Brown Rice Vinegar as an Olfactory Field Attractant for Drosophila suzukii (Matsumura) and Zaprionus indianus Gupta (Diptera: Drosophilidae) in Cherimoya in Maui, Hawaii, with Implications for Attractant Specificity between Species and Estimation of Relative Abundance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trap Assembly
2.2. Attractant Solutions
2.3. Trap Installation and Maintenance
2.4. Species Identification
2.5. Experimental Design
2.6. Statistical Analysis
3. Results
3.1. Drosophila suzukii: Mean Captures and Attractant Specificity
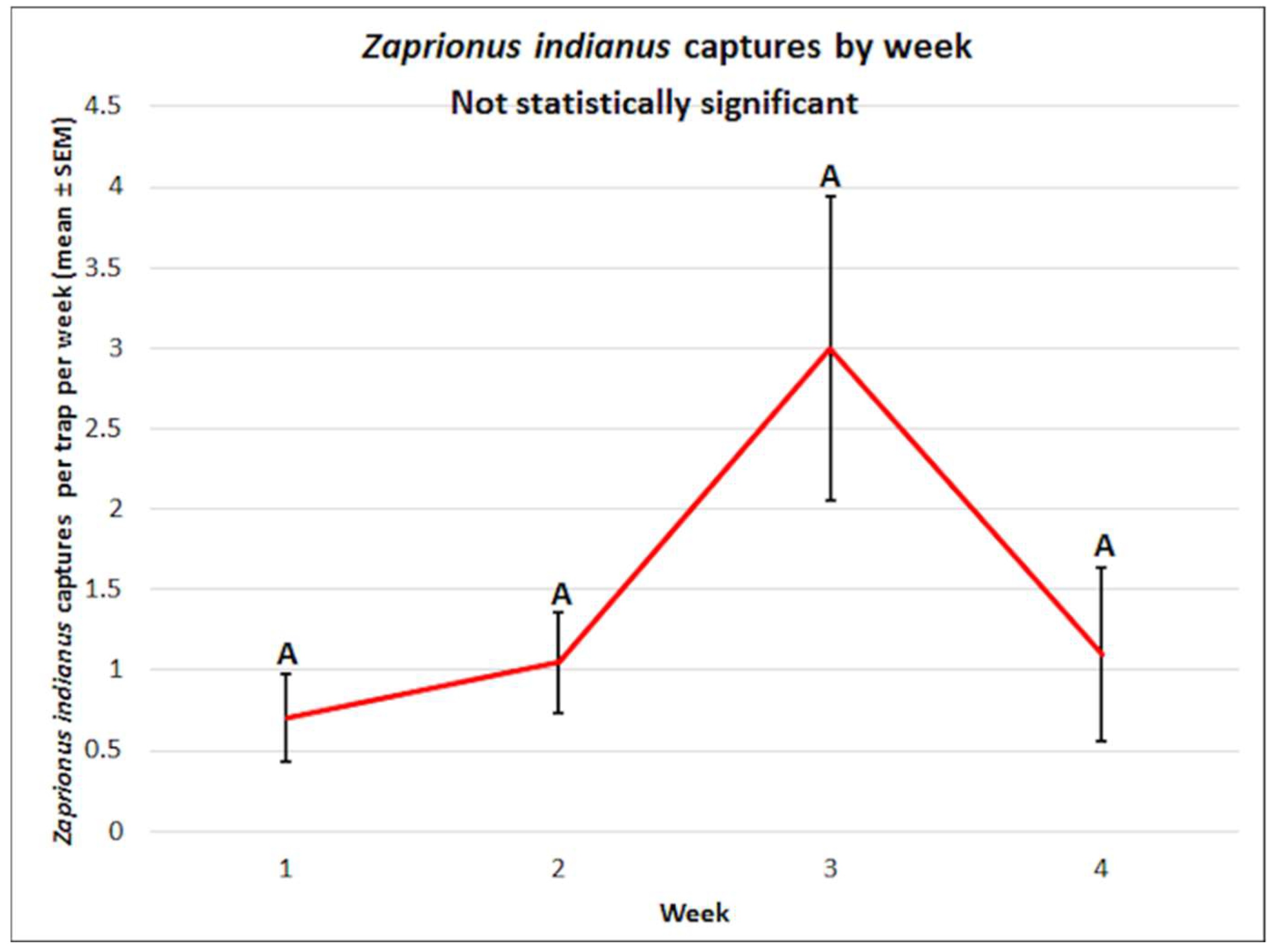
3.2. Zaprionus indianus: Mean Captures and Attractant Specificity
3.3. Non-Target Drosophilid: Mean Captures and Attractant Specificity
3.4. Estimated Relative Abundance of D. suzukii, Z. indianus, and Non-Target Drosophilids in Cherimoya
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hauser, M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Lasa, R.; Tadeo, E. Invasive drosophilid pests Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in Veracruz, Mexico. Fla. Entomol. 2015, 98, 987–988. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; Cristina De Toni, D.; Valente, V.L.S. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Süss, L.; Costanzi, M. Presence of Drosophila suzukii (Matsumura, 1931) (Diptera Drosophilidae) in Liguria (Italy). J. Entomol. Acarol. Res. Ser. II 2010, 42, 185–188. [Google Scholar] [CrossRef]
- Calabria, G.; Maca, J.; Bächli, G.; Serra, L.; Pascual, M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Toševski, I.; Milenković, S.; Krstić, O.; Kosovac, A.; Jakovljević, M.; Mitrović, M.; Cvrković, T.; Jović, J. Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae), a new invasive pest in Serbia. Plant Prot. 2014, 65, 99–103. [Google Scholar] [CrossRef]
- Kaneshiro, K.Y. Drosophila (Sophophora) suzukii (Matsumura). Notes and exhibitions. Proc. Hawaii. Entomol. Soc. 1983, 24, 179. [Google Scholar]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.; Chu, D.; Daane, K.M.; Gilbert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Westphal, M.I.; Browne, M.; MacKinnon, K.; Noble, I. The link between international trade and the global distribution of invasive alien species. Biol. Invasions 2008, 10, 391–398. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, transport, and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Wiman, N.G.; Walton, V.M.; Dalton, D.T.; Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S.; et al. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef] [PubMed]
- Da Mata, R.A.; Tidon, R.; Côrtes, L.G.; De Marco, P., Jr.; Diniz-Filho, J.A.F. Invasive and flexible: Niche shift in the drosophilid Zaprionus indianus (Insecta, Diptera). Biol. Invasions 2010, 12, 1231–1241. [Google Scholar] [CrossRef]
