Impact of Constant Versus Fluctuating Temperatures on the Development and Life History Parameters of Aldrichina grahami (Diptera: Calliphoridae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of Laboratory Colony
2.2. Experiment Condition
- -
- 00:00–06:00 14 °C,
- -
- 06:00–14:00 temperature increasing arithmetically from 14 to 20 °C,
- -
- 14:00–22:00 temperature decreasing arithmetically from 20 to 14 °C,
- -
- 22:00–00:00 14 °C.
2.3. Collection of Developmental Data
2.4. Data Analysis
3. Results
3.1. Constant Temperatures vs. Fluctuating Temperatures
3.2. Development in Constant Temperatures
3.2.1. Developmental Duration and Isomorphen Diagram
3.2.2. Thermal Summation Model
3.2.3. Larval Body Length and Weight Changes over Time and Isomegalen Diagram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villet, M.H. Forensic Entomology: The Utility of Arthropods in Legal Investigations. Afr. Entomol. 2010, 18, 387. [Google Scholar] [CrossRef]
- Marquez-Grant, N. Forensic Entomology: An Introduction. J. Biosoc. Sci. 2012, 44, 637–639. [Google Scholar] [CrossRef]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J.R. Forensic entomology: Applications and limitations. Forensic. Sci. Med. Pat. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.G.V. A Manual of Forensic Entomology; American Journal of Archaeology: London, UK, 1986; p. 287. [Google Scholar]
- Gruner, S.V.; Slone, D.H.; Capinera, J.L. Forensically important Calliphoridae (Diptera) associatedwith pig carrion in rural north-central Florida. J. Med. Entomol. 2007, 44, 509–515. [Google Scholar] [CrossRef]
- Chen, L.S.; Xu, Q.; Shi, F. Estimation time of death by necrophagous flies life cycle. Fa Yi Xue Za Zhi 2010, 26, 332–335. [Google Scholar] [PubMed]
- Nunez-Vazquez, C.; Tomberlin, J.; Garcia-Martinez, O. First Record of the Blow Fly Calliphora grahami from Mexico. Southwest Entomol. 2010, 35, 313–316. [Google Scholar] [CrossRef]
- Artamonov, S.D. Ecological characterization of calliphoridae (calliphoridae, diptera: Insecta) of the Russian Far East. Contemp. Probl. Ecol. 2012, 5, 46–49. [Google Scholar] [CrossRef]
