Behavioral Genetics of the Interactions between Apis mellifera and Varroa destructor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenotyping
2.2. Genotyping
2.3. Testing the Links between Phenotypes and Genotypes
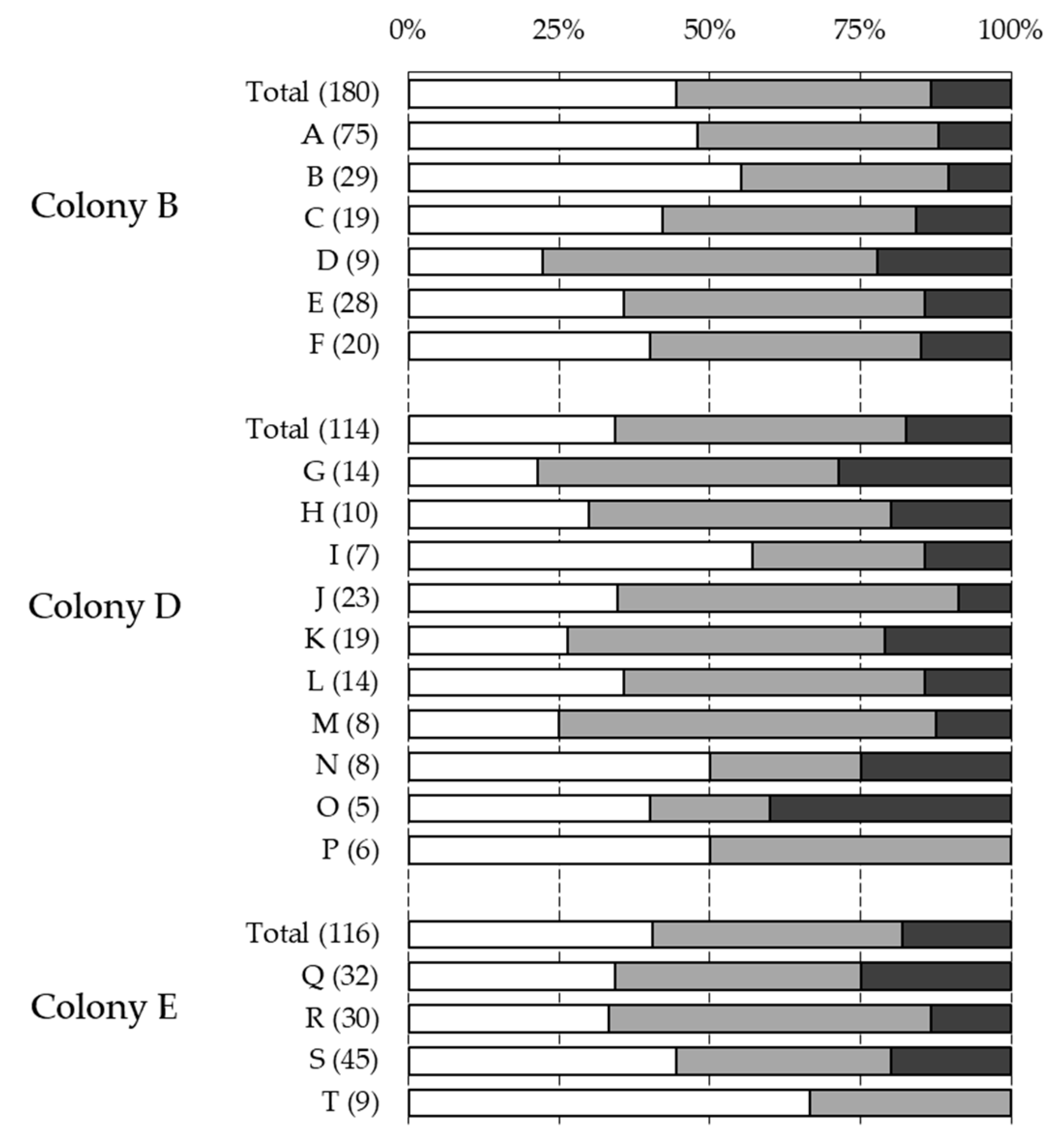
3. Results
3.1. Phenotyping
3.2. Genotyping
3.3. Association between Phenotypes and Genotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1998. [Google Scholar]
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social Immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid-Hempel, P. Evolutionary Parasitology; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Baer, B.; Schmid-Hempel, P. Unexpected consequences of polyandry for parasitism and fitness in the bumblebee, bombus terrestris. Evolution (N.Y) 2001, 55, 1639–1643. [Google Scholar]
- Sherman, P.W.; Seeley, T.D.; Reeve, H.K. Parasites, Pathogens, and Polyandry in Honey Bees. Source Am. Nat. 1998, 151, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.F.; Schmid-Hempel, P. The Evolution of Female Multiple Mating in Social Hymenoptera. Evolution (N.Y) 2003, 57, 2067–2081. [Google Scholar]
- Tarpy, D.R.; Nielsen, R.; Nielsen, D.I. A scientific note on the revised estimates of effective paternity frequency in Apis. Insectes Soc. 2004, 51, 203–204. [Google Scholar] [CrossRef]
- Beaurepaire, A.L.; Kraus, B.F.; Koeniger, G.; Koeniger, N.; Lim, H.; Moritz, R.F.A. Extensive population admixture on drone congregation areas of the giant honeybee, Apis dorsata (Fabricius, 1793). Ecol. Evol. 2014, 4, 4669–4677. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.B.; Koeniger, N.; Tingek, S.; Moritz, R.F.A. Temporal genetic structure of a drone congregation area of the giant Asian honeybee (Apis dorsata). Naturwissenschaften 2005, 92, 578–581. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Pechhacker, H. The nearer the better? Drones (Apis mellifera) prefer nearer drone congregation areas. Insectes Soc. 2005, 52, 31–35. [Google Scholar] [CrossRef]
- Baudry, E.; Solignac, M.; Garnery, L.; Gries, M.; Cornuet, J.-M.; Koeniger, N. Relatedness among honeybees (Apis mellifera) of a drone congregation. Proc. R. Soc. B 1998, 265, 2009–2014. [Google Scholar] [CrossRef] [Green Version]
- Beye, M.; Gattermeier, I.; Hasselmann, M.; Gempe, T.; Schioett, M.; Baines, J.F.; Schlipalius, D.; Mougel, F.; Emore, C.; Rueppell, O.; et al. Exceptionally high levels of recombination across the honey bee genome. Genome Res. 2006, 16, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Mattila, H.R.; Seeley, T.D. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 2007, 317, 362–364. [Google Scholar] [CrossRef]
- Page, R.E.; Robinson, G.E.; Fondrk, M.K.; Nasr, M.E. Effects of worker genotypic diversity on honey bee colony development and behavior (Apis mellifera L.). Behav. Ecol. Sociobiol. 1995, 36, 387–396. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Walz, M.; Tarpy, D.R. Genetic diversity confers colony-level benefits due to individual immunity. Biol. Lett. 2016, 12, 20151007. [Google Scholar] [CrossRef] [PubMed]