- Silva-Soares, N.F.; Nogueira-Alves, A.; Mirth, C.K. Adaption to new nutritional environments: Larval performance, foraging decisions, and adult oviposition choices in Drosophila suzukii. BMC Ecol. 2017, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Nava, D.E.; Nascimento, A.M.; Stein, C.P.; Haddad, M.L.; Bento, J.M.S.; Parra, J.R.P. Biology, thermal requirements, and estimation of the number of generations of Zaprionus indianus (Diptera: Drosophilidae) for the main fig producing regions of Brazil. Fla. Entomol. 2007, 90, 495–501. [Google Scholar] [CrossRef]
- Revadi, S.; Lebreton, S.; Witzgall, P.; Anfora, G.; Dekker, T.; Becher, P. Sexual behavior of Drosophila suzukii. Insects 2015, 6, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 2016, 73, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Stamps, J.A.; Yang, L.H.; Morales, V.M.; Boundy-Mills, K.L. Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS ONE 2012, 7, e42238. [Google Scholar] [CrossRef]
- Jaramillo, S.L.; Mehlferber, E.; Moore, P.J. Life-history trade-offs under different larval diets in Drosophila suzukii (Diptera: Drosophilidae). Physiol. Entomol. 2015, 40, 2–9. [Google Scholar] [CrossRef]
- Burrack, H.J.; Fernandez, G.E.; Spivey, T.; Kraus, D.A. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumura (Diptera: Drosophilidae), an invasive frugivore. Pest. Manag. Sci. 2013, 69, 1173–1180. [Google Scholar] [CrossRef]
- Karageorgi, M.; Bräcker, L.B.; Lebreton, S.; Kadow, I.C.G.; Gompel, N.; Prud’homme, B. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Matavelli, C.; Carvalho, M.A.; Martins, N.E.; Mirth, C.K. Differences in larval nutritional requirements and female oviposition preference reflect the order of fruit colonization of Zaprionus indianus and Drosophila simulans. J. Insect Physiol. 2015, 82, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.; Andreazza, F.; Botton, M.; Baronio, C.A.; Nava, D.E. Susceptibility and interactions of Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in damaging strawberry. Neotrop. Entomol. 2017, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Camejo, L.A.; Maldonado-Morales, G.; Bayman, P. Differential microbial diversity in Drosophila melanogaster: Are fruit flies potential vectors of opportunistic pathogens? Int. J. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ioriatti, C.; Guzzon, R.; Anfora, G.; Ghidoni, F.; Mazzoni, V.; Villegas, T.R.; Dalton, D.T.; Walton, V.M. Drosophila suzukii (Diptera: Drosophilidae) contributes to the development of sour rot in grape. J. Econ. Entomol. 2018, 111, 283–292. [Google Scholar] [CrossRef]
- Bellutti, N.; Gallmetzer, A.; Innerebner, G.; Schmidt, S.; Zelger, R.; Koschier, E.H. Dietary yeast affects preference and performance in Drosophila suzukii. J. Pest Sci. 2018, 91, 651–660. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Loriatti, C.; Vogt, H.; Baufeld, P. In focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Burrack, H.J.; Barrantes, L.D.; Beers, E.H.; Dreves, A.J.; Hamby, K.A.; Haviland, D.R.; Isaacs, R.; Richardson, T.A.; Shearer, P.W.; et al. Evaluation of monitoring traps for Drosophila suzukii (Diptera: Drosophilidae) in North America. J. Econ. Entomol. 2012, 105, 1350–1357. [Google Scholar] [CrossRef]