- Chen, L.S. Necrophagous Flies in China; Guizhou Science and Technology Press: Guiyang, China, 2013. [Google Scholar]
- Wang, J.; Cui, H.; Chen, Y.C.; Xiong, M.J.; Li, J. Chronometrical Morphology of Aldrichina Grahami and Itsapplication in the Determination of Postmortem Interval; Acta Entomologica Sinica: Beijing, China, 2002; Volume 45. [Google Scholar]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J.R. Best practice in forensic entomology-standards and guidelines. Int. J. Leg. Med. 2007, 121, 90–104. [Google Scholar] [CrossRef]
- Byrd, J.H.; Butler, J.F. Effects of temperature on Sarcophaga haemorrhoidalis (Diptera: Sarcophagidae) development. J. Med. Entomol. 1998, 35, 694–698. [Google Scholar] [CrossRef]
- Nabity, P.D.; Higley, L.G.; Heng-Moss, T.M. Effects of temperature on development of Phormia regina (Diptera: Calliphoridae) and use of developmental data in determining time intervals in forensic entomology. J. Med. Entomol. 2006, 43, 1276–1286. [Google Scholar] [CrossRef]
- Midgley, J.M.; Villet, M.H. Development of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) at constant temperatures. Int. J. Leg. Med. 2009, 123, 285–292. [Google Scholar] [CrossRef]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic. Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Spafford, H.; Dadour, I.R. Temperature-dependant development of Nasonia vitripennison five forensically important carrion fly species. Entomol. Exp. Et Appl. 2010, 135, 37–47. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Lyu, Z.; Li, X.B.; Li, K.; Yao, L.; Wan, L.H. Technical note: Development of Hemipyrellialigurriens (Wiedemann) (Diptera: Calliphoridae) at constant temperatures: Applications in estimating postmortem interval. Forensic. Sci. Int. 2015, 253, 48–54. [Google Scholar] [CrossRef]
- Byrd, J.H.; Allen, J.C. The development of the black blow fly, Phormia regina (Meigen). Forensic Sci. Int. 2001, 12, 79–88. [Google Scholar] [CrossRef]