- Boecking, O.; Genersch, E. Varroosis—The ongoing crisis in bee keeping. J. Verbraucherschutz Leb. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Anderson, D.L.; Trueman, J.W.H. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, D.J.T.; Martin, S.J. The dynamics of virus epidemics in Varroa-infested honey bee colonies. J. Anim. Ecol. 2004, 73, 51–63. [Google Scholar] [CrossRef]
- Mondet, F.; de Miranda, J.R.; Kretzschmar, A.; Le Conte, Y.; Mercer, A.R. On the Front Line: Quantitative Virus Dynamics in Honeybee (Apis mellifera L.) Colonies along a New Expansion Front of the Parasite Varroa destructor. PLoS Pathog. 2014, 10, e1004323. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Beaurepaire, A.L.; Ellis, J.D.; Krieger, K.J.; Moritz, R.F.A. Association of Varroa destructor females in multiply infested cells of the honeybee Apis mellifera. Insect Sci. 2017, 26, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Beaurepaire, A.L.; Krieger, K.J.; Moritz, R.F.A. Seasonal cycle of inbreeding and recombination of the parasitic mite Varroa destructor in honeybee colonies and its implications for the selection of acaricide resistance. Infect. Genet. Evol. 2017, 50, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Nazzi, F.; Le Conte, Y. Ecology of Varroa destructor, the major ectoparasite of the Western honey bee, Apis mellifera. Annu. Rev. Entomol. 2016, 61, 417–432. [Google Scholar] [CrossRef]
- Fries, I.; Rosenkranz, P. Number of reproductive cycles of Varroa jacobsoni in honey-bee (Apis mellifera) colonies. Exp. Appl. Acarol. 1996, 20, 103–112. [Google Scholar] [CrossRef]
- Martin, S.J. A population model for the ectoparasitic mite Varroa jacobsoni in honey bee (Apis mellifera) colonies. Ecol. Model. 1998, 109, 267–281. [Google Scholar] [CrossRef]
- Calis, J.N.M.; Fries, I.; Ryrie, S.C. Population modelling of Varroa jacobsoni Oud. Apidologie 1999, 30, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Hatjina, F.; Costa, C.; Büchler, R.; Uzunov, A.; Drazic, M.; Filipi, J.; Charistos, L.; Ruottinen, L.; Andonov, S.; Meixner, M.D.; et al. Population dynamics of European honey bee genotypes under sdifferent environmental conditions. J. Apic. Res. 2014, 53, 233–247. [Google Scholar] [CrossRef]
- Martin, S.J. Ontogenesis of the mite Varroa jacobsoni Oud. in drone brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 1995, 19, 199–210. [Google Scholar] [CrossRef]
- Martin, S.J. Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 1994, 18, 87–100. [Google Scholar] [CrossRef]
- Fuchs, S. Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie 1990, 21, 193–199. [Google Scholar] [CrossRef]
- Locke, B. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 2016, 47, 467–482. [Google Scholar] [CrossRef]
- Kurze, C.; Routtu, J.; Moritz, R.F.A. Parasite resistance and tolerance in honeybees at the individual and social level. Zoology 2016, 119, 290–297. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; de Guzman, L.I. Breeding for resistance to Varroa destructor in North America. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef]
