sela, I. Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PLoS Pathog. 2012, 8, e1003035. [Google Scholar] [CrossRef] [Green Version]
- An, J.D.; Huang, J.X.; Shao, Y.Q.; Zhang, S.W.; Wang, B.; Liu, X.Y.; Wu, J.; Williams, P.H. The bumblebees of North China (Apidae, Bombus Latreille). Zootaxa 2014, 3830, 1–89. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zhang, H.; Liang, C.; Zou, Y.; Dong, J.; Yuan, X.; Huang, J.; An, J. Foraging preference of the honeybee Apis mellifera and the bumblebee Bombus lantschouensis (Hymenoptera: Apidae) in peach greenhouse. Acta Entomol. Sin. 2015, 58, 1315–1321. [Google Scholar]
- Gurel, F.; Gosterit, A. Effects of different stimulation methods on colony initiation and development of Bombus terrestris L. (Hymenoptera: Apidae) queens. Appl. Entomol. Zool. 2008, 43, 113–117. [Google Scholar] [CrossRef]
- Han, L.; Ding, G.; Liu, Y.; Huang, J.; Wu, J. Characterization of sphingomyelin phosphodiesterase expression in bumblebee (Bombus lantschouensis). J. Insect Sci. 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Almagro, A.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Ivica, L.; Tobias, D.; Peer, B. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2011, 40, D302–D305. [Google Scholar]
- Sudhir, K.; Glen, S.; Li, M.; Christina, K.; Koichiro, T. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 6, 1547–1549. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 4, 783–791. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Yao, Q.; Zhang, J.; Dong, X.; Tian, H.; Chen, J.; Zhang, W. Feeding-based RNA interference of atrehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2010, 19, 777–786. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Li, J.; Huang, J.; Wu, J. Identification of suitable reference genes for miRNA quantitation in bumblebee (Hymenoptera: Apidae) response to reproduction. Apidologie 2019, 50, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Dohanik, V.T.; Gonçalves, W.G.; Oliveira, L.L.; Zanuncio, J.C.; Serrão, J.E. Vitellogenin transcytosis in follicular cells of the honeybee Apis mellifera and the wasp Polistes simillimus. Protoplasma 2018, 255, 1703–1712. [Google Scholar] [CrossRef]
- Boldbaatar, D.; Battsetseg, B.; Matsuo, T.; Hatta, T.; Umemiya-Shirafuji, R.; Xuan, X.; Fujisaki, K. Tick vitellogenin receptor reveals critical role in oocyte development and transovarial transmission of Babesia parasite. Biochem. Cell Biol. 2008, 86, 331–344. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular cloning and developmental expression pattern of the vitellogenin receptor from the cockroach, Leucophaea maderae. Insect Biochem. Mol. Biol. 2007, 37, 235–245. [Google Scholar] [CrossRef]
- Chen, S.; Lee, T.; Ou, Y. Incorporating significant amino acid pairs to identify O-linked glycosylation sites on transmembrane proteins and non-transmembrane proteins. BMC Bioinform. 2010, 11, 536. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Yang, W.; Jiang, X.; Niu, J.; Shen, G.; Ran, C.; Wang, J. The essential role of vitellogenin receptor in ovary development and vitellogenin uptake in Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 2015, 16, 18368–18383. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, N.; Chapman, R.W.; Lindzey, J.K.; Haynes, M.R.; Sullivan, C.V. Molecular characterization and expression of vitellogenin receptor from white perch (Morone americana). Biol. Reprod. 2004, 70, 1720–1730. [Google Scholar] [CrossRef] [Green Version]
- Piulachs, M.D.; Guidugli, K.R.; Barchuk, A.R.; Cruz, J.; Simoes, Z.L.; Belles, X. The vitellogenin of the honey bee, Apis mellifera: Structural analysis of the cDNA and expression studies. Insect Biochem. Mol. Biol. 2003, 33, 459–465. [Google Scholar] [CrossRef]
- Herz, J.; Gotthardt, M.; Willnow, T.E. Cellular signalling by lipoprotein receptors. Curr. Opin. Lipidol. 2000, 11, 161–166. [Google Scholar] [CrossRef]