- Dallwitz, R. The influence of constant and fluctuating temperatures on development rate and survival of pupae of the Australian sheep blowfly Lucilia cuprina. Entomol. Exp. Et Appl. 2011, 36, 89–95. [Google Scholar] [CrossRef]
- Clarkson, C.A.; Hobischak, N.R.; Anderson, G.S. A Comparison of the Development Rate ofProtophormia Terraenovae (Robineau-Desvoidy) Raised under Constant and Fluctuating Temperature Regimes. Can. Soc. Forensic Sci. J. 2004, 37, 95–101. [Google Scholar] [CrossRef]
- Warren, J.A.; Anderson, G.S. Effect of Fluctuating Temperatures on the Development of a Forensically Important Blow Fly, Protophormia terraenovae (Diptera: Calliphoridae). Environ. Entomol. 2013, 42, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Dadour, I.R.; Cook, D.F.; Wirth, N. Rate of development of Hydrotaea rostrata under summer and winter (cyclic and constant) temperature regimes. Med. Vet. Entomol. 2001, 15, 1–6. [Google Scholar] [CrossRef]
- Fan, Z.D. Key to the Common Flies of China; Science Press: Beijing, China, 1997. [Google Scholar]
- Magni, P.A.; Pacini, T.; Pazzi, M.; Vincenti, M.; Dadour, I.R. Development of a GC-MS method for methamphetamine detection in Calliphora vomitoria L. (Diptera: Calliphoridae). Forensic Sci. Int. 2014, 241, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.L.; Wang, J.F.; Wang, M.; Yang, L.J.; Tao, L.Y.; Zhang, Y.N.; Hou, Y.D.; Chu, J.; Hou, Z.L. Development of the green bottle fly Lucilia illustris at constant temperatures. Forensic Sci. Int. 2016, 267, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Adams, Z.J.O.; Hall, M.J.R. Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci. Int. 2003, 138, 50–61. [Google Scholar] [CrossRef]
- Bugelli, V.; Campobasso, C.P.; Verhoff, M.A.; Amendt, J. Effects of different storage and measuring methods on larval length values for the blow flies (Diptera: Calliphoridae) Lucilia sericata and Calliphora vicina. Sci. Justice 2017, 57, 159–164. [Google Scholar] [CrossRef]
- Kotze, Z.; Villet, M.H.; Weldon, C.W. Effect of temperature on development of the blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Int. J. Leg. Med. 2015, 129, 1155–1162. [Google Scholar] [CrossRef] [Green Version]
