Managing Floral Resources in Apple Orchards for Pest Control: Ideas, Experiences and Future Directions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
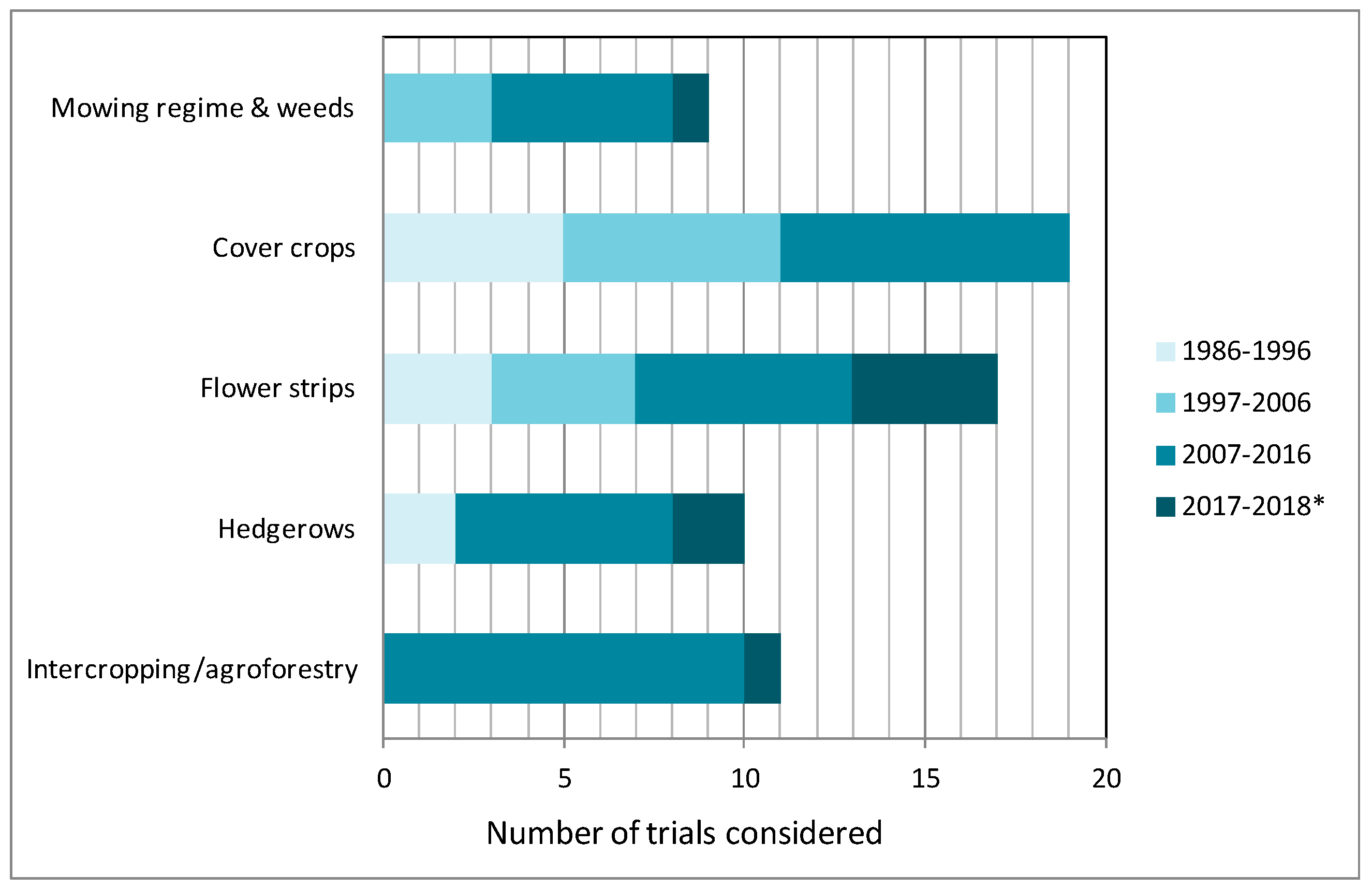
3.1. Adaptation of Mowing Regime and Weed Maintenance
3.2. Cover Crops
3.3. Annual and Perennial Flower Strips
3.4. Hedgerows
3.5. Intercropping and Agroforestry
4. General Conclusions and Further Perspectives
4.1. Effect on Biodiversity and Ecosystem Services
4.2. Effect on Orchard Management
4.3. Considerations for Stakeholders and Policy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baumgärtner, J.; Bieri, M. Fruit tree ecosystem service provision and enhancement. Ecol. Eng. 2006, 27, 118–123. [Google Scholar] [CrossRef]
- Simon, S.; Bouvier, J.C.; Debras, J.F.; Sauphanor, B. Biodiversity and pest management in orchard systems. A review. Agron. Sustain. Dev. 2010, 30, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Plieninger, T.; Levers, C.; Mantel, M.; Costa, A.; Schaich, H.; Kuemmerle, T. Patterns and Drivers of Scattered Tree Loss in Agricultural Landscapes: Orchard Meadows in Germany (1968–2009). PLoS ONE 2015, 10, e0126178. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I.; Leschke, K. Effects of habitat management on vegetation and above-ground nesting bees and wasps of orchard meadows in Central Europe. Biodivers. Conserv. 2003, 12, 1953–1968. [Google Scholar] [CrossRef]
- Porcel, M.; Andersson, G.K.S.; Pålsson, J.; Tasin, M. Organic management in apple orchards: Higher impacts on biological control than on pollination. J. Appl. Ecol. 2018, 55, 2779–2789. [Google Scholar] [CrossRef] [Green Version]
- Samnegård, U.; Alins, G.; Boreux, V.; Bosch, J.; García, D.; Happe, A.K.; Klein, A.M.; Miñarro, M.; Mody, K.; Porcel, M.; et al. Management trade-offs on ecosystem services in apple orchards across Europe: Direct and indirect effects of organic production. J. Appl. Ecol. 2018, 56, 802–811. [Google Scholar] [CrossRef]
- Daniel, C.; Matray, S.; Stoeckli, S.; Niggli, U. Pest management in organic apple, pear and stone fruit. In Handbook of Pest Management in Organic Farming; Vacante, V., Kreiter, S., Eds.; CAB International: Wallingford, UK, 2018; pp. 130–150. [Google Scholar]
- Demestihas, C.; Plenet, D.; Genard, M.; Raynal, C.; Lescourret, F. Ecosystem services in orchards—A review. Agron. Sustain. Dev. 2017, 37. [Google Scholar] [CrossRef]
- Pfiffner, L.; Wyss, E. Use of sown wildflower strips to enhance natural enemies of agricultural pests. In Ecological Engineering for Pest Management. Advances in Habitat Manipulation for Arthropods; Gurr, G.M., Wratten, S.D., Altieri, M.A., Eds.; CSIRO Publishing: Collingwood, Australia, 2004; pp. 165–186. [Google Scholar]
- Nilsson, U.; Porcel, M.; Świergiel, W.; Wivstad, M. Habitat Manipulation—As a Pest Management Tool in Vegetable and Fruit Cropping Systems, with the Focus on Insects and Mites; SLU, EPOK—Centre for Organic Food & Farming: Uppsala, Sweden, 2016; p. 52. [Google Scholar]
- Norton, R.L. Windbreaks—Benefits to orchard and vineyard crops. Agric. Ecosyst. Environ. 1988, 22–23, 205–213. [Google Scholar] [CrossRef]
- Miñarro, M.; Prida, E. Hedgerows surrounding organic apple orchards in north-west Spain: Potential to conserve beneficial insects. Agric. For. Entomol. 2013, 15, 382–390. [Google Scholar] [CrossRef]
- Boller, E.F.; Häni, F.; Poehling, H.-M. Ecological Infrastructures—Ideabook on Functional Biodiversity at the Farm Level; IOBC-WPRS, Ed.; Swiss Centre for Agricultural Extension and Rural Development (LBL): Lindau, Switzerland, 2004; p. 212. [Google Scholar]
