First Insights into the Intrapuparial Development of Bactrocera dorsalis (Hendel): Application in Predicting Emergence Time for Tephritid Fly Control
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Pupariation
3.2. Larval-Pupal Apolysis (0–34 h, 0–1.42 days, 32.4 ± 0.23 h)
3.3. Cryptocephalic Pupa (32–40 h, 1.33–1.67 days, 4.7 ± 0.22 h)
3.4. Phanerocephalic Pupa (36–42 h, 1.5–1.75 days, 5.5 ± 0.20 h)
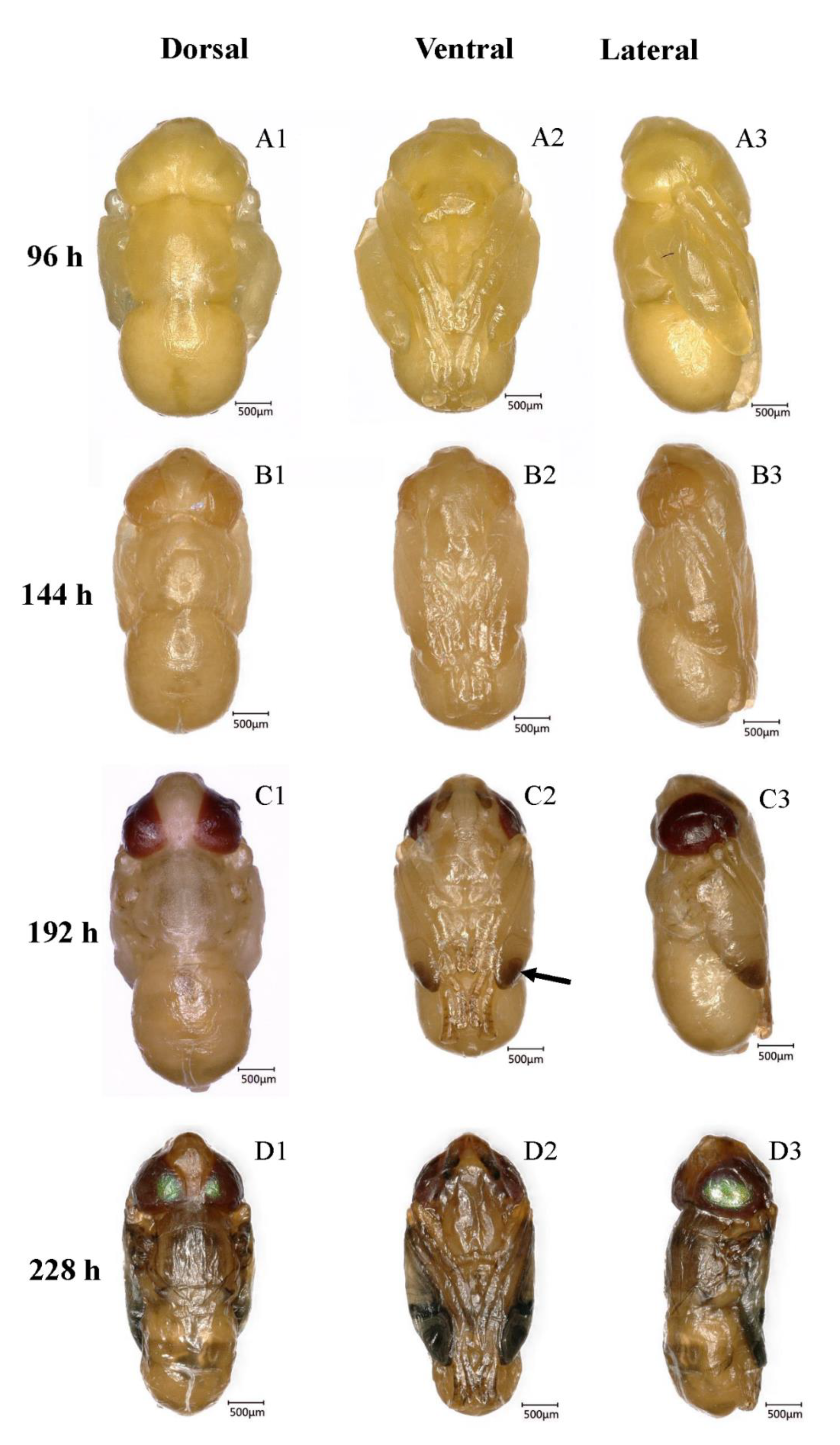
3.5. Pharate Adult (66–228 h, 2.75–9.5 days, 133.2 ± 1.55 h)
3.5.1. Transparent-Eyed Pupa (66–138 h, 2.75–5.75 days, 59.7 ± 1.02 h)
3.5.2. Yellow-Eyed Pupa (144–180 h, 6.0–7.5 days, 34.2 ± 0.63 h)
3.5.3. Reddish Brown-Eyed Pupa (186–222 h, 7.75–9.25 days, 33.9 ± 0.66 h)
3.5.4. Metallic Red-Eyed Pupa (222–228 h, 9.25–9.5 days, 5.4 ± 0.41 h)
3.6. Emergent adult (228–246 h, 9.5–10.25 days, 9.6 ± 0.91 h)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Li, Z.H.; Qin, Y.J.; Zhao, Z.H.; Liu, L.J.; Schutze, M.K. Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) is not invasive through Asia: It’s been there all along. J. Appl. Entomol. 2019, 143, 797–801. [Google Scholar] [CrossRef]
- Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First record of an invasive fruit fly belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.J.; Krosch, M.N.; Schutze, M.K.; Zhang, Y.; Wang, X.X.; Prabhakar, C.S.; Susanto, A.; Hee, A.K.W.; Ekesi, S.; Badji, K.; et al. Population structure of a global agricultural invasive pest, Bactrocera dorsalis (Diptera: Tephritidae). Evol. Appl. 2018, 11, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Vayssieres, J.F.; Korie, S.; Coulibaly, O.; Temple, L.; Boueyi, S.P. The mango tree in central and northern Benin: Cultivar inventory, yield assessment, infested stages and loss due to fruit flies (Diptera Tephritidae). Fruits 2008, 63, 335–348. [Google Scholar] [CrossRef]
- Hou, B.; Xie, Q.; Zhang, R. Depth of pupation and survival of the Oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) pupae at selected soil moistures. Appl. Entomol. Zool. 2006, 41, 515–520. [Google Scholar] [CrossRef]
- Pujol-Luz, J.R.; Barros-Cordeiro, K.B. Intra-puparial development of the females of Chrysomya albiceps (Wiedemann)(Diptera, Calliphoridae). Rev. Bras. Entomol. 2012, 56, 269–272. [Google Scholar] [CrossRef]
- Sinha, S.K.; Mahato, S. Intra-puparial development of flesh fly Sarcophaga dux (Thomson)(Diptera, Sarcophagidae). Curr. Sci. 2016, 111, 1063. [Google Scholar] [CrossRef]
- Frick, K.E.; Simkover, H.G.; Telford, H.S. Bionomics of the Cherry Fruit Flies in Eastern Washington; State College of Washington Tech Bull: Pullman, DC, USA, 1954; pp. 29–36. [Google Scholar]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica 2007, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.W. Bionomics of the Apple Maggot in Eastern New York; Search Agriculture: New York, NY, USA, 1973; pp. 27–29. [Google Scholar]
- Papanastasiou, S.A.; Papadopoulos, N.T. Description of Rhagoletis cerasi (Diptera: Tephritidae) pupal developmental stages: Indications of prolonged diapause. J. Insect Sci. 2014, 14, 156. [Google Scholar] [CrossRef]