- Epsky, N.D.; Gill, M.A.; Cha, D.H.; Landolt, P.J. Trapping the African fig fly (Diptera: Drosophilidae) with combinations of vinegar and wine. Fla. Entomol. 2014, 97, 85–89. [Google Scholar] [CrossRef]
- Kirkpatrick, D.M.; McGhee, P.S.; Hermann, S.L.; Gut, L.J.; Miller, J.R. Alignment of spotted wing drosophila (Diptera: Drosophilidae) on odorless disks varying in color. Environ. Entomol. 2016, 45, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Adams, T.; Rogg, H. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. 2012, 136, 148–154. [Google Scholar] [CrossRef]
- Kleiber, J.R.; Unelius, C.R.; Lee, J.C.; Suckling, D.M.; Qian, M.C.; Bruck, D.J. Attractiveness of fermentation and related products to spotted wing drosophila (Diptera: Drosophilidae). Chem. Ecol. 2014, 43, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Stensmyr, M.C.; Giordano, E.; Balloi, A.; Angioy, A.; Hansson, B.S. Novel natural ligands for Drosophila olfactory receptor neurons. J. Exp. Biol. 2003, 206, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Stökyl, J.; Strutz, A.; Dafni, A.; Svatos, A.; Doubsky, J.; Knaden, M.; Sachse, S.; Hansson, B.S.; Stensmyr, M.C. A deceptive pollination system targeting drosophilids through olfactory mimicry of yeast. Curr. Biol. 2010, 20, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Faucher, C.P.; Hilker, M.; de Bruyne, M. Interactions of carbon dioxide and food odours in Drosophila: Olfactory hedonics and sensory neuron properties. PLoS ONE 2013, 8, e56361. [Google Scholar] [CrossRef] [PubMed]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile codes: Correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci. Rep. 2015, 5, 14059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.; Kim, A.Y.; Jung, J.K.; Donahue, K.M.; Jung, C.; Choi, M.Y.; Koh, Y.H. The biochemical adaptations of spotted wing drosophila (Diptera: Drosophilidae) to fresh fruits reduced fructose concentrations and glutathione-S transferase activities. J. Econ. Entomol. 2016, 109, 973–981. [Google Scholar] [CrossRef]
- Kambysellis, M.P.; Ho, K.; Craddock, E.M.; Piano, F.; Parisi, M.; Cohen, J. Pattern of ecological shifts in the diversification of Hawaiian Drosophila inferred from a molecular phylogeny. Curr. Biol. 1995, 5, 1129–1139. [Google Scholar] [CrossRef]
- O’Grady, P.M.; Lapoint, R.T.; Bonacum, J.; Lasola, J.; Owen, E.; Wu, Y.; DeSalle, R. Phylogenetic and ecological relationships of the Hawaiian Drosophila inferred by mitochondrial DNA analysis. Mol. Phylogenet. Evol. 2011, 58, 244–256. [Google Scholar] [CrossRef]
- Magnacca, K.N.; Price, D.K. Rapid adaptive radiation and host plant conservation in the Hawaiian picture wing Drosophila (Diptera: Drosophilidae). Mol. Phylogenet. Evol. 2015, 92, 226–242. [Google Scholar] [CrossRef]
- Keesey, I.W.; Knaden, M.; Hansson, B.S. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 2015, 41, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Young, Y.; Buchiewicz, N.; Long, T.A.F. Nutritional geometry and fitness consequences in Drosophila suzukii, the Spotted-Wing Drosophila. Ecol. Evol. 2017, 8, 2842–2851. [Google Scholar] [CrossRef] [PubMed]