- Balmer, O.; Pfiffner, L.; Schied, J.; Willareth, M.; Leimgruber, A.; Luka, H.; Traugott, M. Noncrop flowering plants restore top-down herbivore control in agricultural fields. Ecol. Evol. 2013, 3, 2634–2646. [Google Scholar] [CrossRef] [Green Version]
- Prokopy, R.J. Two decades of bottom-up, ecologically based pest management in a small commercial apple orchard in Massachusetts. Agric. Ecosyst. Environ. 2003, 94, 299–309. [Google Scholar] [CrossRef]
- Simon, S.; Lesueur-Jannoyer, M.; Plénet, D.; Lauri, P.-É.; Le Bellec, F. Methodology to design agroecological orchards: Learnings from on-station and on-farm experiences. Eur. J. Agron. 2017, 82, 320–330. [Google Scholar] [CrossRef]
- Bugg, R.L.; Waddington, C. Using cover crops to manage arthropod pests of orchards—A review. Agric. Ecosyst. Environ. 1994, 50, 11–28. [Google Scholar] [CrossRef]
- Prokopy, R.J. Integration in orchard pest and habitat management—A review. Agric. Ecosyst. Environ. 1994, 50, 1–10. [Google Scholar] [CrossRef]
- EBIO-Network. EBIO_Network—European Biodiversity Orchards-Network. Available online: https://ebionetwork.julius-kuehn.de/ (accessed on 8 August 2019).
- Van Rijn, P.C.J.; Wäckers, F.L.; Cadotte, M. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Vattala, H.D.; Wratten, S.D.; Phillips, C.B.; Wackers, F.L. The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biol. Control 2006, 39, 179–185. [Google Scholar] [CrossRef]
- Walton, N.J.; Isaacs, R. Survival of Three Commercially Available Natural Enemies Exposed to Michigan Wildflowers. Environ. Entomol. 2011, 40, 1177–1182. [Google Scholar] [CrossRef]
- He, X.; Sigsgaard, L. A Floral Diet Increases the Longevity of the Coccinellid Adalia bipunctata but Does Not Allow Molting or Reproduction. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef]
- Kugler, H. Blütenökologie; Gustav Fischer Verlag: Stuttgart, Germany, 1970. [Google Scholar]
- Funayama, K. Unmown groundcover conserves adult populations of the predatory ground beetle Chlaenius micans (Coleoptera: Carabidae) in commercial apple orchards. Appl. Entomol. Zool. 2014, 49, 183–187. [Google Scholar] [CrossRef]
- Funayama, K. Influence of mowing on dynamics of native phytoseiid mites and Tetranychus urticae in apple orchards in northern Japan. Exp. Appl. Acarol. 2016, 70, 57–67. [Google Scholar] [CrossRef]
- Funayama, K.; Komatus, M.; Sonoda, S.; Takahashi, I.; Hara, K. Management of apple orchards to conserve generalist phytoseiid mites suppresses two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2015, 65, 43–54. [Google Scholar] [CrossRef]
- Kienzle, J.; Zebitz, C.P.W.; Brass, S. Floral and faunal species diversity and abundance of aphid predators in ecological apple orchards. Biol. Agric. Hortic. 1997, 15, 233–240. [Google Scholar] [CrossRef]
- Kienzle, J.; Zebitz, C.P.W.; Brass, S.; Athanassov, A. Abundance of different tortricid species and their parasitoid antagonists in ecological apple orchards in southern Germany. Biol. Agric. Hortic. 1997, 15, 211–221. [Google Scholar] [CrossRef]
- Horton, D.R.; Broers, D.A.; Lewis, R.R.; Granatstein, D.; Zack, R.S.; Unruh, T.R.; Moldenke, A.R.; Brown, J.J. Effects of mowing frequency on densities of natural enemies in three Pacific Northwest pear orchards. Entomol. Exp. Appl. 2003, 106, 135–145. [Google Scholar] [CrossRef]
