Interspecific Hybridization between the Two Sympatric Termite Reticulitermes Species under Laboratory Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Termites
2.2. Experimental Setup
2.3. Behavioral Observation
2.4. Genotyping Analyses
3. Results
- Agonism reduced mating frequency, while acceptance represents more possibility of mating when interspecific reproductives encounter each other. Our results indicated that the frequencies of acceptance were significantly higher than those of aggression between interspecific partners when they encountered each other (Figure 1; t = −8.35, df = 8, p < 0.0001).
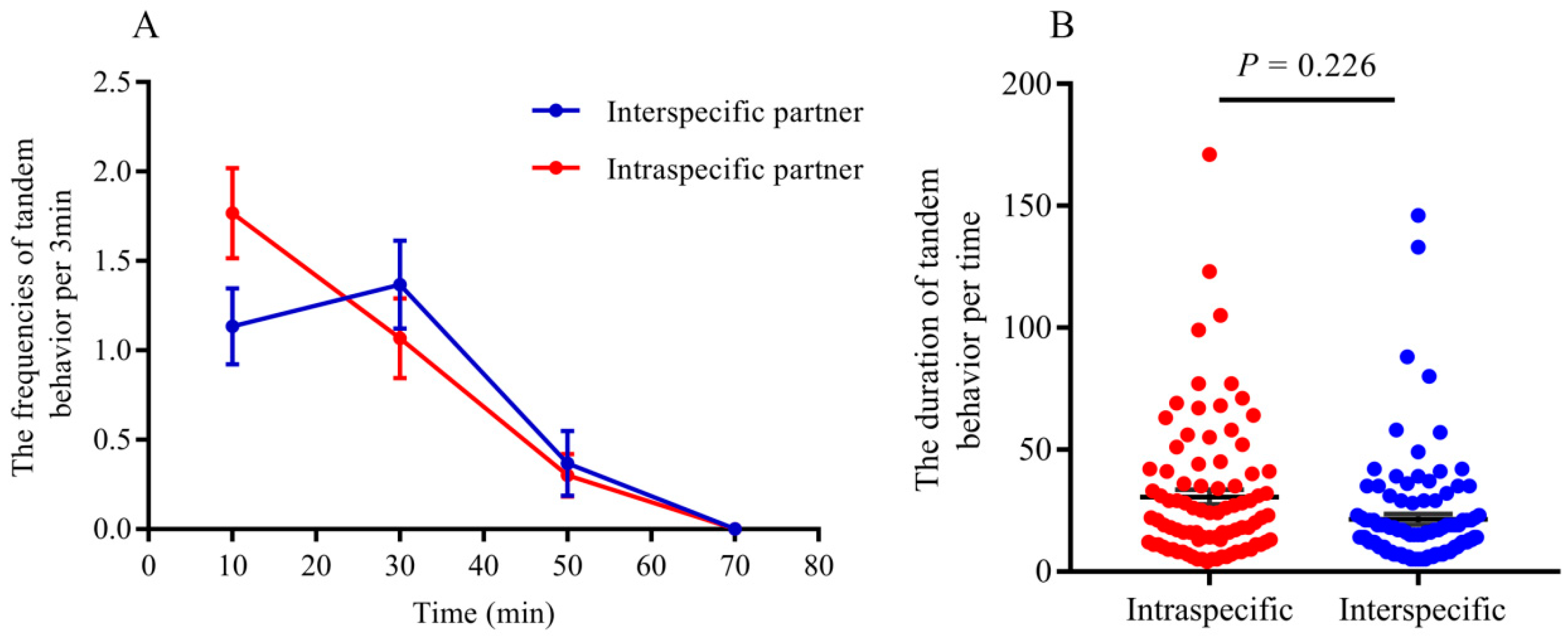
- Tandem behavior showed that conspecific mating preference was indistinct between R. chinensis and R. flaviceps. Similar to tandem behavior in intraspecies, the frequencies of tandem behavior in interspecies decreased with an increase in time, which began with encountering each other and ended after one hour. The frequencies of both conspecific (Figure 2A; GLMM: F = 12.24, p < 0.001) and interspecific tandem behavior (Figure 2A; GLMM: F = 19.25, p < 0.001) were significantly different during each time of observation. However, there were no significant differences in the tandem frequencies at each time of observation between conspecific and heterospecific species in the laboratory (Figure 2A; GLMM: F = 0.34, p = 0.56). Similarly, there were no significant differences in tandem duration at each time between conspecific partners and heterospecific partners (Figure 2B; t = 2.31, p = 0.22). Our results indicated that the preferences for conspecific individuals were absent in tandem behavior.