- Lasa, R.; Tadeo, E.; Toledo-Hérnandez, R.A.; Carmona, L.; Lima, I.; Williams, T. Improved capture of Drosophila suzukii by a trap baited with two attractants in the same device. PLoS ONE 2017, 12, e0188350. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Akasaka, N.; Goda, I.; Sakoda, H.; Fujiwara, S. Effective trapping of fruit flies with cultures of metabolically modified acetic acid bacteria. Appl. Environ. Microbiol. 2015, 81, 2265–2273. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J., Jr.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef]
- Chinnici, F.; Guerrero, E.D.; Sonni, F.; Natali, N.; Marín, R.N.; Riponi, C. Gas Chromatography—Mass Spectrometry (GC-MS) characterization of volatile compounds in quality vinegars with protected European geographical indication. J. Agric. Food Chem. 2009, 57, 4784–4792. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Shearer, P.W.; Barrantes, L.D.; Beers, E.H.; Burrack, H.J.; Dalton, D.T.; Dreves, A.J.; Gut, L.J.; Hamby, K.A.; Haviland, D.R.; et al. Trap designs for monitoring Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2013, 42, 1348–1355. [Google Scholar] [CrossRef]
- Wang, X.; Stewart, T.J.; Biondi, A.; Chavez, B.A.; Ingels, C.; Caprile, J.; Grant, J.A.; Walton, V.M.; Daane, K.M. Population dynamics and ecology of Drosophila suzukii in Central California. J. Pest Sci. 2016, 89, 701–712. [Google Scholar] [CrossRef]
- Kremmer, L.; David, J.; Borowiec, N.; Thaon, M.; Ris, N.; Poirié, M.; Gatti, J. The African fig fly Zaprionus indianus: A new invasive pest in France? Bull. Insectol. 2017, 70, 57–62. [Google Scholar]
- Akasaka, N.; Higashikubo, H.; Ishii, Y.; Sakoda, H.; Fujiwara, S. Polyamines in brown rice vinegar function as potent attractants for the spotted wing drosophila. J. Biosci. Bioeng. 2017, 123, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Willbrand, B.W.; Pfeiffer, D.; Leblanc, L.; Yassin, A. First report of African fig fly, Zaprionus indianus Gupta (Diptera: Drosophilidae), on the island of Maui, Hawaii, USA, in 2017 and potential impacts to the Hawaiian entomofauna. Proc. Hawaii. Entomol. Soc. 2018, 50, 55–65. [Google Scholar]
- Cha, D.H.; Gill, M.A.; Epsky, N.D.; Werle, C.T.; Adamczyk, J.J.; Landolt, P.J. From a non-target to a target: Identification of a fermentation volatile blend attractive to Zaprionus indianus. J. Appl. Entomol. 2015, 139, 114–122. [Google Scholar] [CrossRef]
- Rice, K.B.; Jones, S.K.; Morrison, W., III; Leskey, T.C. Spotted wing drosophila prefer low hanging fruit: Insights into foraging behavior and management strategies. J. Insect Behav. 2017, 30, 645–661. [Google Scholar] [CrossRef]
- Vloch, J. Identifying Drosophila suzukii. Oregon Department of Agriculture 2013. Available online: http://www.oregon.gov/oda/shared/documents/publications/ippm/spottedwingdrosophilaidkey.pdf (accessed on 9 September 2017).