- Marliac, G.; Simon, S.; Mazzia, C.; Penvern, S.; Lescourret, F.; Capowiez, Y. Increased grass cover height in the alleys of apple orchards does not promote Cydia pomonella biocontrol. Biocontrol 2015, 60, 805–815. [Google Scholar] [CrossRef]
- García, R.R.; Miñarro, M. Role of floral resources in the conservation of pollinator communities in cider-apple orchards. Agric. Ecosyst. Environ. 2014, 183, 118–126. [Google Scholar] [CrossRef]
- Saunders, M.E.; Luck, G.W. Interaction effects between local flower richness and distance to natural woodland on pest and beneficial insects in apple orchards. Agric. For. Entomol. 2018, 20, 279–287. [Google Scholar] [CrossRef]
- Granatstein, D.; Davenport, J.R.; Kirby, E. Growing Legumes in Orchard Alleys as an Internal Nitrogen Source. Hortscience 2017, 52, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Granatstein, D.; Sanchez, E. Research knowledge and needs for orchard floor management in organic tree fruit systems. Int. J. Fruit Sci. 2009, 9, 257–281. [Google Scholar] [CrossRef]
- Brown, M.W.; Glenn, D.M. Ground cover plants and selective insecticides as pest management tools in apple orchards. J. Econ. Entomol. 1999, 92, 899–905. [Google Scholar] [CrossRef]
- Fernandez, D.E.; Cichon, L.I.; Sanchez, E.E.; Garrido, S.A.; Gittins, C. Effect of different cover crops on the presence of arthropods in an organic apple (Malus domestica Borkh) orchard. J. Sustain. Agric. 2008, 32, 197–211. [Google Scholar] [CrossRef]
- Fréchette, B.; Cormier, D.; Chouinard, G.; Vanoosthuyse, F.; Lucas, É. Apple aphid, Aphis spp. (Hemiptera: Aphididae), and predator populations in an apple orchard at the non-bearing stage: The impact of ground cover and cultivar. Eur. J. Entomol. 2008, 105, 521–529. [Google Scholar] [CrossRef]
- Haley, S.; Hogue, E.J. Ground cover influence on apple aphid, Aphis pomi DeGeer (Homoptera Aphididae), and its predators in a young apple orchard. Crop Prot. 1990, 9, 225–230. [Google Scholar] [CrossRef]
- Irvin, N.A.; Scarratt, S.L.; Wratten, S.D.; Frampton, C.M.; Chapman, R.B.; Tylianakis, J.M. The effects of floral understoreys on parasitism of leafrollers (Lepidoptera: Tortricidae) on apples in New Zealand. Agric. For. Entomol. 2006, 8, 25–34. [Google Scholar] [CrossRef]
- Irvin, N.A.; Wratten, S.D.; Chapman, R.B.; Frampton, C.M. Effects of floral resources on fitness of the leafroller parasitoid (Dolichogenidea tasmanica) in apples. In Proceedings of the Fifty Second New Zealand Plant Protection Conference, Auckland Airport Centra, Auckland, New Zealand, 10–12 August 1999; pp. 84–88. [Google Scholar]
- Irvin, N.A.; Wratten, S.D.; Frampton, C.M. Understorey management for the enhancement of the leafroller parasitoid Dolichogenidea tasmanica (Cameron) in orchards at Canterbury, New Zealand. In Hymenoptera: Evolution, Biodiversity and Biological Control; Austin, A., Dowton, M., Eds.; CSIRO Publishing: Melbourne, Australia, 2000; pp. 396–403. [Google Scholar]
- Stephens, M.J.; France, C.M.; Wratten, S.D.; Frampton, C. Enhancing Biological Control of Leafrollers (Lepidoptera: Tortricidae) by Sowing Buckwheat (Fagopyrum esculentum) in an Orchard. Biocontrol Sci. Technol. 1998, 8, 547–558. [Google Scholar] [CrossRef]
- Yan, Y.-H.; Yu, Y.; Du, X.-G.; Zhao, B.-G. Conservation and augmentation of natural enemies in pest management of Chinese apple orchards. Agric. Ecosyst. Environ. 1997, 62, 253–260. [Google Scholar] [CrossRef]
- Weber, M.G.; Porturas, L.D.; Heeler, K.H. World List of Plants with Extrafloral Nectaries. Available online: www.extrafloralnectaries.org (accessed on 15 May 2019).