- When conspecific and interspecific partners were present in the same arena, we found that there were the similar frequencies of allogrooming in early encountering between interspecies and intraspecies (Figure 3A; t = 0.43, p = 0.67). The allogrooming frequencies of both interspecies and intraspecies increased with time, whereas the allogrooming frequency of interspecies was almost twice as much as that of intraspecies (Figure 3A; GLMM: F = 25.85, p < 0.001). Significantly higher allogrooming frequency was found in interspecies as compared with interspecies (Figure 3A; GLMM: F = 6.39. p = 0.012). Although the allogrooming duration in interspecific partners was longer than intraspecific partners, no significant differences were observed between them (Figure 3B; t = −0.96, p= 0.34). These results suggested that the two species preferred interspecific versus intraspecific partners, based on allogrooming duration and frequency under laboratory conditions.
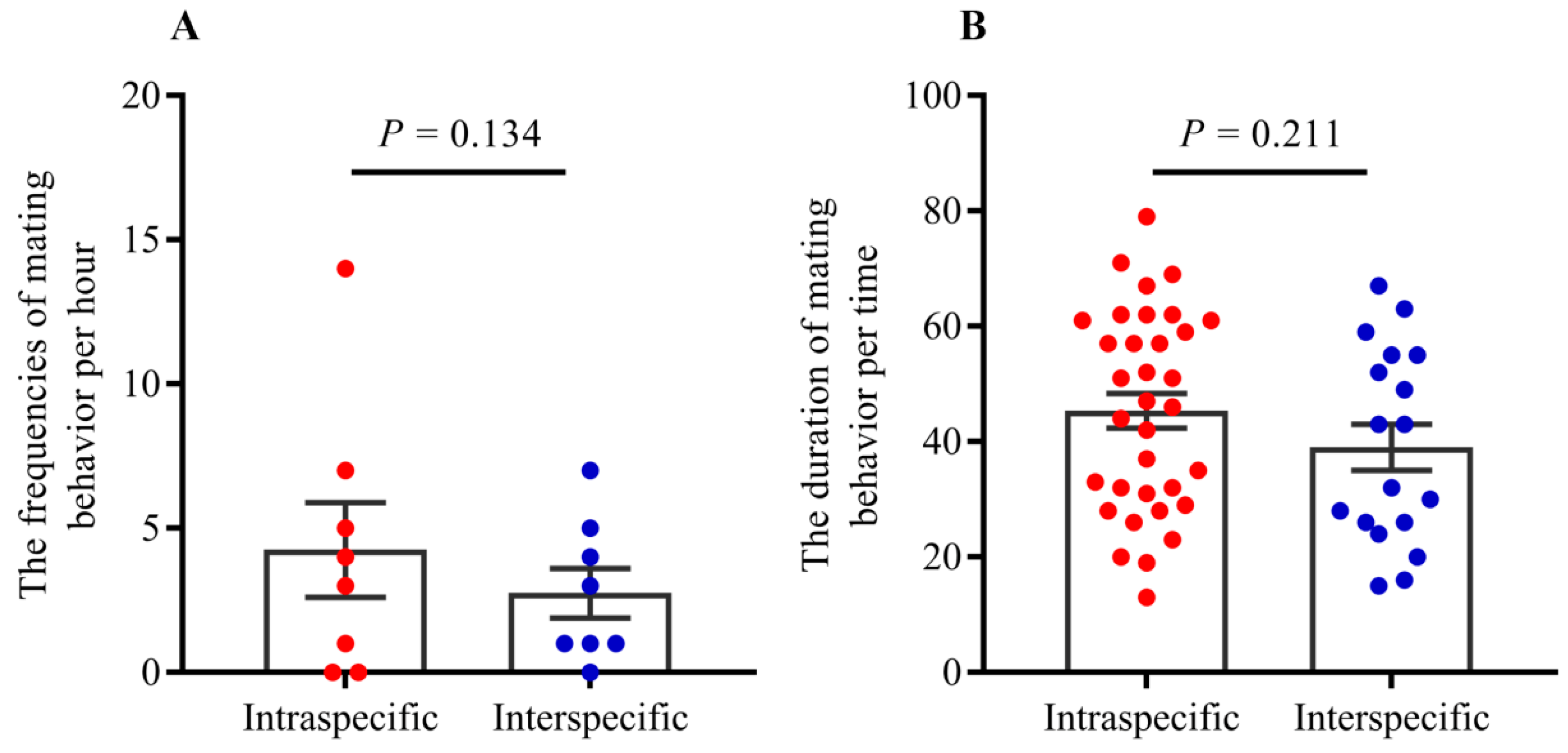
- Both tandem and allogrooming are associated with courtship, which allows termites to prepare for mating. We observed multiple occurrences of mating in each nest after pair formation. Mating behavior happened multiple times (average 2.34 ± 0.83 times, including mating of intraspecies and interspecies) within 1 h (see Supplementary Movie Video S1). Strikingly, both conspecific and interspecific mating was commenced over a short time. Our result indicated that there were 56 mating behavior in period of observation, 39.29% (22/56) mating occurred in interspecies couples and 61.71% (34/56) mating occurred in intraspecies couples. However, there were no significant differences in mating frequency between conspecific and heterospecific partners (Figure 4A; GLMM: F = 2.53, p = 0.13). There were no significant differences in the mating duration between intraspecies and interspecies also (Figure 4B; t = 1.27, p = 0.21). Genotyping analyses of larvae from artificial colonies identified the number of larvae produced by hybridization and conspecific partners in artificial colonies (Table S3). Although the accurate proportion of hybrid offspring in colonies was unknown because of limited diagnostic alleles, we still proposed that in the case of intraspecific mating being present in a colony, the interspecies mating can also produce living offspring in termites (Figure 5).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pfennig, K.S. Facultative mate choice drives Aadaptive hybridization. Science 2007, 318, 965–967. [Google Scholar] [CrossRef]
- Seehausen, O. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 1997, 277, 1808–1811. [Google Scholar] [CrossRef]