- Büchler, R.; Berg, S.; Le Conte, Y. Breeding for resistance to Varroa destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Heritability in Honey Bees (Hymenoptera: Apidae) of Characteristics Associated with Resistance to Varroa jacobsoni (Mesostigmata: Varroidae). J. Econ. Entomol. 1999, 92, 5. [Google Scholar] [CrossRef]
- Locke, B. Inheritance of reduced Varroa mite reproductive success in reciprocal crosses of mite-resistant and mite-susceptible honey bees (Apis mellifera). Apidologie 2015, 47, 583–588. [Google Scholar] [CrossRef]
- Oddie, M.; Büchler, R.; Dahle, B.; Kovacic, M.; Le Conte, Y.; Locke, B.; de Miranda, J.R.; Mondet, F.; Neumann, P. Rapid parallel evolution overcomes global honey bee parasite. Sci. Rep. 2018, 8, 7704. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apic. Res. 2005, 44, 21–23. [Google Scholar] [CrossRef]
- Ibrahim, A.; Spivak, M. The relationship between hygienic behavior and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie 2006, 37, 31–40. [Google Scholar] [CrossRef]
- Danka, R.G.; Harris, J.W.; Dodds, G.E. Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 2016, 47, 483–490. [Google Scholar] [CrossRef]
- Milani, N.; Della Vedova, G.; Nazzi, F. (Z)-8-Heptadecene reduces the reproduction of Varroa destructor in brood cells. Apidologie 2004, 35, 265–273. [Google Scholar] [CrossRef]
- Wagoner, K.M.; Spivak, M.; Rueppell, O. Brood Affects Hygienic Behavior in the Honey Bee (Hymenoptera: Apidae). J. Econ. Entomol. 2018, 111, 2520–2530. [Google Scholar] [CrossRef] [Green Version]
- Oldroyd, B.P.; Thompson, G.J. Behavioural Genetics of the Honey Bee Apis mellifera. Adv. Insect. Phys. 2006, 33, 1–49. [Google Scholar]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S. Standard methods for varroa research. In The COLOSS BEEBOOK, Volume II: Standard Methods for Apis Mellifera Pest and Pathogen Research; Dietemann, V., Ellis, J.D., Neumann, P., Eds.; International Bee Research Association (IBRA): Bristol, UK, 2013. [Google Scholar]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechnique 1991, 10, 506–513. [Google Scholar] [CrossRef]
- Solignac, M.; Vautrin, D.; Loiseau, A.; Mougel, F.; Baudry, E.; Estoup, A.; Garnery, L.; Haberl, M.; Cornuet, J.M. Five hundred and fifty microsatellite markers for the study of the honeybee (Apis mellifera L.) genome. Mol. Ecol. Notes 2003, 3, 307–311. [Google Scholar] [CrossRef]
- Estoup, A.; Garnery, L.; Solignac, M.; Cornuet, J.M. Microsatellite variation in honey bee (Apis mellifera L.) populations: Hierarchical genetic structure and test of the infinite allele and stepwise mutation models. Genetics 1995, 140, 679–695. [Google Scholar]
- Human, H.; Brodschneider, R.; Dietemann, V.; Dively, G.; Ellis, J.D.; Forsgren, E.; Fries, I.; Hatjina, F.; Hu, F.; Jensen, A.B.; et al. Miscellaneous standard methods for Apis mellifera research. In Coloss Beebook Volume II; International Bee Research Association (IBRA): Bristol, UK, 2013. [Google Scholar]
- Goudet, J. FSTAT (Version 1.2): A computer program to calculate F-statistics. Heredity (Edinb) 1995, 96, 485–486. [Google Scholar] [CrossRef]
