Morphology and Distribution of the Antennal Sensilla of Two Species, Megalurothrips usitatus and Thrips palmi (Thysanoptera: Thripidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Scanning Electron Microscopy (SEM)
2.3. Statistical Analysis
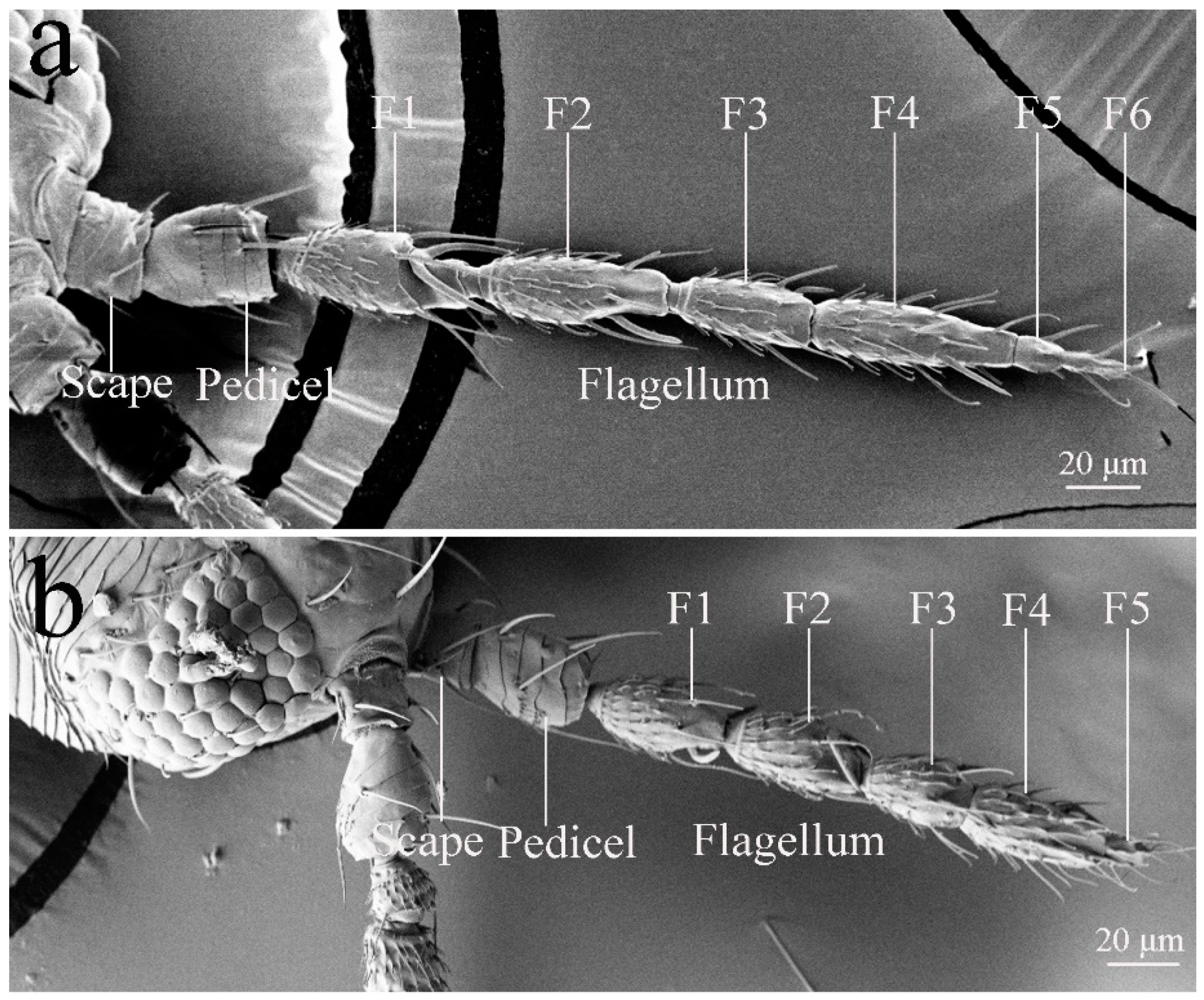
3. Results
3.1. General Morphology of Antennae
3.2. Types of Antennal Sensilla
3.2.1. Böhm Bristles (BB)
3.2.2. Sensilla Campaniformia (Sca)
3.2.3. Sensilla Trichodea (St)
3.2.4. Sensilla Chaetica (Sch)
3.2.5. Sensilla Styloconica (Sst)
3.2.6. Sensilla Basiconica (Sb)
3.2.7. Sensilla Cavity (Scav)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, L.D.; Yan, K.L.; Fu, B.L.; Wu, J.H.; Liu, K.; Lu, Y.Y. The life table parameters of Megalurothrips usitatus (Thysanoptera: Thripidae) on four leguminous crops. Fla. Entomol. 2015, 98, 620–625. [Google Scholar] [CrossRef]
- Macleod, A.; Head, J.; Gaunt, A. An assessment of the potential economic impact of Thrips palmi on horticulture in England and the significance of a successful eradication campaign. Crop Prot. 2004, 23, 601–610. [Google Scholar] [CrossRef]
- Hunter, W.B.; Ullman, D.E. Precibarial and cibarial chemosensilla in the western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Int. J. Insect Morphol. Embryol. 1994, 23, 69–83. [Google Scholar] [CrossRef]
- Zhu, W.J.; Zhou, S.H.; Wang, S.J.; Han, D.Y.; Chen, J.Y.; Fu, Y.G. Ultrastructure and distribution of antennal sensilla of the chilli thrips Scirtothrips dorsalis hood (Thysanoptera: Thripidae). Microsc. Res. Techniq. 2017, 80. [Google Scholar] [CrossRef] [PubMed]
- De Facci, M.; Wallén, R.; Hallberg, E.; Anderbrant, O. Flagellar sensilla of the eusocial gall-inducing thrips Kladothrips intermedius and its kleptoparasite, Koptothrips dyskritus (Thysanoptera: Phlaeothripinae). Arthropod Struct. Dev. 2011, 40, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Ganske, A.S.; Uhl, G. The sensory equipment of a spider-A morphological survey of different types of sensillum in both sexes of Argiope bruennichi (Araneae, Araneidae). Arthropod Struct. Dev. 2018, 47, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Onagbola, E.O.; Fadamiro, H.Y. Scanning electron microscopy studies of antennal sensilla of Pteromalus cerealellae (Hymenoptera: Pteromalidae). Micron 2008, 39, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Weseloh, R.M. Sense organs of the hyperparasite Cheiloneurus noxius (Hymenoptera: Encyrtidae) important in host selection processes. Ann. Entomol. Soc. Am. 1972, 65, 41–46. [Google Scholar] [CrossRef]