- Gotthardt, M.; Trommsdorff, M.; Nevitt, M.F.; Shelton, J.; Richardson, J.A.; Stockinger, W.; Nimpf, J.; Herz, J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 2000, 275, 25616–25624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huang, J.; Cai, W.; Zhao, Z.; Peng, W.; Wu, J. The vitellogenin of the bumblebee, Bombus hypocrita: Studies on structural analysis of the cDNA and expression of the mRNA. J. Comp. Physiol. B 2010, 180, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Raikhel, A.S. Organization and developmental expression of the mosquito vitellogenin receptor gene. Insect Mol. Biol. 2001, 10, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Lee, K.S.; Yoon, H.J.; Kim, I.; Li, J.; Sohn, H.D.; Jin, B.R. Expression profile of the iron-binding proteins transferrin and ferritin heavy chain subunit in the bumblebee Bombus ignitus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 165–170. [Google Scholar] [CrossRef]
- Nunes, F.M.F.; Aleixo, A.C.; Barchuk, A.R.; Bomtorin, A.D.; Grozinger, C.M.; Simões, Z.L.P. Non-target effects of green fluorescent protein (GFP)-derived double-stranded RNA (dsRNA-GFP) used in honey bee RNA interference (RNAi) assays. Insects 2013, 4, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Duncan, E.J.; Hyink, O.; Dearden, P.K. Notch signalling mediates reproductive constraint in the adult worker honeybee. Nat. Commun. 2016, 7, 12427. [Google Scholar] [CrossRef] [Green Version]
- Genissel, A.; Aupinel, P.; Bressac, C.; Tasei, J.N.; Chevrier, C. Influence of pollen origin on performance of Bombus terrestris micro-colonies. Entomol. Exp. Appl. 2010, 104, 329–336. [Google Scholar] [CrossRef]
- Babendreier, D.; Reichhart, B.; Romeis, J.; Bigler, F. Impact of insecticidal proteins expressed in transgenic plants on bumblebee microcolonies. Entomol. Exp. Appl. 2010, 126, 148–157. [Google Scholar] [CrossRef]
- Dance, C.; Botías, C.; Goulson, D. The combined effects of a monotonous diet and exposure to thiamethoxam on the performance of bumblebee micro-colonies. Ecotoxicol. Environ. Saf. 2017, 139, 194–201. [Google Scholar] [CrossRef]



| Purpose | Name | Primer Sequence (5′–3′) |
|---|---|---|
| cDNA cloning | VgRF | ACTCATGTTTGTGCCAACCTG |
| VgRR | TTGGATGGTACAGATCGAAGG | |
| RACE | 5′VgR9F | TCGAGGTGCAAGCATGAGGATCAGTT |
| 3′VgR9R | GCGTTTCTTTGGCATGTTACGCTCT | |
| UPM-Long | CTAATACGACTCACTATAGC | |
| UPM-Short | TCACCGCATTCATCTTCC | |
| Real-time PCR | FVgRF | GTGTGCCTGTTATCTAATGCTGAT |
| FVgRR | TTCATCTTCACCGTTAGGACAATC | |
| β-actinF | CGACTACCTCATGAAGATT | |
| β-actinR | CGACGTAACAAAGTTTCTC | |
| GAPDH-F | GCTGGAGCTGAATATGTTGTAGAATC | |
| GAPDH-R | AGTAGTGCAGGAAGCATTAGAGATAACT | |
| RNAi | RNAiVgR1F | GTTTCAATGTAAAAACGGCGACT |
| RNAiVgR1R | TCGTTCTTTGGACAATCTGTAACG | |
| T7RNAiVgR1F | GGATCCTAATACGACTCACTATAGGGTTTCAATGTAAAAACGGCGACT | |
| T7RNAiVgR1R | GGATCCTAATACGACTCACTATAGGTCGTTCTTTGGACAATCTGTAACG | |
| GFP-F | CCACAAGTTCAGCGTGTCCG | |
| GFP-R | AAGTTCACCTTGATGCCGTTCT | |
| T7GFP-F | GGATCCTAATACGACTCACTATAGCCACAAGTTCAGCGTGTCCG | |
| T7GFP-R | GGATCCTAATACGACTCACTATAGAAGTTCACCTTGATGCCGTTCT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Wang, M.; Li, J.; He, S.; Huang, J.; Wu, J. Characterization of a Vitellogenin Receptor in the Bumblebee, Bombus lantschouensis (Hymenoptera, Apidae). Insects 2019, 10, 445. https://doi.org/10.3390/insects10120445
Du L, Wang M, Li J, He S, Huang J, Wu J. Characterization of a Vitellogenin Receptor in the Bumblebee, Bombus lantschouensis (Hymenoptera, Apidae). Insects. 2019; 10(12):445. https://doi.org/10.3390/insects10120445
Chicago/Turabian StyleDu, Lin, Mingming Wang, Jilian Li, Shaoyu He, Jiaxing Huang, and Jie Wu. 2019. "Characterization of a Vitellogenin Receptor in the Bumblebee, Bombus lantschouensis (Hymenoptera, Apidae)" Insects 10, no. 12: 445. https://doi.org/10.3390/insects10120445
APA StyleDu, L., Wang, M., Li, J., He, S., Huang, J., & Wu, J. (2019). Characterization of a Vitellogenin Receptor in the Bumblebee, Bombus lantschouensis (Hymenoptera, Apidae). Insects, 10(12), 445. https://doi.org/10.3390/insects10120445





