Exploring an Odor-Baited “Trap Bush” Approach to Aggregate Plum Curculio (Coleoptera: Curculionidae) Injury in Blueberries
Abstract
1. Introduction
2. Materials and Methods
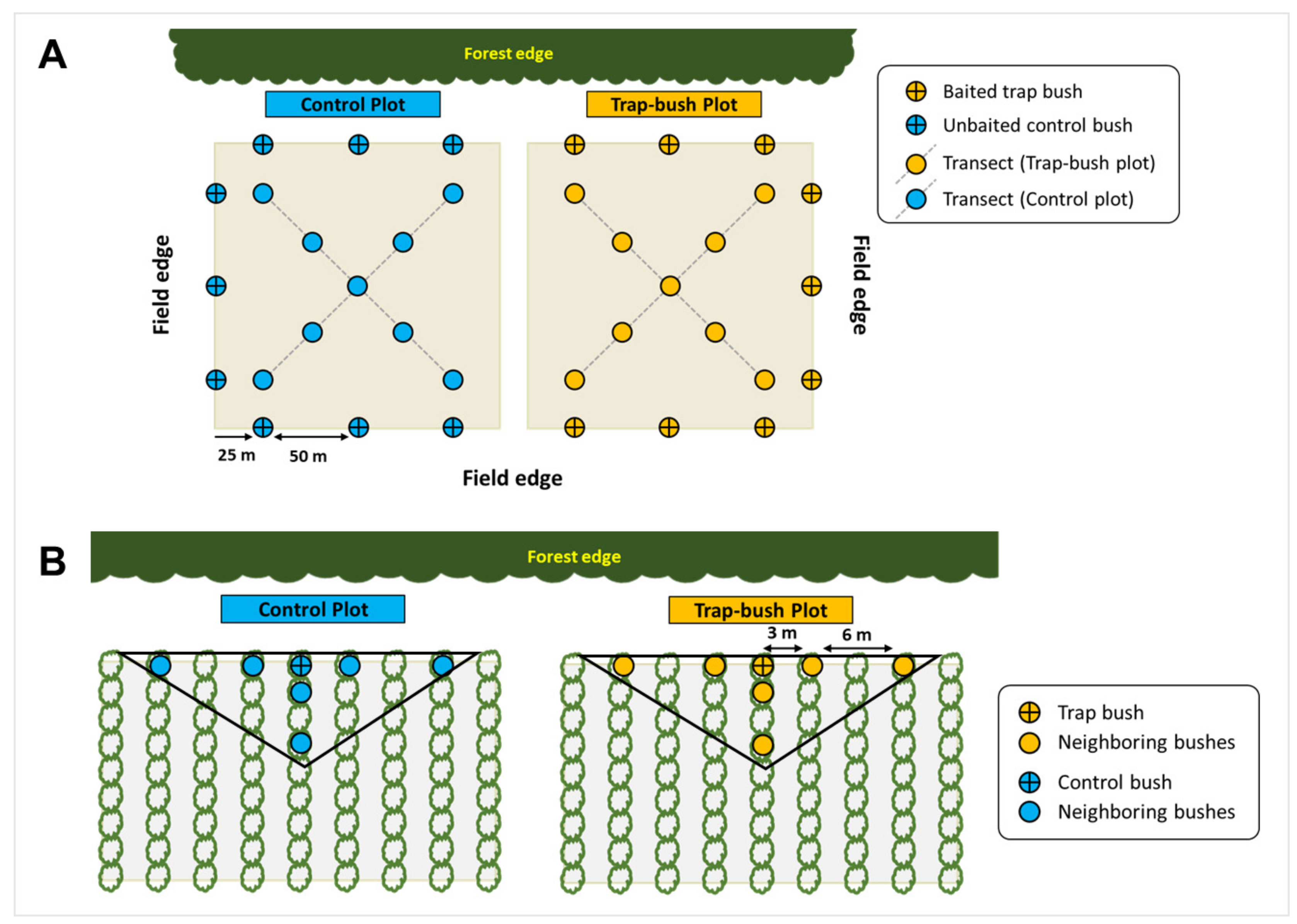
2.1. Sites and Study Design
2.2. Fruit Evaluation