- Bin, F.; Colazza, S.; Isidoro, N.; Solinas, M.; Vinson, S.B. Antennal chemosensilla and glands, and their possible meaning in the reproductive behaviour of Trissolcus basalis (Woll) (Hymenoptera: Scelionidae). Entomologica 1989, 30, 33–97. [Google Scholar]
- Isidoro, N.; Bin, F.; Colazza, S.; Vinson, S.B. Morphology of antennal gustatory sensilla and glands in some parasitoid Hymenoptera with hypothesis on their role in sex and host recognition. J. Hymenopt. Res. 1996, 5, 206–239. [Google Scholar]
- Bartlet, E.; Romani, R.; Williams, I.H.; Isidoro, N. Functional anatomy of sensory structures on the antennae of Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae). Int. J. Insect Morphol. Embryol. 1999, 28, 291–300. [Google Scholar] [CrossRef]
- Slifer, E.H.; Sekhon, S.S. Sense organs on the antennae of two species of thrips (Thysanoptera, insecta). J. Morphol. 1974, 143, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Govindarajulu, V. Management of blossom thrips, Megalurothrips usitatus on pigeonpea. Trop Pest Manag. 1990, 36, 312–313. [Google Scholar] [CrossRef]
- Duraimurugan, P.; Tyagi, K. Pest spectra, succession and its yield losses in mungbean and urdbean underchanging climatic scenario. Legum. Res. 2014, 37, 212. [Google Scholar] [CrossRef]
- Dialoke, S. The population of leaf beetles (Leptualaca fassicollis thoms Coleoptera: Chrysomelidae) and flower thrips (Megalurothrips usitatus Bagnall Thysanoptera: Thripide) on pigeonpea under the influence of plant density and planting date in a rain forest zone, Nigeria. J. Biol. Agric. Healthc. 2013, 3, 81–86. [Google Scholar]
- Aliakbarpour, H.; Rawi, C.S.M. The species composition of thrips (Insecta: Thysanoptera) inhabiting mango orchards in Pulau Pinang, Malaysia. Trop. Life Sci. Res. 2012, 23, 45. [Google Scholar] [PubMed]
- Cardona, C.; Frei, A.; Bueno, J.M.; Diaz, J.; Gu, H.; Dorn, S. Resistance to Thrips palmi (Thysanoptera: Thripidae) in beans. J. Econ. Entomol. 2002, 95, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.J.C.; Matthews, L.; Collins, D.W. A review of the pest status and control options for Thrips palmi. Crop Prot. 2007, 26, 1089–1098. [Google Scholar] [CrossRef]