- Fisher, H.S.; Wong, B.B.; Rosenthal, G.G. Alteration of the chemical environment disrupts communication in a freshwater fish. Proc. Biol. Sci. 2006, 273, 1187–1193. [Google Scholar] [CrossRef]
- Rosenthal, G.G. Individual mating decisions and hybridization. J. Evol. Biol. 2013, 26, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Capblancq, T.; Despres, L.; Rioux, D.; Mavarez, J. Hybridization promotes speciation in Coenonympha butterflies. Mol. Ecol. 2015, 24, 6209–6222. [Google Scholar] [CrossRef] [PubMed]
- Hartke, T.R.; Rosengaus, R.B. Heterospecific pairing and hybridization between Nasutitermes corniger and N. ephratae. Die Naturwissenschaften 2011, 98, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Chouvenc, T.; Helmick, E.E.; Su, N.Y. Hybridization of two major termite invaders as a consequence of human activity. PLoS ONE 2015, 10, e0120745. [Google Scholar] [CrossRef]
- Aldrich, B.T.; Kambhampati, S. Preliminary analysis of a hybrid zone between two subspecies of Zootermopsis nevadensis. Insect. Soc. 2009, 56, 439–450. [Google Scholar] [CrossRef]
- Hartke, T.R.; Baer, B. The mating biology of termites: A comparative review. Anim. Behav. 2011, 82, 927–936. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, K.; Tan, Y.-L.; Xing, L.-X. The complete mitochondrial genome of the subterranean termite, Reticulitermes chinensis Snyder (Isoptera: Rhinotermitidae). Mitochondrial DNA 2014, 27, 1428–1429. [Google Scholar] [CrossRef]
- Zhao, S.; Dang, Y.; Zhang, H.; Guo, X.; Su, X. The complete mitochondrial genome of the subterranean termite Reticulitermes flaviceps (Isoptera: Rhinotermitidae). Conserv. Genet. Resour. 2016, 8, 451–453. [Google Scholar] [CrossRef]
- Miyata, H.; Furuichi, H.; Kitade, O. Patterns of neotenic differentiation in a subterranean termite, Reticulitermes speratus (Isoptera: Rhinotermitidae). Entomol. Sci. 2004, 7, 309–314. [Google Scholar] [CrossRef]
- Wu, J.; Su, X.; Kong, X.; Liu, M.; Xing, L. Multiple male and female reproductive strategies and the presence of a polyandric mating system in the termite Reticulitermes labralis (Isoptera: Rhinotermitidae). Sociobiology 2013, 60, 459–465. [Google Scholar] [CrossRef][Green Version]
- Vargo, E.L.; Husseneder, C. Biology of subterranean termites: Insights from molecular studies of Reticulitermes and Coptotermes. Annu. Rev. Entomol. 2009, 54, 379–403. [Google Scholar] [CrossRef]
- Huang, Q.; Li, G.; Husseneder, C.; Lei, C. Genetic analysis of population structure and reproductive mode of the termite Reticulitermes chinensis snyder. PLoS ONE 2013, 8, e69070. [Google Scholar] [CrossRef]