2.3. Data Analyses
3. Results
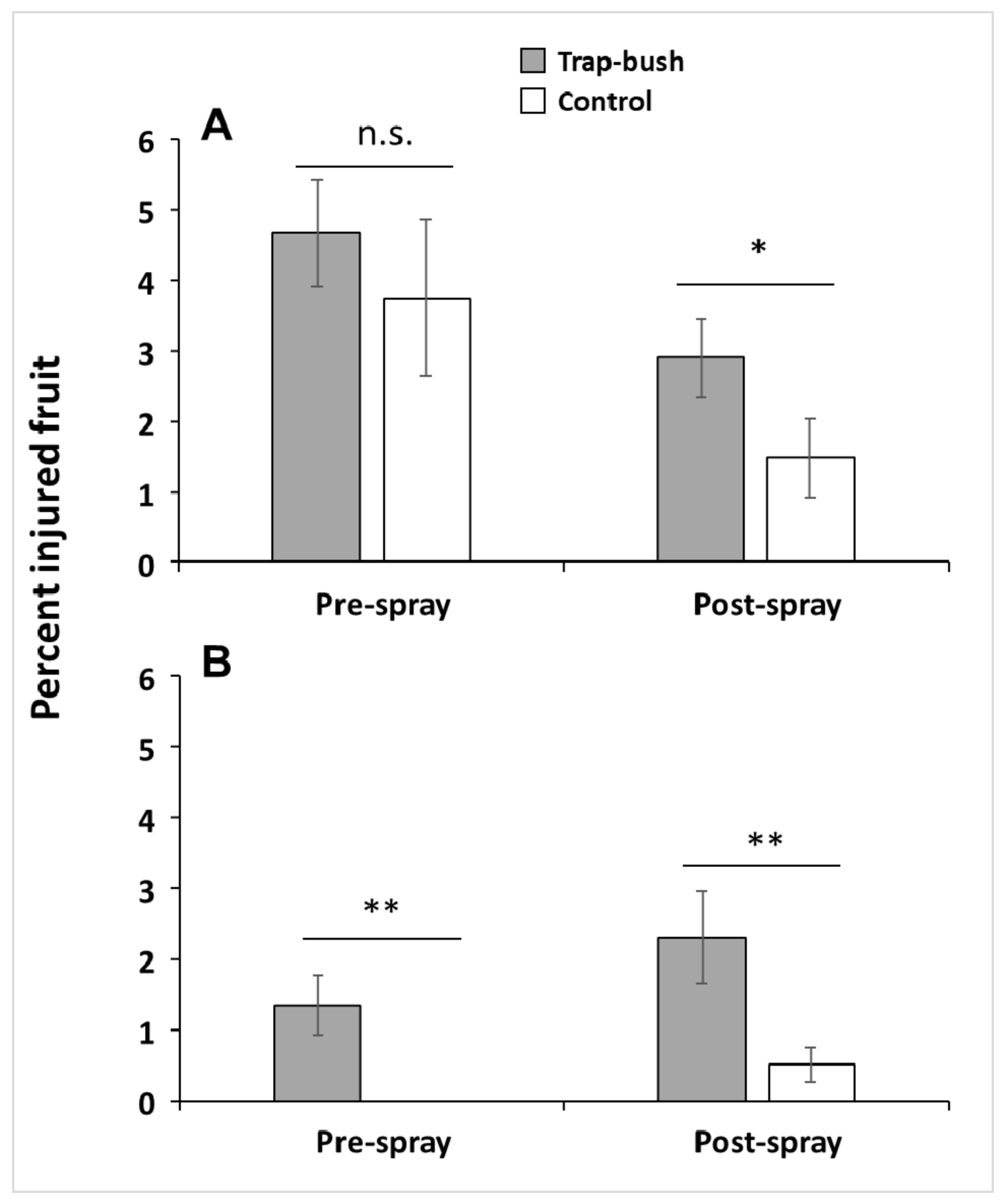
3.1. Does Fruit Injury Differ Between Trap and Control Bushes (Hypothesis 1)?
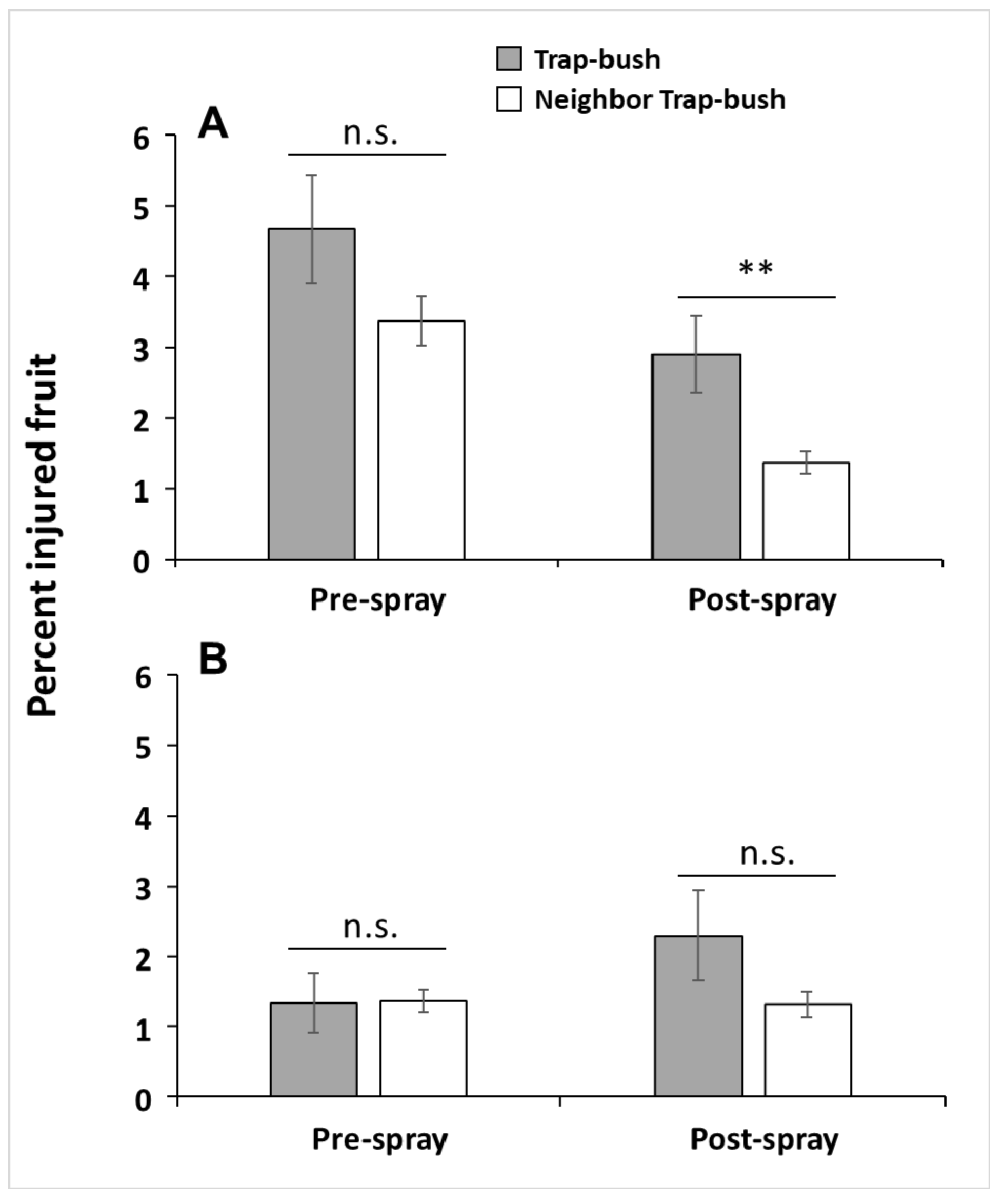
3.2. Does Fruit Injury Differ Between Bushes Neighboring Trap Bushes and Those Neighboring Control Bushes (Hypothesis 2a)?
3.3. Does Fruit Injury Differ Between Trap Bushes and Their Neighboring Bushes (Hypothesis 2b)?
3.4. Does Fruit Injury Differ Between Interior Bushes from Trap-tree Plots and Interior Bushes from Control Plots (Hypothesis 3)?