- Mcdonald, J.R.; Head, J.; Bale, J.S.; Walters, K.F.A. Cold tolerance, overwintering and establishment potential of Thrips palmi. Physiol. Entomol. 2005, 25, 159–166. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Antennae and sensilla. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.J., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 6, pp. 1–69. [Google Scholar]
- Ding, Y.H.; ZhuGe, P.P.; Wang, M.Q.; Zhang, G.A. Observation of antennal sensilla of Frankliniella occidentalis with scanning electron microscopy. Chin. Bull. Entomol. 2011, 47, 165–171. [Google Scholar]
- Li, W.N.; Feng, J.N. Ultrastructure of antennal sensilla in three species of Frankliniella Karny (Thysanoptera: Thripidae). Acta Entomol. Sin. 2013, 56, 1088–1100. [Google Scholar]
- Krishnan, A.; Prabhakar, S.; Sudarsan, S.; Sane, S.P. The neural mechanisms of antennal positioning in flying moths. J. Exp. Biol. 2012, 215, 3096–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Specie | Gender | Scape (μm) | Pedicel (μm) | Flagellum (μm) | Total Length (μm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |||||
| M | female (n = 10) | 32.79 ± 0.63 | 37.43 ± 0.68 | 55.38 ± 0.97 | 59.43 ± 1.34 | 38.07 ± 1.08 | 58.02 ± 0.33 | 15.25 ± 0.49 | 23.47 ± 0.44 | 319.84 ± 2.17 |
| male (n = 10) | 21.62 ± 0.96 | 30.53 ± 0.66 | 44.30 ± 0.93 | 47.77 ± 1.98 | 30.90 ± 1.03 | 48.09 ± 1.43 | 12.06 ± 0.46 | 16.22 ± 1.86 | 252.66 ± 6.71 | |
| T | female (n = 10) | 17.35 ± 1.31 | 30.50 ± 0.99 | 38.50 ± 0.86 | 39.01 ± 1.26 | 31.19 ± 0.52 | 43.04 ± 0.80 | 14.31 ± 0.34 | - | 213.89 ± 1.92 |
| male (n = 10) | 17.63 ± 0.73 | 31.58 ± 1.77 | 36.06 ± 2.40 | 39.60 ± 1.02 | 30.88 ± 1.21 | 42.38 ± 1.68 | 14.76 ± 0.69 | - | 212.88 ± 3.22 | |
| Antennal Segments | Species (n = 10) | BB | Sca | St | Sst | Scav | Sch | Sb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | ||||||||
| Scape | M | 5 | - | - | - | - | 10 | - | - | - | - | |
| T | 5 | - | - | - | - | 8 | - | - | - | - | ||
| Pedicel | M | - | 1 | - | - | - | 7 | - | - | - | - | |
| T | - | 1 | - | - | - | 8 | - | - | - | - | ||
| Flagellum | F1 | M | - | - | - | - | 1 | 6 | - | 1 | - | - |
| T | - | - | - | - | 1 | 6 | - | 1 | - | - | ||