- Gruner, S.V.; Slone, D.H.; Capinera, J.L.; Turco, M.P. Development of the Oriental Latrine Fly, Chrysomya megacephala (Diptera: Calliphoridae), at Five Constant Temperatures. J. Med. Entomol. 2017, 54, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, T.; Takai, K. A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ. Entomol. 2000, 29, 671–682. [Google Scholar] [CrossRef]
- Norris, K.R. The Bionomics of Blow Flies. Annu. Rev. Entomol. 1965, 10, 47–68. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Blanford, S.; Bell, A.S.; Blanford, J.I.; Read, A.F.; Thomas, M.B. Influence of climate onmalaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA 2010, 107, 15135–15139. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Shiao, S.F.; Okuyama, T. Development of insects under fluctuating temperature: A review and case study. J. Appl. Entomol. 2015, 139, 592–599. [Google Scholar] [CrossRef]
- Cloudsley-Thompson, J. The significance of fluctuating temperatures on the physiology and ecology of insects. Entomologist 1953, 86, 183–189. [Google Scholar]
- Kingsolver, J.G.; Ragland, G.J.; Diamond, S.E. Evolution in a Constant Environment: Thermal Fluctuations and Thermal Sensitivity of Laboratory and Field Populations of Manduca Sexta. Evolution 2009, 63, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.D.; Trumble, J.T. Predicting population dynamics of the parasitoid Cotesia marginiventris (Hymenoptera: Braconidae) resulting from novel interactions of temperature and selenium. Biocontrol Sci. Technol. 2010, 20, 391–406. [Google Scholar] [CrossRef]
- Arias, M.B.; Poupin, M.J.; Lardies, M.A. Plasticity of life-cycle, physiological thermal traits and Hsp70 gene expression in an insect along the ontogeny: Effect of temperature variability. J. Biol. 2011, 36, 355–362. [Google Scholar] [CrossRef]
- Fischer, K.; Kolzow, N.; Holtje, H.; Karl, I. Assay conditions in laboratory experiments: Is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia 2011, 166, 23–33. [Google Scholar] [CrossRef]