- Jones, I.M.; Koptur, S.; von Wettberg, E.J. The use of extrafloral nectar in pest management: Overcoming context dependence. J. Appl. Ecol. 2017, 54, 489–499. [Google Scholar] [CrossRef]
- Bugg, R.L.; Ellis, R.T.; Carlson, R.W. Ichneumonidae (Hymenoptera) Using Extrafloral Nectar of Faba Bean (Vicia faba L., Fabaceae) in Massachusetts. Biol. Agric. Hortic. 1989, 6, 107–114. [Google Scholar] [CrossRef]
- Walach, S.; Herz, A. Suitability of extrafloral nectaries of the faba bean as nutritional resource for the parasitoid Ascogaster quadridentata (Hymenoptera, Braconidae). Mitt. Dtsch. Ges. Allg. Angew. Ent. 2015, 20, 145–148. [Google Scholar]
- Altieri, M.A.; Schmidt, L.L. Cover crops affect insect and spider populations in apple orchards. Calif. Agric. 1986, 40, 15–17. [Google Scholar]
- Mullinix, K.; Brunner, J.F.; Isman, M.B. Apple leafroller (Lepidoptera: Tortricidae) populations and parasitism in an orchard managed with either a grass or alfalfa cover and without insecticides over four growing seasons. Int. J. Fruit Sci. 2011, 11, 99–110. [Google Scholar] [CrossRef]
- Mullinix, K.; Isman, M.B.; Brunner, J.F. Key and Secondary Arthropod Pest Population Trends in Apple Cultivated over Four Seasons with No Insecticides and a Legume Cover. J. Sustain. Agric. 2010, 34, 584–594. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Damaged-self recognition in common bean (Phaseolus vulgaris) shows taxonomic specificity and triggers signaling via reactive oxygen species (ROS). Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Gontijo, L.M.; Beers, E.H.; Snyder, W.E. Flowers promote aphid suppression in apple orchards. Biol. Control 2013, 66, 8–15. [Google Scholar] [CrossRef]
- Bone, N.J.; Thomson, L.J.; Ridland, P.M.; Cole, P.; Hoffmann, A.A. Cover crops in Victorian apple orchards: Effects on production, natural enemies and pests across a season. Crop Prot. 2009, 28, 675–683. [Google Scholar] [CrossRef]
- Jaworska, K. The cover of herbaceous plants in an IPM apple orchard and its influence on the occurrence of rodents. IOBC/WPRS Bull. 1996, 19, 431–432. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Goulet, H.; O’Hara, J.; Masner, L.; Racette, G. Towards insecticide free apple orchards: Flowering plants to attract beneficial arthropods. Biocontrol Sci. Technol. 2004, 14, 25–37. [Google Scholar] [CrossRef]
- Cahenzli, F.; Sigsgaard, L.; Daniel, C.; Herz, A.; Jamar, L.; Kelderer, M.; Jacobsen, S.K.; Kruczyńska, D.; Matray, S.; Porcel, M.; et al. Perennial flower strips for pest control in organic apple orchards—A pan-European study. Agric. Ecosyst. Environ. 2019, 278, 43–53. [Google Scholar] [CrossRef]
- Wyss, E. The effects of weed strips on aphids and aphidophagous predators in an apple orchard. Entomol. Exp. Appl. 1995, 75, 43–49. [Google Scholar] [CrossRef]
- Wyss, E. The effects of artificial weed strips on diversity and abundance of the arthropod fauna in a Swiss experimental apple orchard. Agric. Ecosyst. Environ. 1996, 60, 47–59. [Google Scholar] [CrossRef]
- Wyss, E.; Niggli, U.; Nentwig, W. The impact of spiders on aphid populatons in a strip-managed apple orchard. J. Appl. Entomol.-Z. Angew. Entomol. 1995, 119, 473–478. [Google Scholar] [CrossRef]
- Cross, J.V.; Cubison, S.; Harris, A.; Harrington, R. Autumn control of rosy apple aphid, Dysaphis plantaginea (Passerini), with aphicides. Crop Prot. 2007, 26, 1140–1149. [Google Scholar] [CrossRef]
- Cahenzli, F.; Pfiffner, L.; Daniel, C. Reduced crop damage by self-regulation of aphids in an ecologically enriched, insecticide-free apple orchard. Agron. Sustain. Dev. 2017, 37. [Google Scholar] [CrossRef]
- Sackett, T.E.; Buddle, C.M.; Vincentb, C. Dynamics of spider colonization of apple orchards from adjacent deciduous forest. Agric. Ecosyst. Environ. 2009, 129, 144–148. [Google Scholar] [CrossRef]
- Smith, R.B.; Mommsen, T.P. Pollen feeding in an orb-weaving spider. Science 1984, 226, 1330–1332. [Google Scholar] [CrossRef]
- Taylor, R.M.; Pfannenstiel, R.S. Nectar feeding by wandering spiders on cotton plants. Environ. Entomol. 2008, 37, 996–1002. [Google Scholar] [CrossRef]
- Falta, V.; Holy, K.; Vavra, R. Enhancing abundance of natural enemies in apple orchard using flowering strips. In Proceedings of the Ecofruit. 14th International Conference on Organic Fruit-Growing. Proceedings for the Conference, Hohenheim, Germany, 22–24 February 2010; pp. 395–398. [Google Scholar]
- Dib, H.; Libourel, G.; Warlop, F. Entomological and functional role of floral strips in an organic apple orchard: Hymenopteran parasitoids as a case study. J. Insect Conserv. 2012, 16, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Rousselin, A.; Bevacqua, D.; Sauge, M.-H.; Lescourret, F.; Mody, K.; Jordan, M.-O. Harnessing the aphid life cycle to reduce insecticide reliance in apple and peach orchards. A review. Agron. Sustain. Dev. 2017, 37. [Google Scholar] [CrossRef]