- Park, S.D.E. Excel Microsatellite Toolkit 2008. Computer Program and Documentation Distributed by the Author. Available online: http://animalgenomics.ucd.ie/sdepark/ms-toolkit/ (accessed on 30 May 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Kulincevic, J.M.; Rinderer, T.E.; Urosevic, D.J. Seasonality and colony variation of reproducing and non-reproducing Varroa jacobsoni females in Western honey bee (Apis mellifera) worker brood. Apidologie 1988, 20, 173–180. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Engels, W. Infertility of Varroa jacobsoni females after invasion into Apis mellifera worker brood a tolerance factor against varroatosis. Apidologie 1994, 25, 402–411. [Google Scholar] [CrossRef]
- Page, R.E.; Kimsey, R.B.; Laidlaw, H.H. Migration and dispersal of spermatozoa in the spermathecae of queen honeybees (Apis mellifera L.). Experientia 1984, 40, 182–184. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Lattorff, H.M.G.; Neumann, P.; Kraus, F.B.; Radloff, S.E.; Hepburn, H.R. Rare royal families in honeybees, Apis mellifera. Naturwissenschaften 2005, 92, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Withrow, J.W.; Tarpy, D.R. Cryptic “royal” subfamilies in honey bee (Apis mellifera) colonies. PLoS ONE 2018, 13, e0199124. [Google Scholar] [CrossRef]
- Beetsma, J.; Boot, W.J.; Calis, J.N.M. Invasion behaviour of Varroa jacobsoni Oud.: From bees into brood cells. Apidologie 1999, 30, 125–140. [Google Scholar] [CrossRef]
- Le Conte, Y.; Arnold, G.; Trouiller, J.; Masson, C.; Chappe, B.; Ourisson, G. Attraction of the Parasitic Mite Varroa to the Drone Larvae of Honey Bees by Simple Aliphatic Esters. Science 1989, 245, 638–639. [Google Scholar] [CrossRef]
- Salvy, M.; Capowiez, Y.; Le Conte, Y.; Clément, J.L. Does the spatial distribution of the parasitic mite Varroa jacobsoni oud. (Mesostigmata: Varroidae) in worker brood of honey bee Apis mellifera L. (Hymenoptera: Apidae) rely on an aggregative process? Naturwissenschaften 1999, 86, 540–543. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Resistance to Varroa destructor (Mesostigmata: Varroidae) When Mite-Resistant Queen Honey Bees (Hymenoptera: Apidae) Were Free-Mated with Unselected Drones. J. Econ. Entomol. 2001, 94, 1319–1323. [Google Scholar] [CrossRef]
- Conlon, B.; Frey, E.; Rosenkranz, P.; Locke, B.; Moritz, R.F.A.; Routtu, J. The role of epistatic interactions underpinning resistance to parasitic Varroa mites in haploid honey bee (Apis mellifera) drones. J. Evol. Biol. 2018, 31, 801–809. [Google Scholar] [CrossRef]
- Conlon, B.; Aurori, A.; Giurgiu, A.-I.; Kefuss, J.; Dezmirean, D.S.; Moritz, R.F.A.; Routtu, J. A gene for resistance to the Varroa mite (Acari) in honey bee (Apis mellifera) pupae. Mol. Ecol. 2019. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Pietravalle, S.; Brown, M.A.; Budge, G.E. Honey Bee Colonies Headed by Hyperpolyandrous Queens Have Improved Brood Rearing Efficiency and Lower Infestation Rates of Parasitic Varroa Mites. PLoS ONE 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Seeley, T.D. Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs. monandrous queens. Naturwissenschaften 2006, 93, 195–199. [Google Scholar] [CrossRef]


| Colony | Sampling | NInf | % Inf | % Multi | Reproduction | % Recapped | % Inf Recapped |