- Kjaersgaard, A.; Pertoldi, C.; Loeschcke, V.; Blanckenhorn, W.U. The Effect of Fluctuating Temperatures During Development on Fitness-Related Traits of Scatophaga stercoraria (Diptera: Scathophagidae). Environ. Entomol. 2013, 42, 1069–1078. [Google Scholar] [CrossRef]
- Economos, A.C.; Lints, F.A. Developmental temperature and life span in Drosophila melanogaster. II. Oscillating temperature. Gerontology 1986, 32, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kersting, U.; Satar, S.; Uygun, N. Effect of temperature on development rate and fecundity of apterous Aphis gossypii Glover (Hom., Aphididae) reared on Gossypium hirsutum L. J. Appl. Entomol. 1999, 123, 23–27. [Google Scholar] [CrossRef]
- Garcia-Ruiz, E.; Marco, V.; Perez-Moreno, I. Effects of variable and constant temperatures on theembryonic development and survival of a new grape pest, Xylotrechus arvicola (Coleoptera: Cerambycidae). Env. Entomol. 2011, 40, 939–947. [Google Scholar] [CrossRef]
- Jensen, J.L.W.V. Sur les fonctions convexes et les inégalités entre les valeurs moyennes. Acta Math. 1906, 30, 175–193. [Google Scholar] [CrossRef]
- Ruel, J.J.; Ayres, M.P. Jensen’s inequality predicts effects of environmental variation. Trends Ecol. Evol. 1999, 14, 361–366. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.J.; Zhang, Y.N.; Tao, L.Y.; Wang, J.F. Development of Musca domestica at constanttemperatures and the first case report of its application for estimating the minimum postmortem interval. Forensic Sci. Int. 2018, 285, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Effects of temperature on the growth and development of four common necrophagous flies and their signincance in forensic medicine. Chin. J. Forensic Med. 1998, 13, 8. (In Chinese) [Google Scholar]
- Jiang, W.; Cui, H.U.; Yu, C.; Jian, M.; Jun, L.I. Application of the pupal morphogenesis of Aldrichina grahami (Aldrich) to the deduction of postmortem interval. Acta Entomol. Sin. 2002, 45, 696–699. [Google Scholar]
- Yu, W.; Wang, J.; Talatsiyeit; Min, W.; Cui, H.; Ye, G. The observation and analysis of the growth and development rules of six species of necrophagous flies in Hangzhou and Guangzhou. Chin. J. Forensic Med. 2015, 4, 350–353. [Google Scholar]