- Albert, L.; Franck, P.; Gilles, Y.; Plantegenest, M. Impact of Agroecological Infrastructures on the Dynamics of Dysaphis plantaginea (Hemiptera: Aphididae) and Its Natural Enemies in Apple Orchards in Northwestern France. Environ. Entomol. 2017, 46, 528–537. [Google Scholar] [CrossRef]
- Pfiffner, L.; Cahenzli, F.; Steinemann, B.; Jamar, L.; Bjørn, M.C.; Porcel, M.; Tasin, M.; Telfser, J.; Kelderer, M.; Lisek, J.; et al. Design, implementation and management of perennial flower strips to promote functional agrobiodiversity in organic apple orchards: A pan-European study. Agric. Ecosyst. Environ. 2019, 278, 61–71. [Google Scholar] [CrossRef]
- Kienzle, J.; Foell, M.; Karrer, E.; Krismann, A. Establishment of permanent weed strips with autochthonous nectar plants and their effect on the occurrence of aphid predators. In Proceedings of the 16th International Conference on Organic Fruit-Growing, Stuttgart-Hohenheim, Germany, 17–19 February 2014; pp. 31–39. [Google Scholar]
- Vogt, H.; Weigel, A. Is it possible to enhance the biological control of aphids in an apple orchard with flowering strips? IOBC/Wprs Bull. 1999, 22, 39–46. [Google Scholar]
- Markó, V.; Keresztes, B. Flowers for better pest control? Ground cover plants enhance apple orchard spiders (Araneae), but not necessarily their impact on pests. Biocontrol Sci. Technol. 2014, 24, 574–596. [Google Scholar] [CrossRef]
- Markó, V.; Jenser, G.; Mihályi, K.; Hegyi, T.; Balázs, K. Flowers for better pest control? Effects of apple orchard groundcover management on mites (Acari), leafminers (Lepidoptera, Scitellidae), and fruit pests. Biocontrol Sci. Technol. 2012, 22, 39–60. [Google Scholar] [CrossRef]
- Markó, V.; Jenser, G.; Kondorosy, E.; Ábrahám, L.; Balázs, K. Flowers for better pest control? The effects of apple orchard ground cover management on green apple aphids (Aphis spp.) (Hemiptera: Aphididae), their predators and the canopy insect community. Biocontrol Sci. Technol. 2013, 23, 126–145. [Google Scholar] [CrossRef]
- Fitzgerald, J.D.; Solomon, M.G. Can Flowering Plants Enhance Numbers of Beneficial Arthropods in UK Apple and Pear Orchards? Biocontrol Sci. Technol. 2004, 14, 291–300. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Tylianakis, J.; Kean, J.; Keller, M. Providing plant foods for insect natural enemies in farming systems: Balancing practicalities and theory. In Plant-Derived Food and Plant-Carnivore Mutualism; Wäckers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 326–341. [Google Scholar]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wackers, F. Getting More Power from Your Flowers: Multi-Functional Flower Strips Enhance Pollinators and Pest Control Agents in Apple Orchards. Insects 2017, 8, 101. [Google Scholar] [CrossRef]
- Bischoff, A.; Steinger, T.; Muller-Scharer, H. The Importance of Plant Provenance and Genotypic Diversity of Seed Material Used for Ecological Restoration. Restor. Ecol. 2010, 18, 338–348. [Google Scholar] [CrossRef]
- Bischoff, A.; Vonlanthen, B.; Steinger, T.; Mueller-Schaerer, H. Seed provenance matters—Effects on germination of four plant species used for ecological restoration. Basic Appl. Ecol. 2006, 7, 347–359. [Google Scholar] [CrossRef]
- Debras, J.F.; Senoussi, R.; Rieux, R.; Buisson, E.; Dutoit, T. Spatial distribution of an arthropod community in a pear orchard (Southern France). Identification of a hedge effect. Agric. Ecosyst. Environ. 2008, 127, 166–176. [Google Scholar] [CrossRef]
- Debras, J.-F.; Dussaud, A.; Rieux, R.; Dutoit, T. A prospective research on the hedgerow’s ‘source’ function. C. R. Biol. 2007, 330, 664–673. [Google Scholar] [CrossRef]
- Heath, S.K.; Soykan, C.U.; Velas, K.L.; Kelsey, R.; Kross, S.M. A bustle in the hedgerow: Woody field margins boost on farm avian diversity and abundance in an intensive agricultural landscape. Biol. Conserv. 2017, 212, 153–161. [Google Scholar] [CrossRef]
- Duso, C.; Malagnini, V.; Paganelli, A.; Aldegheri, L.; Bottini, M.; Otto, S. Pollen availability and abundance of predatory phytoseiid mites on natural and secondary hedgerows. Biocontrol 2004, 49, 397–415. [Google Scholar] [CrossRef]
- Coli, W.M.; Ciurlino, R.A.; Hosmer, T. Effect of understory and border vegetation composition on phytophagous and predatory mites in Massachusetts commerical apple orchards. Agric. Ecosyst. Environ. 1994, 50, 49–60. [Google Scholar] [CrossRef]
- Tuovinen, T. Influence of surrounding trees and bushes on the phytoseiid mite fauna on apple orchard trees in Finland. Agric. Ecosyst. Environ. 1994, 50, 39–47. [Google Scholar] [CrossRef]
- Ricci, B.; Franck, P.; Bouvier, J.-C.; Casado, D.; Lavigne, C. Effects of hedgerow characteristics on intra-orchard distribution of larval codling moth. Agric. Ecosyst. Environ. 2011, 140, 395–400. [Google Scholar] [CrossRef]