- Van der Linde, K. Zaprionus indianus: Species identification and taxonomic position. Drosoph. Inf. Serv. 2010, 93, 95–98. [Google Scholar]
- Yassin, A.; David, J.R. Revision of the Afrotropical species of Zaprionus (Diptera, Drosophilidae) with descriptions of two new species and notes on internal reproductive structures and immature stages. ZooKeys 2010, 51, 33–72. [Google Scholar] [CrossRef]
- Oregon State University. Common Adult Features of Family Drosophilidae—Pomace/Vinegar Flies. 2013. Available online: http://uspest.org/swd/pubs/Drosophila_ID_Key_10-14-2014.pdf (accessed on 1 November 2017).
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulson, J.R.; Stevens, M.H.H.; White, J.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute: Cary, NC, USA, 2006; ISBN 9781590475003. [Google Scholar]
- Gbur, E.; Stroup, W.W.; McCarter, K.S.; Durham, S.; Young, L.J. Analysis of Generalized Linear Mixed Models in the Agricultural and Natural Resources Sciences; ASA: Madison, WI, USA, 2012; ISBN 9780891181828. [Google Scholar]
- Revadi, S.; Vitagliano, S.; Stacconi, M.V.R.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.M.; Rota-Stabelli, O.; Angeli, S.; et al. Olfactory responses to Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, W.; Zhang, F.; Kenis, M.; Griepink, F.; Zhang, J.; Chen, L.; Xiao, C. Identification of active components from volatiles of Chinese bayberry, Myrica rubra attractive to Drosophila suzukii. Arthropod Plant Interact. 2018, 12, 435–442. [Google Scholar] [CrossRef]
- Tidon, R.; Leite, D.F.; Leão, B.F.D. Impact of the colonisation of Zaprionus (Diptera, Drosophilidae) in different ecosystems of the Neotropical Region: 2 years after the invasion. Biol. Conserv. 2003, 112, 299–305. [Google Scholar] [CrossRef]
- Shrader, M.E. Drosophila suzukii (Matsumura) (Diptera: Drosophilidae): Risk Assessment for an Invasive Vinegar Fly in Virginia Vineyards. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2017; 141p. [Google Scholar]











| Attractant Type | Drosophila suzukii Male | Drosophila suzukii Female | Zaprionus indianus Male | Zaprionus indianus Female | Non-Target Drosophilids | Non-Target Other | Total Captures |
|---|---|---|---|---|---|---|---|
| Apple cider vinegar | 99 | 50 | 14 | 8 | 210 | 11 | 392 |
| Brown rice vinegar | 173 | 91 | 8 | 3 | 294 | 21 | 590 |
| Red wine | 123 | 81 | 5 | 5 | 700 | 76 | 990 |
| Apple cider vinegar + red wine | 190 | 92 | 12 | 22 | 626 | 59 | 1001 |
| Brown rice vinegar + red wine | 300 | 164 | 21 | 19 | 667 | 51 | 1222 |
| Distilled water | 1 | 2 | 0 | 0 | 5 | 9 | 17 |
| Total | 886 | 480 | 60 | 57 | 2502 | 227 | 4212 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willbrand, B.N.; Pfeiffer, D.G. Brown Rice Vinegar as an Olfactory Field Attractant for Drosophila suzukii (Matsumura) and Zaprionus indianus Gupta (Diptera: Drosophilidae) in Cherimoya in Maui, Hawaii, with Implications for Attractant Specificity between Species and Estimation of Relative Abundance. Insects 2019, 10, 80. https://doi.org/10.3390/insects10030080
Willbrand BN, Pfeiffer DG. Brown Rice Vinegar as an Olfactory Field Attractant for Drosophila suzukii (Matsumura) and Zaprionus indianus Gupta (Diptera: Drosophilidae) in Cherimoya in Maui, Hawaii, with Implications for Attractant Specificity between Species and Estimation of Relative Abundance. Insects. 2019; 10(3):80. https://doi.org/10.3390/insects10030080
Chicago/Turabian StyleWillbrand, Brittany N., and Douglas G. Pfeiffer. 2019. "Brown Rice Vinegar as an Olfactory Field Attractant for Drosophila suzukii (Matsumura) and Zaprionus indianus Gupta (Diptera: Drosophilidae) in Cherimoya in Maui, Hawaii, with Implications for Attractant Specificity between Species and Estimation of Relative Abundance" Insects 10, no. 3: 80. https://doi.org/10.3390/insects10030080