- Wang, Y.; Zhang, Y.N.; Liu, C.; Hu, G.L.; Wang, M.; Yang, L.J.; Chu, J.; Wang, J.F. Development of Aldrichina grahami (Diptera: Calliphoridae) at Constant Temperatures. J. Med. Entomol. 2018, 55, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Kikuo, M.; Kiichi, U. Studies on the life history of Aldrichina grahami (Aldrich, 1930): Part I. The rearing results of flies kept at various conditions of temperature. Jap. J. Sanit. Zool. 1962, 13, 248–252. [Google Scholar]
- Sarup, P.; Loeschcke, V. Developmental acclimation affects clinal variation in stress resistance traits in Drosophila buzzatii. J. Evol. Biol 2010, 23, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Bozinovic, F.; Bastias, D.A.; Boher, F.; Clavijo-Baquet, S.; Estay, S.A.; Angilletta, M.J. The Mean and Variance of Environmental Temperature Interact to Determine Physiological Tolerance and Fitness. Physiol. Biochem. Zool. 2011, 84, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Overgaard, J.; Hoffmann, A.A.; Kristensen, T.N. Assessing population and environmental effects on thermal resistance in Drosophila melanogaster using ecologically relevant assays. J. Biol 2011, 36, 409–416. [Google Scholar] [CrossRef]
- Grassberger, M.; Reiter, C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen-and isomorphen-diagram. Forensic Sci. Int. 2001, 120, 32–36. [Google Scholar] [CrossRef]
- Reiter, C. Zum Wachstumsverhalten der Maden der blauen Schmeißfliege Calliphora vicina. Z. Für Rechtsmed. 1984, 91, 295–308. [Google Scholar] [CrossRef]

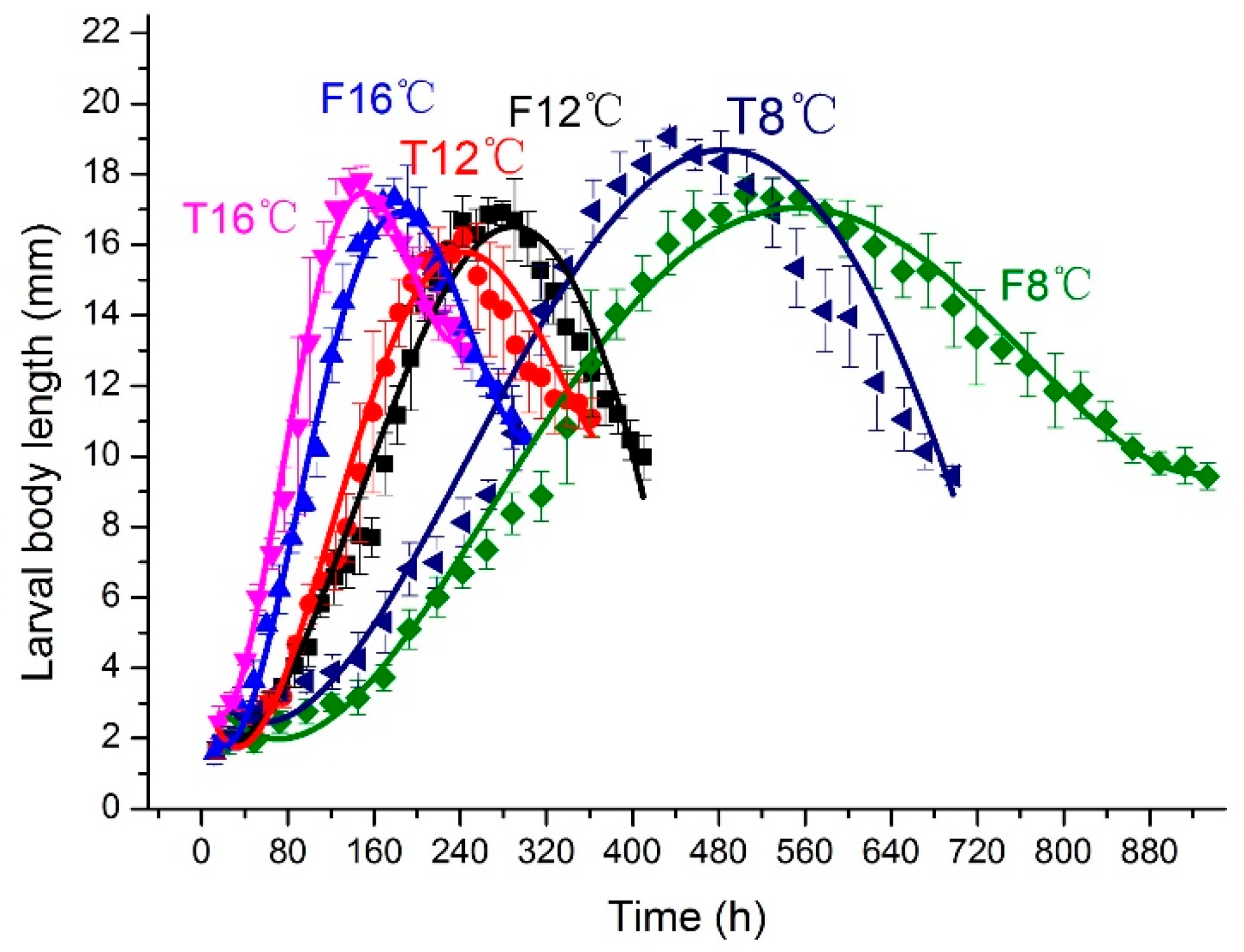
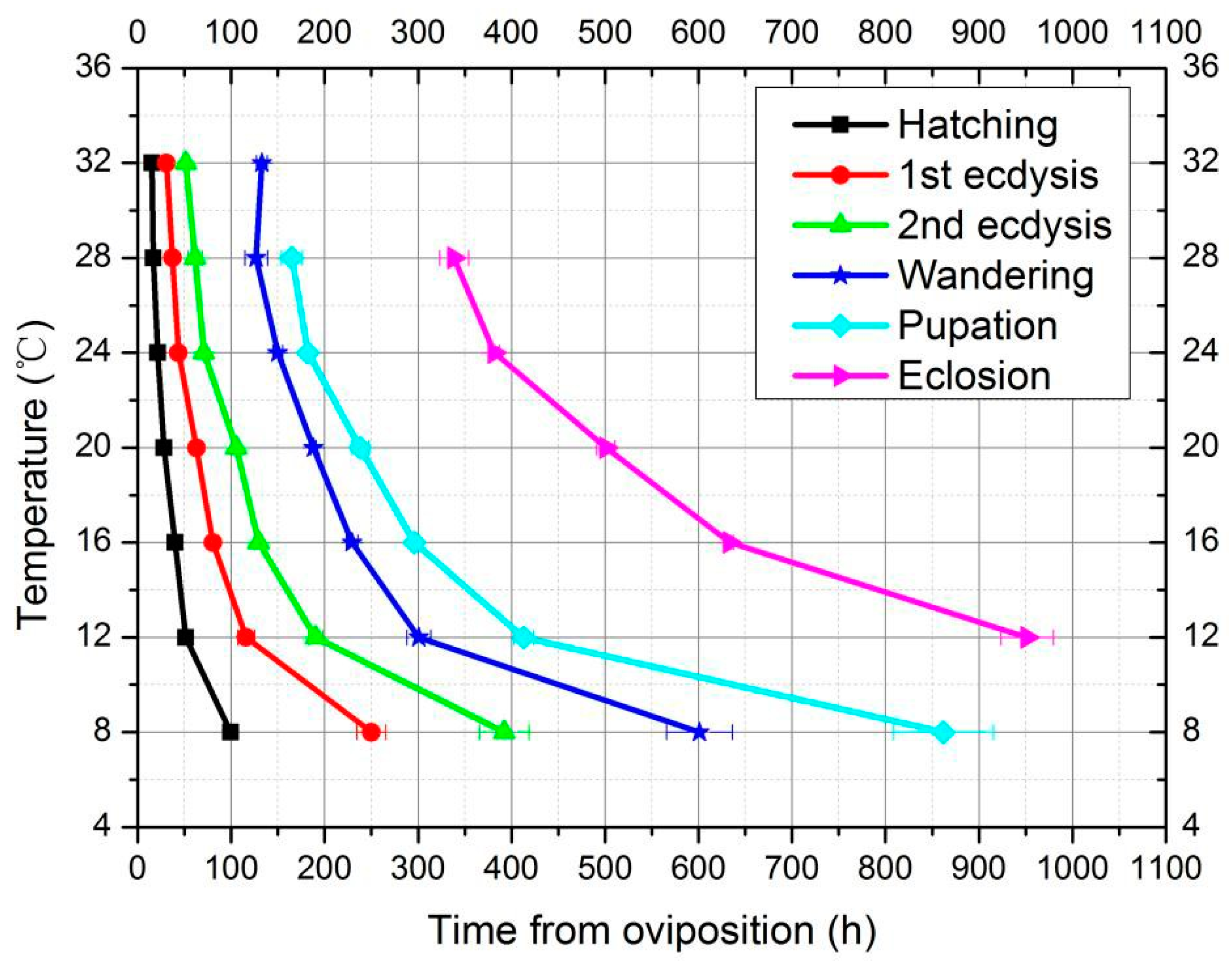
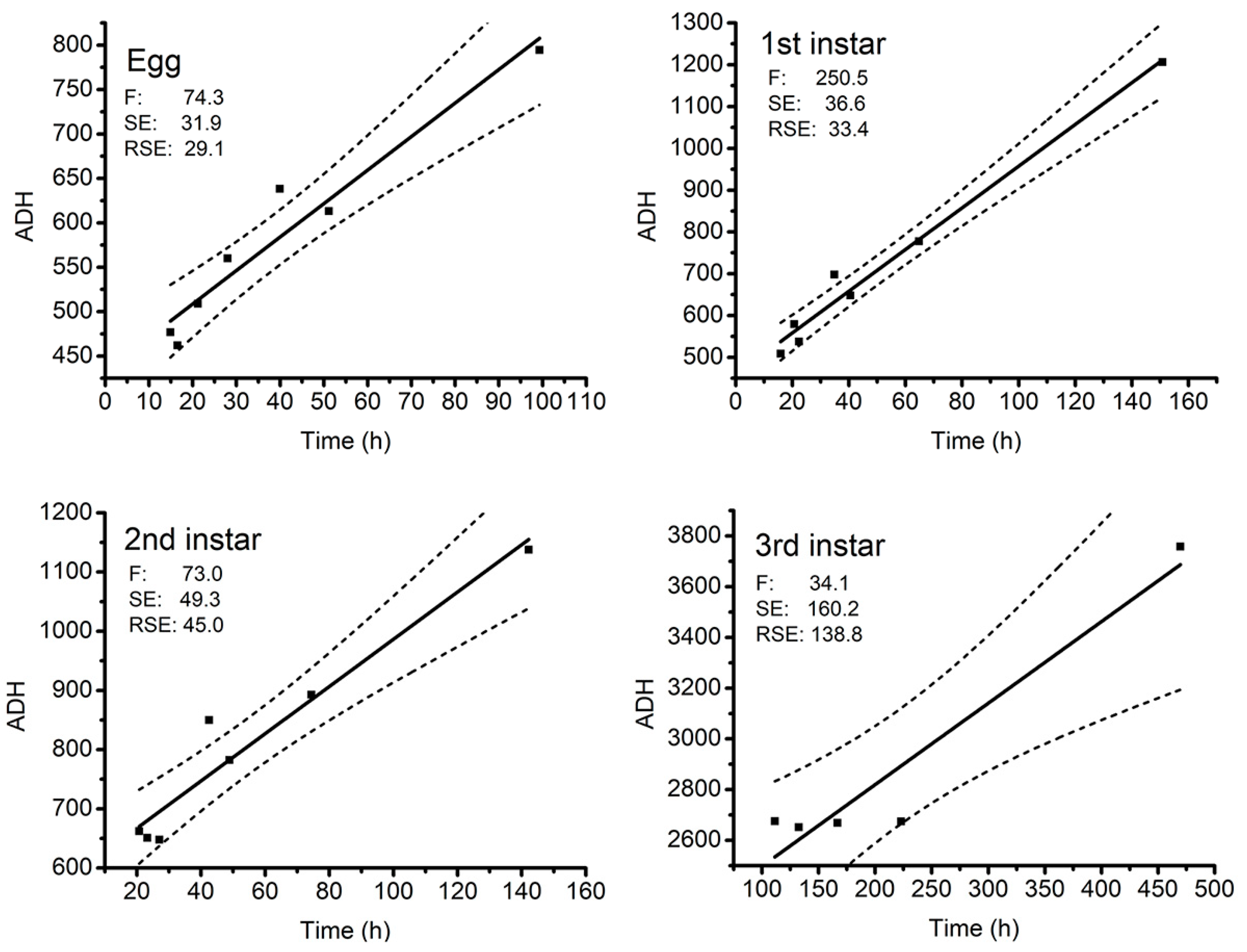
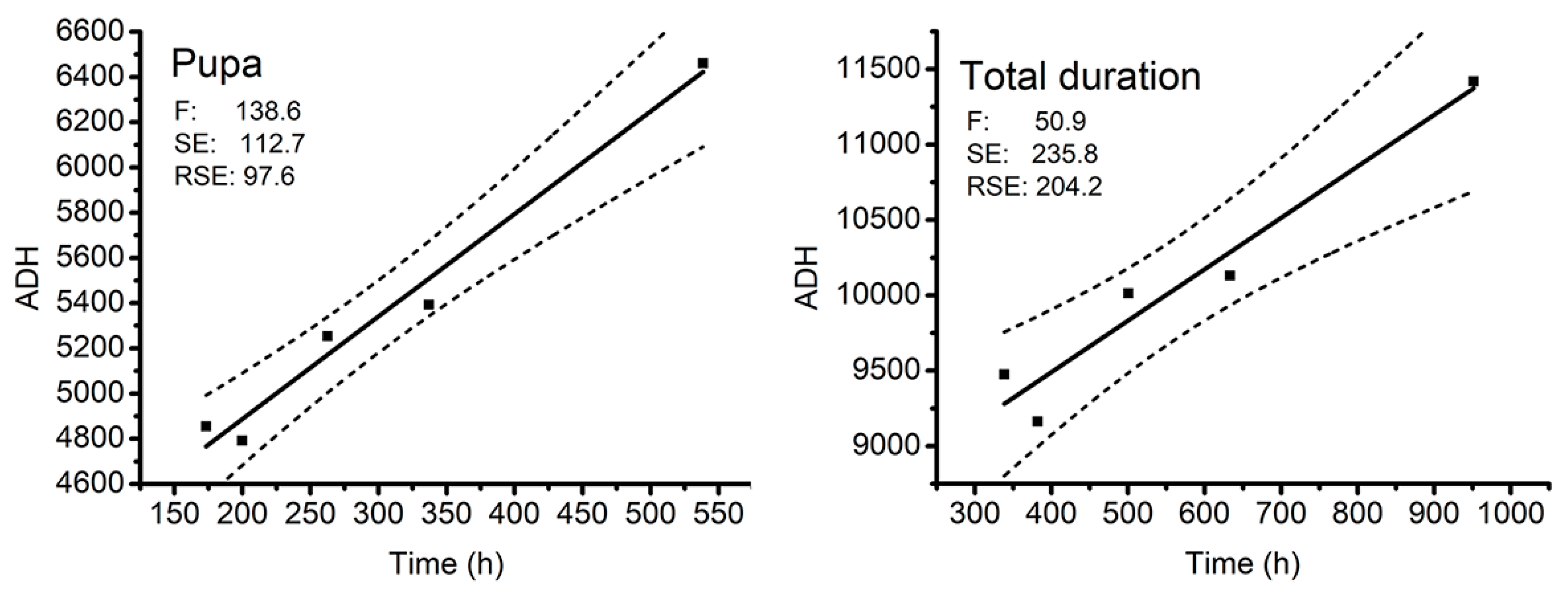


| Developmental Stages (h) | |||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Egg | First-Instar | Second-Instar | Third-Instar | Wandering | Pupa | Total Duration |
| 8 | 99.3 ± 4.3 a | 150.8 ± 13.5 a | 142.2 ± 18.3 a | 208.6 ± 20.1 a | 261.2 ± 20.7 a | X | |
| 6–12 | 133.9 ± 16.1 b | 166.8 ± 3.6 b | 155.6 ± 2.9 b | 253.0 ± 20.2 b | 354.6 ± 15.4 b | X | |