- Maalouly, M.; Franck, P.; Bouvier, J.C.; Toubon, J.F.; Lavigne, C. Codling moth parasitism is affected by semi-natural habitats and agricultural practices at orchard and landscape levels. Agric. Ecosyst. Environ. 2013, 169, 33–42. [Google Scholar] [CrossRef]
- Piekarska-Boniecka, H.; Siatkowski, I.; Zyprych-Walczak, J.; Trzcinski, P.; Rzanska-Wieczorek, M. The phenology of occurrence of dominant predatory Syrphidae (Diptera) species in apple orchards and on their edges. Acta Sci. Pol.-Hortorum Cultus 2017, 16, 23–38. [Google Scholar]
- Trzcinski, P.; Piekarska-Boniecka, H. Dynamics of predatory Syrphidae in the apple orchard and neighbouring shrubberies. J. Plant Prot. Res. 2013, 53, 119–123. [Google Scholar] [CrossRef]
- Lefebvre, M.; Papaïx, J.; Mollot, G.; Deschodt, P.; Lavigne, C.; Ricard, J.-M.; Mandrin, J.-F.; Franck, P. Bayesian inferences of arthropod movements between hedgerows and orchards. Basic Appl. Ecol. 2017, 21, 76–84. [Google Scholar] [CrossRef]
- Brown, M.W.; Mathews, C.R. Conservation Biological Control of Rosy Apple Aphid, Dysaphis plantaginea (Passerini), in Eastern North America. Environ. Entomol. 2007, 36, 1131–1139. [Google Scholar] [CrossRef]
- Brown, M.W.; Mathews, C.R. Conservation biological control of spirea aphid, Aphis spiraecola (Hemiptera: Aphididae) on apple by providing natural alternative food resources. Eur. J. Entomol. 2008, 105, 537–540. [Google Scholar] [CrossRef]
- Brown, M.W.; Mathews, C.R.; Krawczyk, G. Extrafloral Nectar in an Apple Ecosystem to Enhance Biological Control. J. Econ. Entomol. 2010, 103, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Spellman, B.; Brown, M.W.; Mathews, C.R. Effect of floral and extrafloral resources on predation of Aphis spiraecola by Harmonia axyridis on apple. Biocontrol 2006, 51, 715–724. [Google Scholar] [CrossRef]
- Brown, M.W.; Mathews, C.R.; Krawczyk, G. Analyzing the results of a biodiversity experiment: Enhancing parasitism of Platynota idaeusalis (Lepidoptera: Tortricidae). IOBC/WPRS Bull. 2010, 54, 13. [Google Scholar]
- Brown, M.W. Role of biodiversity in integrated fruit production in eastern North American orchards. Agric. For. Entomol. 2012, 14, 89–99. [Google Scholar] [CrossRef]
- Song, B.; Jiao, H.; Tang, G.; Yao, Y. Combining repellent and attractive aromatic plants to enhance biological control of three tortricid species (Lepidoptera: Tortricidae) in an apple orchard. Fla. Entomol. 2014, 97, 1679–1689. [Google Scholar] [CrossRef]
- Song, B.; Tang, G.; Sang, X.; Zhang, J.; Yao, Y.; Wiggins, N. Intercropping with aromatic plants hindered the occurrence of Aphis citricola in an apple orchard system by shifting predator–prey abundances. Biocontrol Sci. Technol. 2013, 23, 381–395. [Google Scholar] [CrossRef]
- Song, B.; Zhang, J.; Wiggins, N.L.; Yao, Y.; Tang, G.; Sang, X. Intercropping with Aromatic Plants Decreases Herbivore Abundance, Species Richness, and Shifts Arthropod Community Trophic Structure. Environ. Entomol. 2012, 41, 872–879. [Google Scholar] [CrossRef]
- Tang, G.B.; Song, B.Z.; Zhao, L.L.; Sang, X.S.; Wan, H.H.; Zhang, J.; Yao, Y.C. Repellent and attractive effects of herbs on insects in pear orchards intercropped with aromatic plants. Agrofor. Syst. 2013, 87, 273–285. [Google Scholar] [CrossRef]
- Imbert, C.; Warlop, F.; Husson, L.; Lavigne, C. Does the association between fruit trees and vegetables promote functional biodiversity and biocontrol of vegetables pests? IOBC/WPRS Bull. 2017, 122, 123–126. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef] [Green Version]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutiérrez, C.; López, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Iverson, A.L.; Marín, L.E.; Ennis, K.K.; Gonthier, D.J.; Connor-Barrie, B.T.; Remfert, J.L.; Cardinale, B.J.; Perfecto, I. REVIEW: Do polycultures promote win-wins or trade-offs in agricultural ecosystem services? A meta-analysis. J. Appl. Ecol. 2014, 51, 1593–1602. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Bhagwat, S.A.; Buchori, D.; Faust, H.; Hertel, D.; Holscher, D.; Juhrbandt, J.; Kessler, M.; Perfecto, I.; et al. Multifunctional shade-tree management in tropical agroforestry landscapes—A review. J. Appl. Ecol. 2011, 48, 619–629. [Google Scholar] [CrossRef]
- Addison, J.A.; Hardman, J.M.; Walde, S.J. Pollen availability for predaceous mites on apple: Spatial and temporal heterogeneity. Exp. Appl. Acarol. 2000, 24, 1–18. [Google Scholar] [CrossRef]
- Nyffeler, M.; Olson, E.J.; Symondson, W.O.C. Plant-eating by spiders. J. Arachnol. 2016, 44, 15–27. [Google Scholar] [CrossRef]
- Araj, S.E.; Wratten, S.; Lister, A.; Buckley, H. Adding floral nectar resources to improve biological control: Potential pitfalls of the fourth trophic level. Basic Appl. Ecol. 2009, 10, 554–562. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Bärtschi, C.; Collatz, J.; Entling, M.H.; Jacot, K. Perennial, species-rich wildflower strips enhance pest control and crop yield. Agric. Ecosyst. Environ. 2016, 220, 97–103. [Google Scholar] [CrossRef]