- Shen, G.M.; Dou, W.; Huang, Y.; Jiang, X.Z.; Smagghe, G.; Wang, J.J. In silico cloning and annotation of genes involved in the digestion, detoxification and RNA interference mechanism in the midgut of Bactrocera dorsalis [Hendel (Diptera: Tephritidae)]. Insect Mol. Biol. 2013, 22, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, L.; Dou, W.; Jiang, H.-B.; Wei, D.-D.; Wei, D.; Niu, J.-Z.; Wang, J.-J. Determination of instars of Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2017, 100, 270–276. [Google Scholar] [CrossRef]
- Liang, G.; Chen, J.; Yang, J.; Huang, J.; Ji, Q. Advances in research of Bactrocera dorsalis(hendel) in China. Entomol. J. East China 2003, 12, 90–98. [Google Scholar]
- Liu, H.; Zhang, D.J.; Xu, Y.J.; Wang, L.; Cheng, D.F.; Qi, Y.X.; Zeng, L.; Lu, Y.Y. Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J. Integr. Agric. 2018, 18, 771–787. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; McInnis, D. Pre-release consumption of methyl eugenol increases the mating competitiveness of sterile males of the oriental fruit fly, Bactrocera dorsalis, in large field enclosures. J. Insect Sci. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Burikam, I.; Sarnthoy, O.; Charernsom, K.; Kanno, T.; Homma, H. Cold temperature treatment for mangosteens infested with the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 1992, 85, 2298–2301. [Google Scholar] [CrossRef]
- Armstrong, J.W.; Hu, B.K.; Brown, S.A. Single-temperature forced hot-air quarantine treatment to control fruit flies (Diptera: Tephritidae) in papaya. J. Econ. Entomol. 1995, 88, 678–682. [Google Scholar] [CrossRef]
- Wharton, R. Classical biological control of fruit-infesting Tephritidae. Fruit Flies Biol. Nat. Enemies Control 1989, 3, 303–313. [Google Scholar]
- Karabey, T.; Sert, O. The analysis of pupal development period in Lucilia sericata (Diptera: Calliphoridae) forensically important insect. Int. J. Legal Med. 2018, 132, 1185–1196. [Google Scholar] [CrossRef]
- Barros-Cordeiro, K.B.; Pujol-Luz, J.R.; Name, K.P.O.; Báo, S.N. Intra-puparial development of the Cochliomyia macellaria and Lucilia cuprina (Diptera, Calliphoridae). Rev. Bras. Entomol. 2016, 60, 334–340. [Google Scholar] [CrossRef]
- Moffatt, C.; Heaton, V.; Haan, D.D. The distribution of blow fly (Diptera: Calliphoridae) larval lengths and its implications for estimating post mortem intervals. Int. J. Legal Med. 2016, 130, 287. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.W. The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J. Morphol. 2010, 59, 351–399. [Google Scholar] [CrossRef]
- Hodgetts, R.B.; Sage, B.; O‘Connor, J.D. Ecdysone titers during postembryonic development of Drosophila melanogaster. Dev. Biol. 1977, 60, 310–317. [Google Scholar] [CrossRef]
- Chen, B.; Li, W.H.; He, Z.B. Developmental morphology of non-diapausing pupae in onion maggot, Delia antiqua. J. Southwest Univ. 2012, 34, 1–8. [Google Scholar]
- Ruhm, M.E.; Calkins, C.O. Eye-color changes in Ceratitis capitata pupae, a technique to determine pupal development. Entomol. Exp. Appl. 1981, 29, 237–240. [Google Scholar] [CrossRef]
- Rabossi, A.; Boccaccio, G.L.; Wappner, P.; Quesada-Allué, L.A. Morphogenesis and cuticular markers during the larval-pupal transformation of the medfly Ceratitis capitata. Entomol. Exp. Appl. 2011, 60, 135–141. [Google Scholar] [CrossRef]