| 12 | 51.1 ± 4.0 c | 64.8 ± 5.9 c | 74.4 ± 5.0 c | 110.5 ± 11.7 c | 112.4 ± 11.3 c | 538.4 ± 22.1 | 951.6 ± 28.3 |
| 10–16 | 56.7 ± 3.8 cd | 74.2 ± 5.2 d | 90.5 ± 7.1 d | 142.0 ± 10.3 d | 133.2 ± 7.9 d | X | |
| 16 | 39.9 ± 2.5 e | 40.5 ± 3.1 e | 48.9 ± 1.2 e | 100.2 ± 7. 3e | 66.6 ± 6.5 e | 337.1 ± 5.1 e | 633.2 ± 3.6 e |
| 14–20 | 44.0 ± 1.6 e,f | 46.8 ± 1.5 ef | 55.3 ± 3.7 ef | 110.4 ± 7.4 ef | 87.0 ± 3.3 f | 389.1 ± 10.4 f | 732.6 ±14.0 f |
| 20 | 28.0 ± 1.3 | 34.9 ± 2.1 | 42.5 ± 3.5 | 82.5 ± 3.3 | 50.1 ± 6.1 | 262.7 ± 3.9 | 500.7 ± 9.8 |
| 24 | 21.2 ± 0.9 | 22.4 ± 0.4 | 27.0 ± 1.7 | 79.2 ± 4.2 | 32.3 ± 3.7 | 199.7 ± 5.1 | 381.8 ± 5.1 |
| 28 | 16.5 ± 1.6 | 20.7 ± 3.7 | 23.25 ± 4.6 | 65.4 ± 7.2 | 38.4 ± 4.6 | 173.4 ± 12.2 ▲ | 338.4 ± 15.5 |
| 32 | 14.9 ± 0.7 | 15.9 ± 0.9 | 20.7 ± 2.2 | 81.3 ± 4.5 | 83.2 ± 4.9 * | X | |
| 36 | X | X | X | X | X | X | |
| Temperature (°C) | Equation | df | R2 |
|---|---|---|---|
| F8 | L = 3.567 − 0.048T + 4.0 × 10−4T2 − 6.8 × 10−7T3 + 3.3 × 10−10T4 | 34 | 0.991 |
| T8 | L = 3.650 − 0.044T + 4.6 × 10−4T2 − 7.8 × 10−7T3 + 3.4 × 10−10T4 | 24 | 0.976 |
| F12 | L = 2.449 − 0.046T + 9.9 × 10−4T2 − 2.9 × 10−6T3 + 2.0 × 10−9T4 | 29 | 0.989 |
| T12 | L = 3.498 − 0.112T + 2.1 × 10−3T2 − 7.8 × 10−6T3 + 8.6 × 10−9T4 | 25 | 0.990 |
| F16 | L = 3.134 − 0.130T + 3.5 × 10−3T2 − 1.8 × 10−5T3 + 2.7 × 10−8T4 | 20 | 0.991 |
| T16 | L = 4.149 − 0.168T + 5.4 × 10−3T2 − 3.5 × 10−5T3 + 6.4 × 10−8T4 | 15 | 0.998 |
| Developmental Stages | K (Degree Hours) | D0 (°C) | R2 | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Egg | 433.2 | 20.8 | 3.77 | 0.43 | 0.92 |
| First instar | 458.6 | 21.0 | 4.99 | 0.32 | 0.98 |
| Second instar | 587.4 | 31.4 | 3.99 | 0.47 | 0.92 |
| Third instar | 2176.5 | 141.1 | 3.21 | 0.55 | 0.89 |
| Pupa | 3980.4 | 126.9 | 4.54 | 0.39 | 0.97 |
| Total duration | 8125.2 | 288.4 | 3.41 | 0.48 | 0.93 |
| Temperature (°C) | Equation | df | R2 |
|---|---|---|---|
| 8 | L = 3.650 − 0.044T + 4.6 × 10−4T2 − 7.8 × 10−7T3+3.4 × 10−10T4 | 24 | 0.976 |
| 12 | L = 3.498 − 0.112T + 2.1 × 10−3T2 − 7.8 × 10−6T3+8.6 × 10−9T4 | 25 | 0.990 |