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mampe, C.D.; Neunzig, H.H. The biology, parasitism, and population sampling of the plum curculio on blueberry in North Carolina. J. Econ. Entomol. 1967, 60, 807–812. [Google Scholar] [CrossRef]
- Racette, G.; Chouinard, G.; Vincent, C.; Hill, B.S. Ecology and management of plum curculio, Conotrachelus nenuphar (Coleoptera: Curculionidae) in apple orchards. Phytoprotection 1992, 73, 85–100. [Google Scholar] [CrossRef]
- Vincent, C.; Chouinard, G.; Hill, S.B. Progress in plum curculio management: A review. Agric. Ecosyst. Environ. 1999, 73, 167–175. [Google Scholar] [CrossRef]
- Leskey, T.C.; Chouinard, G.; Vincent, C. Managing the apple maggot fly and the plum curculio: Honoring the legacy of R.J. Prokopy. In Biorational Tree-Fruit Management; Aluja, M., Leskey, T.C., Vincent, C.C., Eds.; CABI Publishers: Wallingford, UK, 2009; pp. 110–144. [Google Scholar]
- Prokopy, R.J. Two decades of bottom-up, ecologically based pest management in a small commercial apple orchard in Massachusetts. Agric. Ecosyst. Environ. 2003, 94, 299–309. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Mason, J.L.; Christie, M.; Wright, S.E. Arthropod pest and natural enemy abundance under second-level versus first-level integrated pest management practices in apple orchards: A 4-year study. Agric. Ecosyst. Environ. 1996, 57, 35–47. [Google Scholar] [CrossRef]
- Reissig, W.H.; Nyrop, J.P.; Straub, R. Oviposition model for timing insecticide sprays against plum curculio in New York State. Environ. Entomol. 1998, 27, 1053–1061. [Google Scholar] [CrossRef]
- Horton, D.; Brannen, P.; Bellinger, B.; Ritchie, D. Southeastern Peach, Nectarine, and Plum Pest Management and Culture Guide; Bulletin 1171; University of Georgia: Athens, GA, USA, 2011. [Google Scholar]
- Tewari, S.; Polk, D.; Rodriguez-Saona, C. Plum Curculio: A Key Pest of Blueberries in New Jersey; New Jersey Agricultural Experiment Station, Rutgers University Cooperative Extension Fact Sheet FS1229; New Jersey Agricultural Experiment Station: New Brunswick, NJ, USA, 2014. [Google Scholar]
- Polavarapu, S.; Kyryczenko-Roth, V.; Barry, J.D. Phenology and infestation patterns of plum curculio (Coleoptera: Curculionidae) on four highbush blueberry cultivars. J. Econ. Entomol. 2004, 97, 1899–1905. [Google Scholar] [CrossRef][Green Version]
- Oudemans, P.; Besancon, T.; Pavlis, G.; Rodriguez-Saona, C. Commercial Blueberry Pest Control Recommendations for New Jersey, 2018; New Jersey Agricultural Experiment Station, Rutgers University Cooperative Extension Bulletin E265; New Jersey Agricultural Experiment Station: New Brunswick, NJ, USA, 2018. [Google Scholar]
- Rodriguez-Saona, C.; Vincent, C.; Isaacs, R. Blueberry IPM: Past successes and future challenges. Annu. Rev. Entomol. 2019, 64, 95–114. [Google Scholar] [CrossRef]
- Shearer, P.W.; Atanassov, A.; Rucker, A. Eliminating organophosphate and carbamate insecticides from New Jersey, USA, peach culture. Acta Hortic. 2006, 713, 391–395. [Google Scholar] [CrossRef]
- Smith, E.H.; Flessel, J.K. Hibernation of the plum curculio and its spring migration to host trees. J. Econ. Entomol. 1968, 61, 193–203. [Google Scholar] [CrossRef]
- Chouinard, G.; Hill, S.B.; Vincent, C.; Barthakur, N.N. Border-row sprays against the plum curculio (Coleoptera: Curculionidae) in apple orchards: A behavioral study. J. Econ. Entomol. 1992, 85, 1307–1317. [Google Scholar] [CrossRef]
- Chouinard, G.; Hill, S.B.; Vincent, C. Spatial distribution and movements of plum curculio adults within caged apple trees. Entomol. Exp. Appl. 1994, 70, 129–142. [Google Scholar] [CrossRef]