- Santos, L.A.O.; Botelho Costa, M.; Lavigne, C.; Fernandes, O.A.; Bischoff, A.; Franck, P. Influence of the margin vegetation on the conservation of aphid biological control in apple orchards. J. Insect Conserv. 2018, 22, 465–474. [Google Scholar] [CrossRef]
- Schirmel, J.; Albrecht, M.; Bauer, P.M.; Sutter, L.; Pfister, S.C.; Entling, M.H. Landscape complexity promotes hoverflies across different types of semi-natural habitats in farmland. J. Appl. Ecol. 2018, 55, 1747–1758. [Google Scholar] [CrossRef]
- Haenke, S.; Kovacs-Hostyanszki, A.; Fruend, J.; Batary, P.; Jauker, B.; Tscharntke, T.; Holzschuh, A. Landscape configuration of crops and hedgerows drives local syrphid fly abundance. J. Appl. Ecol. 2014, 51, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Haenke, S.; Scheid, B.; Schaefer, M.; Tscharntke, T.; Thies, C. Increasing syrphid fly diversity and density in sown flower strips within simple vs. complex landscapes. J. Appl. Ecol. 2009, 46, 1106–1114. [Google Scholar] [CrossRef]
- Happe, A.K.; Roquer-Beni, L.; Bosch, J.; Alins, G.; Mody, K. Earwigs and woolly apple aphids in integrated and organic apple orchards: Responses of a generalist predator and a pest prey to local and landscape factors. Agric. Ecosyst. Environ. 2018, 268, 44–51. [Google Scholar] [CrossRef]
- Duru, M.; Therond, O.; Fares, M. Designing agroecological transitions; A review. Agron. Sustain. Dev. 2015, 35, 1237–1257. [Google Scholar] [CrossRef] [Green Version]
- Uyttenbroeck, R.; Hatt, S.; Paul, A.; Boeraeve, F.; Piqueray, J.; Francis, F.; Danthine, S.; Frederich, M.; Dufrene, M.; Bodson, B.; et al. Pros and cons of flowers strips for farmers. A review. Biotechnol. Agron. Soc. 2016, 20, 225–235. [Google Scholar]
- Wiman, M.R.; Kirby, E.M.; Granatstein, D.M.; Sullivan, T.P. Cover crops influence meadow vole presence in organic orchards. HortTechnology 2009, 19, 558–562. [Google Scholar] [CrossRef]
- Penvern, S.; Fernique, S.; Cardona, A.; Herz, A.; Ahrenfeldt, E.; Dufils, A.; Jamar, L.; Korsgaard, M.; Kruczyńska, D.; Matray, S.; et al. Farmers’ management of functional biodiversity goes beyond pest management in organic European apple orchards. Agric. Ecosyst. Environ. 2019, 284, 106555. [Google Scholar] [CrossRef]
- Kirmer, A.; Rydgren, K.; Tischew, S. Smart management is key for successful diversification of field margins in highly productive farmland. Agric. Ecosyst. Environ. 2018, 251, 88–98. [Google Scholar] [CrossRef]
- Buri, P.; Humbert, J.Y.; Stanska, M.; Hajdamowicz, I.; Tran, E.; Entling, M.H.; Arlettaz, R. Delayed mowing promotes planthoppers, leafhoppers and spiders in extensively managed meadows. Insect Conserv. Divers. 2016, 9, 536–545. [Google Scholar] [CrossRef]
- Dib, H.; Simon, S.; Sauphanor, B.; Capowiez, Y. The role of natural enemies on the population dynamics of the rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in organic apple orchards in south-eastern France. Biol. Control 2010, 55, 97–109. [Google Scholar] [CrossRef]
- Ioriatti, C.; Tasin, M. Hail nets enhance disruption of sexual communication by synthetic pheromone in codling moth. Entomol. Gen. 2018, 37, 7–18. [Google Scholar] [CrossRef]
- Jehle, J.A.; Schulze-Bopp, S.; Undorf-Spahn, K.; Fritsch, E. Evidence for a Second Type of Resistance against Cydia pomonella Granulovirus in Field Populations of Codling Moths. Appl. Environ. Microbiol. 2017, 83, 13. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Cross, J.V.; Marko, V. Can artificial nectaries outcompete aphids in ant-aphid mutualism? Applying artificial sugar sources for ants to support better biological control of rosy apple aphid, Dysaphis plantaginea Passerini in apple orchards. Crop Prot. 2015, 77, 127–138. [Google Scholar] [CrossRef]
- Simpson, M.; Gurr, G.M.; Simmons, A.T.; Wratten, S.D.; James, D.G.; Leeson, G.; Nicol, H.I.; Orre-Gordon, G.U.S. Attract and reward: Combining chemical ecology and habitat manipulation to enhance biological control in field crops. J. Appl. Ecol. 2011, 48, 580–590. [Google Scholar] [CrossRef]
- Swiergiel, W.; Manduric, S.; Rämert, B.; Porcel, M.; Tasin, M. Development of sustainable plant protection programs through multi-actor Co-innovation: An 8-year case study in Swedish apple production. J. Clean. Prod. 2019, 234, 1178–1191. [Google Scholar] [CrossRef]
- Klotz, S.; Kühn, I.; Durka, W. BIOLFLOR—Eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland. Schriftenreihe für Vegetationskunde 2002, 38, 334. [Google Scholar]
- Fernandez-Triana, J.; Goulet, H.; Bostanian, N.J.; Boudreault, C. Diversity of Microgastrinae (Hymenoptera: Braconidae) in apple orchards of southern Quebec, Canada. Biocontrol Sci. Technol. 2009, 19, 237–248. [Google Scholar] [CrossRef]