| 16 | L = 4.149 − 0.168T + 5.4 × 10−3T2 − 3.5 × 10−5T3+6.4 × 10−8T4 | 15 | 0.998 |
| 20 | L = −3.777 + 0.395T – 2.2 × 10−3T2 + 1.3 × 10−6T3+1.0 × 10−8T4 | 12 | 0.979 |
| 24 | L = 1.550 + 0.0024T – 6.7 × 10−3T2 − 7.4 × 10−5T3+2.2 × 10−7T4 | 8 | 0.989 |
| 28 | L = 3.983 − 0.189T + 1.2 × 10−2T2 − 1.3 × 10−4T3+4.1 × 10−7T4 | 7 | 0.990 |
| 32 | L = −3.340 + 0.591T – 6.3 × 10−3T2 + 2.8 × 10−5T3−4.8 × 10−8T4 | 11 | 0.990 |
| Temperature (°C) | Equation | df | R2 | Errors as Weight |
|---|---|---|---|---|
| 8 | W = 6858.957 − 82.870T + 0.342T2 − 5.5 × 10−4T3 + 3.1 × 10−7T4 | 15 | 0.966 | no weighting |
| 12 | W = 359.021 − 13.670T + 0.156T2 − 5.3 × 10−4T3 + 5.7 × 10−7T4 | 22 | 0.993 | no weighting |
| 16 | W = 86.127 − 7.780T + 0.191T2 − 8.0 × 10−4T3 + 7.1 × 10−7T4 | 15 | 0.988 | instrumental |
| 20 | W = 35.782 − 15.203T + 0.549T2 − 4.1 × 10−3T3 + 9.1 × 10−6T4 | 11 | 0.939 | no weighting |
| 24 | W = 703.987 − 58.104T + 1.516T2 − 1.2 × 10−2T3 + 3.3 × 10−5T4 | 7 | 0.981 | no weighting |
| 28 | W = 260.311 − 31.548T + 1.089T2 − 0.01T3 + 2.9 × 10−5T4 | 7 | 0.998 | instrumental |
| 32 | W = 63.503 − 9.915T + 0.490T2 − 4.2 × 10−3T3 + 1.0 × 10−5T4 | 11 | 0.980 | instrumental |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Yang, L.; Ren, L.; Shang, Y.; Wang, S.; Guo, Y. Impact of Constant Versus Fluctuating Temperatures on the Development and Life History Parameters of Aldrichina grahami (Diptera: Calliphoridae). Insects 2019, 10, 184. https://doi.org/10.3390/insects10070184
Chen W, Yang L, Ren L, Shang Y, Wang S, Guo Y. Impact of Constant Versus Fluctuating Temperatures on the Development and Life History Parameters of Aldrichina grahami (Diptera: Calliphoridae). Insects. 2019; 10(7):184. https://doi.org/10.3390/insects10070184
Chicago/Turabian StyleChen, Wei, Li Yang, Lipin Ren, Yanjie Shang, Shiwen Wang, and Yadong Guo. 2019. "Impact of Constant Versus Fluctuating Temperatures on the Development and Life History Parameters of Aldrichina grahami (Diptera: Calliphoridae)" Insects 10, no. 7: 184. https://doi.org/10.3390/insects10070184