| F2 | M | - | - | - | - | - | 6 | - | 1 | 1 | - | |
| T | - | - | - | - | - | 6 | - | 1 | 1 | - | ||
| F3 | M | - | - | - | 1 | - | 6 | - | - | - | 1 | |
| T | - | - | - | 1 | - | 6 | - | - | - | 1 | ||
| F4 | M | - | - | - | 1 | - | 8 | - | - | - | 2 | |
| T | - | - | - | 1 | - | 6 | - | - | - | - | ||
| F5 | M | - | - | - | - | - | 2 | - | - | - | 1 | |
| T | - | - | 4 | - | - | - | 4 | - | - | 1 | ||
| F6 | M | - | - | 2 | - | - | - | 3 | - | - | - | |
| Species | BB | St | Sst | Sca | Sb | Sch | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | |||||
| M (n = 10) | 3.04 ± 0.12 | 19.34 ± 1.53 | 11.37 ± 1.10 | 3.86 ± 0.13 | 36.35 ± 2.64 | 8.15 ± 1.23 | 27.55 ± 2.46 | 43.68 ± 1.56 | 32.05 ± 2.16 |
| T (n = 10) | 2.59 ± 0.16 | - | 8.55 ± 0.22 | 3.75 ± 0.17 | 16.16 ± 0.73 | 5.77 ± 0.35 | - | 26.56 ± 1.11 | 16.31 ± 0.61 |
| Species (n = 10) | BB | St | Sst | Sb | Sch | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | |||||
| Proximal | M | 0.81 ± 0.04 | 1.16 ± 0.09 | 3.98 ± 2.05 | 4.31 ± 0.33 | 2.01 ± 0.22 | 3.12 ± 0.30 | - | 1.73 ± 0.13 |
| T | - | - | 1.24 ± 0.04 | 0.89 ± 0.05 | - | - | - | - | |
| Middle | M | 0.38 ± 0.13 | - | 1.39 ± 0.13 | 3.10 ± 0.20 | 1.75 ± 0.27 | 2.13 ± 0.19 | - | 1.57 ± 0.12 |
| T | - | - | 1.27 ± 0.06 | - | - | - | - | - | |
| Distal | M | - | - | 1.11 ± 0.11 | 1.26 ± 0.11 | 0.92 ± 0.14 | 1.38 ± 0.18 | - | 0.82 ± 0.09 |
| T | - | - | - | - | - | - | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-S.; Shaukat, A.; Han, Y.; Yang, B.; Tang, L.-D.; Wu, J.-H. Morphology and Distribution of the Antennal Sensilla of Two Species, Megalurothrips usitatus and Thrips palmi (Thysanoptera: Thripidae). Insects 2019, 10, 251. https://doi.org/10.3390/insects10080251
Wang X-S, Shaukat A, Han Y, Yang B, Tang L-D, Wu J-H. Morphology and Distribution of the Antennal Sensilla of Two Species, Megalurothrips usitatus and Thrips palmi (Thysanoptera: Thripidae). Insects. 2019; 10(8):251. https://doi.org/10.3390/insects10080251
Chicago/Turabian StyleWang, Xiao-Shuang, Ali Shaukat, Yun Han, Bo Yang, Liang-De Tang, and Jian-Hui Wu. 2019. "Morphology and Distribution of the Antennal Sensilla of Two Species, Megalurothrips usitatus and Thrips palmi (Thysanoptera: Thripidae)" Insects 10, no. 8: 251. https://doi.org/10.3390/insects10080251