- Hernandez-Cumplido, J.; Leskey, T.C.; Holdcraft, R.; Zaman, F.U.; Hahn, N.G.; Rodriguez-Saona, C. Tempo-spatial dynamics of adult plum curculio (Coleoptera: Curculionidae) based on semiochemical-baited trap captures in blueberries. Environ. Entomol. 2017, 46, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.C.; Prokopy, R.J.; Wright, S.E.; Phelan, P.L.; Haynes, L.W. Evaluation of individual components of plum odor as potential attractants for adult plum curculios. J. Chem. Ecol. 2001, 27, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Eller, F.J.; Bartelt, R.J. Grandisoic acid, a male-produced aggregation pheromone for the plum curculio, Conotrachelus nenuphar. J. Nat. Prod. 1996, 59, 451–453. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Chandler, B.W.; Dynok, S.A.; Piñero, J.C. Odor-baited trap trees: A new approach to monitoring plum curculio (Coleoptera: Curculionidae). J. Econ. Entomol. 2003, 96, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Prokopy, R.J.; Jacome, I.; Gray, E.; Trujillo, G.; Ricci, M.; Piñero, J.C. Using odor-baited trap trees as sentinels to monitor plum curculio (Coleoptera: Curculionidae) in apple orchards. J. Econ. Entomol. 2004, 97, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.C.; Agnello, A.M.; Tuttle, A.; Leskey, T.C.; Faubert, H.; Koehler, G.; Los, L.; Morin, G.; Leahy, K.; Cooley, D.R.; et al. Effectiveness of odor-baited trap trees for plum curculio (Coleoptera: Curculionidae) monitoring in commercial apple orchards in the northeast. J. Econ. Entomol. 2011, 104, 1613–1621. [Google Scholar] [CrossRef]
- Piñero, J.C.; Prokopy, R.J. Field evaluation of plant odor and pheromonal combinations for attracting plum curculios. J. Chem. Ecol. 2003, 12, 2735–2748. [Google Scholar] [CrossRef]
- Leskey, T.; Piñero, J.; Prokopy, R. Odor-baited trap trees: A novel management tool for plum curculio (Coleoptera: Curculionidae). J. Econ. Entomol. 2008, 101, 1302–1309. [Google Scholar] [CrossRef]
- Leskey, T.C.; Zhang, A.; Herzog, M. Nonfruiting host tree volatile blends: Novel attractants for the plum curculio (Coleoptera: Curculionidae). Environ. Entomol. 2005, 34, 785–793. [Google Scholar] [CrossRef]
- Leskey, T.C.; Zhang, A. Impact of temperature on plum curculio (Coleoptera: Curculionidae) response to odor-baited traps. J. Econ. Entomol. 2007, 100, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.J.; Vandervoort, C.; Wise, J.C. Curative activity of insecticides against plum curculio (Coleoptera: Curculionidae) in tart cherries. J. Econ. Entomol. 2009, 102, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Wise, J.C.; Polk, D.; Leskey, T.C.; Vandervoort, C. Lethality of reduced-risk insecticides against plum curculio (Coleoptera: Curculionidae) in blueberries, with emphasis on their curative activity. Pest Manag. Sci. 2013, 69, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 82, 3–10. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Software: Vienna, Austria, 2017. [Google Scholar]
- Shelton, A.M.; Badenes-Perez, E. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef]
- Hodgson, E.W.; Pitts-Singer, T.L.; Barbour, J.D. Effects of the insect growth regulator, novaluron on immature alfalfa leafcutting bees, Megachile rotundata. J. Insect Sci. 2011, 11, 43. [Google Scholar] [CrossRef]
- Pitts-Singer, T.L.; Barbour, J.D. Effects of residual novaluron on reproduction in alfalfa leafcutting bees, Megachile rotundata F. (Megachilidae). Pest Manag. Sci. 2017, 73, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.D.; Mullin, C.A.; Frazier, M.T.; Reynolds, R.D. Field residues and effects of the insect growth regulator novaluron and its major co-formulant n-methyl-2-pyrrolidone on honey bee reproduction and development. J. Econ. Entomol. 2017, 110, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Ilan, D.I.; Leskey, T.C.; Wright, S.E. Virulence of entomopathogenic nematodes to plum curculio, Conotrachelus nenuphar: Effects of strain, temperature, and soil type. J. Nematol. 2011, 43, 187–195. [Google Scholar] [PubMed]