- Liu, S.S.; De Barro, P.J.; Xu, J.; Luan, J.B.; Zang, L.S.; Ruan, Y.M.; Wan, F.H. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef]
- Ritchie, M.G. Sexual Selection and Speciation. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 79–102. [Google Scholar] [CrossRef]
- Jiang, Y.; Bolnick, D.I.; Kirkpatrick, M. Assortative mating in animals. Am. Nat. 2013, 181, 125–138. [Google Scholar] [CrossRef]
- Harari, A.R.; Handler, A.M.; Landolt, P.J. Size-assortative mating, male choice and female choice in the curculionid beetle Diaprepes abbreviatus. Anim. Behav. 1999, 58, 1191–1200. [Google Scholar] [CrossRef]
- Shine, R.; O’Connor, D.; Lemaster, M.P.; Mason, R.T. Pick on someone your own size: Ontogenetic shifts in mate choice by male garter snakes result in size-assortative mating. Anim. Behav. 2001, 61, 1133–1141. [Google Scholar] [CrossRef]
- Slade, B.; Parrott, M.L.; Paproth, A.; Magrath, M.J.; Gillespie, G.R.; Jessop, T.S. Assortative mating among animals of captive and wild origin following experimental conservation releases. Biol. Lett. 2014, 10, 20140656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, G.H.; Liu, L.; Lei, C.L.; Huang, Q.Y. A trade-off between antipredatory behavior and pairing competition produced by male-male tandem running in three Reticulitermes species. Insect. Sci. 2015, 22, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, A.S.; Shpak, M. On the origin of species by means of assortative mating. Proc. Biol. Sci. 1998, 265, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Kirkpatrick, M. The relationship between intraspecific assortative mating and reproductive isolation between divergent populations. Curr. Zool. 2012, 58, 484–492. [Google Scholar] [CrossRef][Green Version]
- Willis, P.M.; Ryan, M.J.; Rosenthal, G.G. Encounter rates with conspecific males influence female mate choice in a naturally hybridizing fish. Behav. Ecol. 2011, 22, 1234–1240. [Google Scholar] [CrossRef]
- Willis, P.M.; Rosenthal, G.G.; Ryan, M.J. An indirect cue of predation risk counteracts female preference for conspecifics in a naturally hybridizing fish Xiphophorus birchmanni. PLoS ONE 2012, 7, e34802. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Kuno, E.; Nishida, T. Homosexual tandem running as selfish herd in Reticulitermes speratus: Novel antipredatory behavior in termites. J. Theor. Biol. 2002, 214, 63–70. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.Y.; Tang, Q.B.; Lei, C.L.; Huang, Q.Y. The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 2019, 11, 244. [Google Scholar] [CrossRef]