- Hallman, G.J.; Worley, J.W. Gamma radiation doses to prevent adult emergence from immatures of Mexican and West Indian fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 1999, 4, 967–973. [Google Scholar] [CrossRef]
- Fraenkel, G.; Bhaskaran, G. Pupariation and pupation in cyclorrhaphous flies (Diptera): terminology and interpretation. Ann. Entomol. Soc. Am. 1973, 66, 418–422. [Google Scholar] [CrossRef]
- Thomas, D.B.; Hallman, G.J. Developmental arrest in Mexican fruit fly (Diptera: Tephritidae) irradiated in grapefruit. Ann. Entomol. Soc. Am. 2001, 104, 1367–1372. [Google Scholar] [CrossRef]
- Barros-Cordeiro, K.B.; Báo, S.N.; Pujol-Luz, J.R. Intra-puparial development of the black soldier-fly, Hermetia illucens. J. Insect Sci. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Scriber, J.M.; Sonke, B. Effects of diurnal temperature range on adult size and emergence times from diapausing pupae in Papilio glaucus and P. canadensis (Papilionidae). Insect Sci. 2011, 18, 435–442. [Google Scholar] [CrossRef]
- Salazar-Souza, M.; Couri, M.S.; Aguiar, V.M. Chronology of the intrapuparial development of the blowfly Chrysomya albiceps (Diptera: Calliphoridae): Application in forensic entomology. J. Med. Entomol. 2018, 55, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Longnecker, M.; Tomberlin, J.K. Effects of temperature and tissue type on Chrysomya rufifacies (Diptera: Calliphoridae) (Macquart) development. Forensic Sci. Int. 2014, 245, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Dambroski, H.; Feder, J. Host plant and latitude-related diapause variation in Rhagoletis pomonella: A test for multifaceted life history adaptation on different stages of diapause development. J. Evol. Biol. 2010, 20, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Blanckenhorn, W.U. The consistency of quantitative genetic estimates in field and laboratory in the yellow dung fly. Genetica 2002, 114, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Parsch, J.; Russell, J.A.; Beerman, I.; Hartl, D.L.; Stephan, W. Deletion of a conserved regulatory element in the Drosophila Adh gene leads to increased alcohol dehydrogenase activity but also delays development. Genetics 2000, 156, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Jennings, K.C.; Foran, D.R. Aging blow fly eggs using gene expression: A feasibility study. J. Forensic Sci. 2010, 52, 1350–1354. [Google Scholar] [CrossRef]
- Boehme, P.; Spahn, P.; Amendt, J.; Zehner, R. The analysis of temporal gene expression to estimate the age of forensically important blow fly pupae: Results from three blind studies. Int. J. Legal Med. 2014, 128, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Boehme, P.; Spahn, P.; Amendt, J. Differential gene expression during metamorphosis: A promising approach for age estimation of forensically important Calliphora vicina pupae (Diptera: Calliphoridae). Int. J. Legal Med. 2013, 127, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z.Y.; Xia, S.X.; Wang, J.F.; Zhang, Y.N.; Tao, L.Y. Estimating the age of Lucilia illustris during the intrapuparial period using two approaches: Morphological changes and differential gene expression. Forensic Sci. Int. 2018, 287, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.S.; Simonsen, T.J.; Abel, R.L.; Hall, M.J.; Schwyn, D.A.; Wicklein, M. Virtual forensic entomology: Improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Sci. Int. 2012, 220, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutty, G.N.; Brough, A.; Biggs, M.J.; Robinson, C.; Lawes, S.D.; Hainsworth, S.V. The role of micro-computed tomography in forensic investigations Forensic Sci. Int. 2013, 225, 60–66. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Simonsen, T.J.; Wicklein, M.; Hall, M.J.R. Age estimation during the blow fly intra-puparial period: A qualitative and quantitative approach using micro-computed tomography. Int. J. Legal Med. 2017, 131, 1429–1448. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Simonsen, T.J.; Mjr, H. Looking into the puparium: Micro-CT visualization of the internal morphological changes during metamorphosis of the blow fly, Calliphora vicina, with the first quantitative analysis of organ development in cyclorrhaphous dipterans. J. Morphol. 2017, 278, 629. [Google Scholar] [CrossRef] [PubMed]
- Martín-vega, D.; Hall, M.J.R.; Simonsen, T.J. Resolving confusion in the use of concepts and terminology in intrapuparial development studies of cyclorrhaphous Diptera. J. Med. Entomol. 2016, 53, 1249–1251. [Google Scholar] [CrossRef] [PubMed]



| Development Stages (hours) | |||||||
|---|---|---|---|---|---|---|---|
| Species | Temperature (°C) | LPA | CCP | PCP | PHA | EAD | REF |
| Drosophila melanogaster | 25 | 4 | 4–6 | 12 | 24 | 76 | [25] |
| Musca domestica | 30 | 4 | 4–9 | 16–18 | 28–30 | 96–110 | [30] |
| Lucilia sericata | 25 | 11 | 11–21 | 22–47 | 69–167 | 175 | [21] |
| Sarcophaga dux | 22 | 24 | 24 | 48 | 192 | 240 | [8] |
| Sarcophaga (Neobellieria) bullata Parker | 24 | 20 | 20–28 | 46–48 | 168–192 | 324–348 | [30] |
| Chrysomya albiceps | 28 | 0–4 | 4–6 | 6–10 | 24–96 | 99 | [7] |
| Cochliomyia macellaria | 23 | 0–27 | 6–42 | 12–78 | 15–90 | 120 | [32] |
| Lucilia cuprina | 23 | 0–36 | 6–39 | 15–34 | 18–192 | 210 | [22] |
| Ceratitis capitata | 23 | 0–40 | 40–46 | 46–48 | 48–264 | 288 | [27,28] |
| Bactrocera dorsalis | 27 | 0–34 | 32–40 | 36–42 | 66–228 | 228–246 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, T.-X.; Zhang, Y.-X.; Dou, W.; Jiang, X.-Y.; Wang, J.-J. First Insights into the Intrapuparial Development of Bactrocera dorsalis (Hendel): Application in Predicting Emergence Time for Tephritid Fly Control. Insects 2019, 10, 283. https://doi.org/10.3390/insects10090283
Jing T-X, Zhang Y-X, Dou W, Jiang X-Y, Wang J-J. First Insights into the Intrapuparial Development of Bactrocera dorsalis (Hendel): Application in Predicting Emergence Time for Tephritid Fly Control. Insects. 2019; 10(9):283. https://doi.org/10.3390/insects10090283
Chicago/Turabian StyleJing, Tian-Xing, Ying-Xin Zhang, Wei Dou, Xin-Yi Jiang, and Jin-Jun Wang. 2019. "First Insights into the Intrapuparial Development of Bactrocera dorsalis (Hendel): Application in Predicting Emergence Time for Tephritid Fly Control" Insects 10, no. 9: 283. https://doi.org/10.3390/insects10090283