- Shapiro-Ilan, D.I.; Wright, S.E.; Tuttle, A.F.; Cooley, D.R.; Leskey, T.C. Using entomopathogenic nematodes for biological control of plum curculio, Conotrachelus nenuphar: Effects of irrigation and species in apple orchards. Biol. Control 2013, 67, 123–129. [Google Scholar] [CrossRef]
- Piñero, J.C.; Prokopy, R.J. Temporal dynamics of plum curculio, Conotrachelus nenuphar (Herbst.) (Coleoptera: Curculionidae), immigration into an apple orchard in Massachusetts. Environ. Entomol. 2006, 35, 413–422. [Google Scholar] [CrossRef]
- Leskey, T.C.; Wright, S.E. Influence of host tree proximity on adult plum curculio (Coleoptera: Curculionidae) responses to monitoring traps. Environ. Entomol. 2004, 33, 389–396. [Google Scholar] [CrossRef]
- Leskey, T.C.; Wright, S.E.; Hock, V.; Chouinard, G.; Cormier, D.; Leahy, K.; Cooley, D.; Tuttle, A.; Eaton, A.; Zhang, A. Evaluating electrophysiological and behavioral responses to volatiles for improvement of odor-baited trap-tree management of Conotrachelus nenuphar (Herbst) (Coleoptera: Curculionidae). Environ. Entomol. 2014, 43, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, P.G.; Bortolotti, P.P.; Nannini, R.; Marianelli, L.; Roversi, P.F. Efficacy of long lasting insecticide nets in killing Halyomorpha halys in pear orchards. Outlooks Pest Manag. 2018, 29, 70–74. [Google Scholar]


| Sampling Year/Date 1 | Variables 2 | Df 3 | F | p4 |
|---|---|---|---|---|
| 2013/pre-spray | Treatment | 1 | 0.58 | 0.451 |
| Location | 1 | 12.72 | <0.001 | |
| Farm | 3 | 2.18 | 0.101 | |
| Treatment × Location | 1 | 0.92 | 0.342 | |
| Treatment × Farm | 3 | 0.32 | 0.805 | |
| Location × Farm | 3 | 0.46 | 0.714 | |
| Treatment × Location × Farm | 3 | 1.11 | 0.352 | |
| 2013/post-spray | Treatment | 1 | 4.19 | 0.046 |
| Location | 1 | 2.48 | 0.121 | |
| Farm | 3 | 4.20 | 0.009 | |
| Treatment × Location | 1 | 2.06 | 0.157 | |
| Treatment × Farm | 3 | 0.29 | 0.832 | |
| Location × Farm | 3 | 0.61 | 0.610 | |
| Treatment × Location × Farm | 3 | 4.28 | 0.009 | |
| 2014/pre-spray | Treatment | 1 | 8.45 | 0.005 |
| Location | 1 | 0.00 | 0.985 | |
| Farm | 3 | 2.43 | 0.075 | |
| Treatment × Location | 1 | 0.06 | 0.802 | |
| Treatment × Farm | 3 | 1.54 | 0.216 | |
| Location × Farm | 3 | 0.53 | 0.665 | |
| Treatment × Location × Farm | 3 | 0.40 | 0.754 | |
| 2014/post-spray | Treatment | 1 | 5.18 | 0.027 |
| Location | 1 | 0.33 | 0.569 | |
| Farm | 3 | 0.36 | 0.779 | |
| Treatment × Location | 1 | 0.48 | 0.492 | |
| Treatment × Farm | 3 | 0.85 | 0.475 | |
| Location × Farm | 3 | 0.79 | 0.506 | |
| Treatment × Location × Farm | 3 | 0.49 | 0.694 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Saona, C.; Nielsen, A.; Shapiro-Ilan, D.; Tewari, S.; Kyryczenko-Roth, V.; Firbas, N.; Leskey, T. Exploring an Odor-Baited “Trap Bush” Approach to Aggregate Plum Curculio (Coleoptera: Curculionidae) Injury in Blueberries. Insects 2019, 10, 113. https://doi.org/10.3390/insects10040113
Rodriguez-Saona C, Nielsen A, Shapiro-Ilan D, Tewari S, Kyryczenko-Roth V, Firbas N, Leskey T. Exploring an Odor-Baited “Trap Bush” Approach to Aggregate Plum Curculio (Coleoptera: Curculionidae) Injury in Blueberries. Insects. 2019; 10(4):113. https://doi.org/10.3390/insects10040113
Chicago/Turabian StyleRodriguez-Saona, Cesar, Anne Nielsen, David Shapiro-Ilan, Sunil Tewari, Vera Kyryczenko-Roth, Nicolas Firbas, and Tracy Leskey. 2019. "Exploring an Odor-Baited “Trap Bush” Approach to Aggregate Plum Curculio (Coleoptera: Curculionidae) Injury in Blueberries" Insects 10, no. 4: 113. https://doi.org/10.3390/insects10040113
APA StyleRodriguez-Saona, C., Nielsen, A., Shapiro-Ilan, D., Tewari, S., Kyryczenko-Roth, V., Firbas, N., & Leskey, T. (2019). Exploring an Odor-Baited “Trap Bush” Approach to Aggregate Plum Curculio (Coleoptera: Curculionidae) Injury in Blueberries. Insects, 10(4), 113. https://doi.org/10.3390/insects10040113