- Arnqvist, G. Assortative mating by fitness and sexually antagonistic genetic variation. Evolution 2011, 65, 2111–2116. [Google Scholar] [CrossRef]
- Mallet, J. Hybridization as an invasion of the genome. Trends Ecol. Evol. 2005, 20, 229–237. [Google Scholar] [CrossRef]
- Kulmuni, J.; Pamilo, P. Introgression in hybrid ants is favored in females but selected against in males. Proc. Natl. Acad. Sci. USA 2014, 111, 12805–12810. [Google Scholar] [CrossRef] [PubMed]
- Mavárez, J.; Salazar, C.A.; Bermingham, E.; Salcedo, C.; Jiggins, C.; Linares, M. Speciation by hybridization in Heliconius butterflies. Nature 2006, 441, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Saether, S.A.; Saetre, G.P.; Borge, T.; Wiley, C.; Svedin, N.; Andersson, G.; Qvarnstrom, A. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 2007, 318, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Korb, J.; Hartfelder, K. Life history and development—a framework for understanding developmental plasticity in lower termites. Biol. Rev. 2008, 83, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Thorne, B.L.; Breisch, N.L.; Haverty, M.I. Longevity of kings and queens and first time of production of fertile progeny in dampwood termite (Isoptera; Termopsidae; Zootermopsis) colonies with different reproductive structures. J. Anim. Ecol. 2002, 71, 1030–1041. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hasegawa, E.; Yamamoto, Y.; Kawatsu, K.; Vargo, E.L.; Yoshimura, J.; Matsuura, K. Sex ratio biases in termites provide evidence for kin selection. Nat. Commun. 2013, 4, 2048. [Google Scholar] [CrossRef]
- Charlesworth, D.; Willis, J.H. The genetics of inbreeding depression. Nat. Rev. Genet. 2009, 10, 783–796. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Xu, H.; Hassan, A.; Huang, Q. Interspecific Hybridization between the Two Sympatric Termite Reticulitermes Species under Laboratory Conditions. Insects 2020, 11, 14. https://doi.org/10.3390/insects11010014
Wu J, Xu H, Hassan A, Huang Q. Interspecific Hybridization between the Two Sympatric Termite Reticulitermes Species under Laboratory Conditions. Insects. 2020; 11(1):14. https://doi.org/10.3390/insects11010014
Chicago/Turabian StyleWu, Jia, Huan Xu, Ali Hassan, and Qiuying Huang. 2020. "Interspecific Hybridization between the Two Sympatric Termite Reticulitermes Species under Laboratory Conditions" Insects 11, no. 1: 14. https://doi.org/10.3390/insects11010014
APA StyleWu, J., Xu, H., Hassan, A., & Huang, Q. (2020). Interspecific Hybridization between the Two Sympatric Termite Reticulitermes Species under Laboratory Conditions. Insects, 11(1), 14. https://doi.org/10.3390/insects11010014