- Brown, M.W.; Schmitt, J.J. Seasonal and diurnal dynamics of beneficial insect populations in apple orchards under different management intensity. Environ. Entomol. 2001, 30, 415–424. [Google Scholar] [CrossRef]


| Family | Species | Reward System | Flower Type [128] | Managed As | Reference |
|---|---|---|---|---|---|
| Apiaceae | Ammi majus L. | Nectar, pollen | Disk flowers with nectar open | Cover crop, flower strip | [54,78] |
| Apiaceae | Anethum graveolens L. | Nectar, pollen | Disk flowers with nectar open | Cover crop, flower strip | [36,78,97] |
| Apiaceae | Carum carvi L. | Nectar, pollen | Disk flowers with nectar open | Flower strip | [57,71,72] |
| Apiaceae | Coriandrum sativum L. | Nectar, pollen | Disk flowers with nectar open | Cover crop, flower strip | [28,41,78] |
| Apiaceae | Daucus carota L. | Nectar, pollen | Disk flowers with nectar open | Flower strip | [57,60,66,72,78] |
| Apiaceae | Foeniculum vulgare Mill. | Nectar plentiful, pollen | Disk flowers with nectar open | Cover crop, flower strip | [54,72,78] |
| Apiaceae | Pastinaca sativa L. | Nectar plentiful, pollen | Disk flowers with nectar open | Flower strip | [60,78] |
| Asteraceae | Achilea millefolium L. | Pollen, nectar | Flower heads, ray and disk flowers | Cover crop, flower strip | [54,56,67,69,78] |
| Asteraceae | Cichorium intybus L. | Pollen, nectar | Flower heads, only ray flowers | Cover crop, flower strip | [54,57,60,78] |
| Asteraceae | Leucanthemum vulgare agg. | Pollen, nectar | Flower heads, ray and disk flowers | Flower strip | [57,60,71,72] |
| Asteraceae | Matricaria chamomilla L. (M. recutita L.) | Pollen, nectar | Flower heads, ray and disk flowers | Flower strip | [60,72] |
| Asteraceae | Tanacetum vulgare L. | Pollen, nectar | Flower heads, only ray flowers | Flower strip | [56,78] |
| Brassicaceae | Brassica napus, Sinapis alba, Sinapis arvensis | Nectar open, pollen | Disk flowers with nectar open | Cover crop, flower strip | [36,54,72] |
| Brassicaceae | Lobularia maritima (L.) | Pollen, nectar | Disk flowers with nectar ± hidden | Cover crop, flower strip | [40,53,78] |
| Boraginaceae | Phacelia tanacetifolia Benth. | Nectar, plentiful | Funnel flowers, corolla tube long | Cover crop, flower strip | [38,40,78,97] |
| Caryophyllaceae | Silene vulgaris (Moench) | Nectar | Stalk disc flowers, stamina and pistil outside tube | Flower strip | [69,71] |
| Dipsacaceae | Knautia arvensis (L.) | Nectar, pollen | Flower heads | Flower strip | [60,72,78] |
| Fabaceae | Lotus corniculatus L. | Nectar, pollen | Flag blossom | Flower strip | [57,60,72,78] |
| Fabaceae | Medicago sativa; Medicago lupulina L. | Nectar, pollen, extrafloral nectaries? | Flag blossom | Cover crop, flower strip | [37,44,50,51,57,60,72,78] |
| Fabaceae | Trifolium repens L., Trifolium fragiferum L., Trifolium sp. | Pollen, nectar and extrafloral nectaries? | Flag blossom | Cover crop, flower strip | [39,72,78,129] |
| Fabaceae | Trigonella foenum-graecum L. | Pollen, nectar | Flag blossom | Cover crop | [54] |
| Fabaceae | Vicia faba L., Vicia sativa, Vicia dasycarpa, Vicia cracca, Vicia sepium | Pollen, nectar hidden, long corolla, extrafloral nectaries | Flag blossom | Cover crop | [42,49,57,72,78,129] |
| Polygonaceae | Fagopyrum esculentum Moench | Pollen, nectar open, plentiful | Disk flowers with nectar open | Cover crop, flower strip | [36,38,40,42,43,54,78,97] |
| “Aromatic plants”, mainly Lamiaceae | Mentha canadensis, Ageratum houstoniuma, Ocimum basilicum, O. citriodourm, Nepeta cataria, Tagetes patula, Satureja hortensis | Pollen, nectar, Volatiles (repellent?) | Various, depending on species | Intercrop | [98,99,100,101] |
| Rosaceae | Pyrus communis L., Prunus avium L., Prunus persica (L.) Batsch | Pollen, nectar, extrafloral nectaries | disk flowers with nectar ± hidden in centre of flower | Intercrop | [92,93,94,97,130] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herz, A.; Cahenzli, F.; Penvern, S.; Pfiffner, L.; Tasin, M.; Sigsgaard, L. Managing Floral Resources in Apple Orchards for Pest Control: Ideas, Experiences and Future Directions. Insects 2019, 10, 247. https://doi.org/10.3390/insects10080247
Herz A, Cahenzli F, Penvern S, Pfiffner L, Tasin M, Sigsgaard L. Managing Floral Resources in Apple Orchards for Pest Control: Ideas, Experiences and Future Directions. Insects. 2019; 10(8):247. https://doi.org/10.3390/insects10080247
Chicago/Turabian StyleHerz, Annette, Fabian Cahenzli, Servane Penvern, Lukas Pfiffner, Marco Tasin, and Lene Sigsgaard. 2019. "Managing Floral Resources in Apple Orchards for Pest Control: Ideas, Experiences and Future Directions" Insects 10, no. 8: 247. https://doi.org/10.3390/insects10080247
APA StyleHerz, A., Cahenzli, F., Penvern, S., Pfiffner, L., Tasin, M., & Sigsgaard, L. (2019). Managing Floral Resources in Apple Orchards for Pest Control: Ideas, Experiences and Future Directions. Insects, 10(8), 247. https://doi.org/10.3390/insects10080247