|---|---|---|---|---|---|---|---|
| A | I | 22 | 26.51% | 9.09% | 0.65 | 16.87% | 22.73% |
| I | 25 | 28.74% | 20.00% | 0.85 | 34.48% | 36.00% | |
| II | 25 | 17.86% | 24.00% | 0.47 | 15.71% | 36.00% | |
| B * | I | 24 | 29.63% | 16.67% | 0.60 | 2.47% | 8.33% |
| I | 27 | 34.18% | 25.93% | 0.55 | 10.13% | 18.52% | |
| II | 28 | 18.92% | 28.57% | 0.65 | 6.76% | 14.29% | |
| III | 39 | 11.08% | 20.51% | 0.42 | 10.23% | 2.56% | |
| C | I | 29 | 30.85% | 31.03% | 0.65 | 23.40% | 17.24% |
| II | 27 | 38.03% | 25.93% | 0.40 | 42.25% | 70.37% | |
| III | 41 | 31.06% | 29.27% | 0.55 | 53.03% | 36.59% | |
| D * | I | 28 | 21.71% | 28.57% | 0.85 | 6.98% | 14.29% |
| II | 20 | 19.05% | 0.00% | 0.85 | 12.38% | 0.00% | |
| IV | 59 | 40.14% | 32.20% | 0.75 | 35.37% | 59.32% | |
| E * | II | 22 | 23.16% | 9.09% | 0.90 | 13.68% | 9.09% |
| IV | 33 | 54.10% | 39.39% | 0.65 | 90.16% | 90.91% | |
| IV | 58 | 38.41% | 32.76% | 0.69 | 82.78% | 100.00% | |
| F | III | 45 | 13.80% | 26.67% | 0.42 | 28.53% | 11.11% |
| III | 53 | 15.32% | 20.75% | 0.55 | 47.69% | 50.94% | |
| Total | 605 | 23.03% | 25.12% | 0.63 | 29.27% | 38.84% | |
| Colony | NDE | NTotal | Patrilines | |||
|---|---|---|---|---|---|---|
| NInd | N | N > 5 | NSE | |||
| Colony B | 0.71% | 213 | 202 | 18 | 6 | 2.44 |
| Colony D | 0.25% | 179 | 149 | 25 | 10 | 1.10 |
| Colony E | 1.70% | 164 | 135 | 13 | 4 | 1.45 |
| Overall | 0.12% | 556 | 486 | 57 | 20 | 5.52 |
| Model | Factors | d.f. | Deviance | Resid. Dev. | p-Values |
|---|---|---|---|---|---|
| Infestation Status | Colony | 2 | 3.060 | 550.39 | 0.216 |
| Colony:Patriline | 17 | 13.468 | 528.32 | 0.704 | |
| Date | 3 | 8.598 | 541.79 | 0.035 | |
| Infestation Level | Colony | 2 | 4.2518 | 429.47 | 0.096 |
| Colony:Patriline | 17 | 14.309 | 396.26 | 0.540 | |
| Date | 3 | 18.898 | 410.57 | <0.001 | |
| Reproduction | Colony | 2 | 10.086 | 211.53 | 0.006 |
| Colony:Patriline | 13 | 14.310 | 187.90 | 0.352 | |
| Date | 3 | 9.315 | 202.21 | 0.025 | |
| Recapping | Colony | 2 | 69.684 | 227.01 | <0.001 |
| Colony:Patriline | 13 | 14.507 | 133.94 | 0.269 | |
| Date | 3 | 78.564 | 148.45 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaurepaire, A.; Sann, C.; Arredondo, D.; Mondet, F.; Le Conte, Y. Behavioral Genetics of the Interactions between Apis mellifera and Varroa destructor. Insects 2019, 10, 299. https://doi.org/10.3390/insects10090299
Beaurepaire A, Sann C, Arredondo D, Mondet F, Le Conte Y. Behavioral Genetics of the Interactions between Apis mellifera and Varroa destructor. Insects. 2019; 10(9):299. https://doi.org/10.3390/insects10090299
Chicago/Turabian StyleBeaurepaire, Alexis, Christina Sann, Daniela Arredondo, Fanny Mondet, and Yves Le Conte. 2019. "Behavioral Genetics of the Interactions between Apis mellifera and Varroa destructor" Insects 10, no. 9: 299. https://doi.org/10.3390/insects10090299
APA StyleBeaurepaire, A., Sann, C., Arredondo, D., Mondet, F., & Le Conte, Y. (2019). Behavioral Genetics of the Interactions between Apis mellifera and Varroa destructor. Insects, 10(9), 299. https://doi.org/10.3390/insects10090299







