Larval Migration Behaviour of Busseola fusca (Lepidoptera: Noctuidae) on Bt and Non-Bt Maize under Semi-Field and Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stock Colonies of B. fusca
2.2. Production of Neonate B. fusca Larvae for Experiments
2.3. Maize Plants
2.4. Experiment Protocols
2.4.1. Greenhouse Experiment
2.4.2. Field Experiment
2.5. Data Analysis
2.5.1. Weekly Monitoring of Plants for Leaf Damage: Damage Frequency
2.5.2. Weekly Monitoring of Plants for Leaf Damage: Damage Rating
2.5.3. Destructive Sampling: Larval Survival and Migration Distance
3. Results
3.1. Greenhouse Trial: Effect of Plant Growth Stage
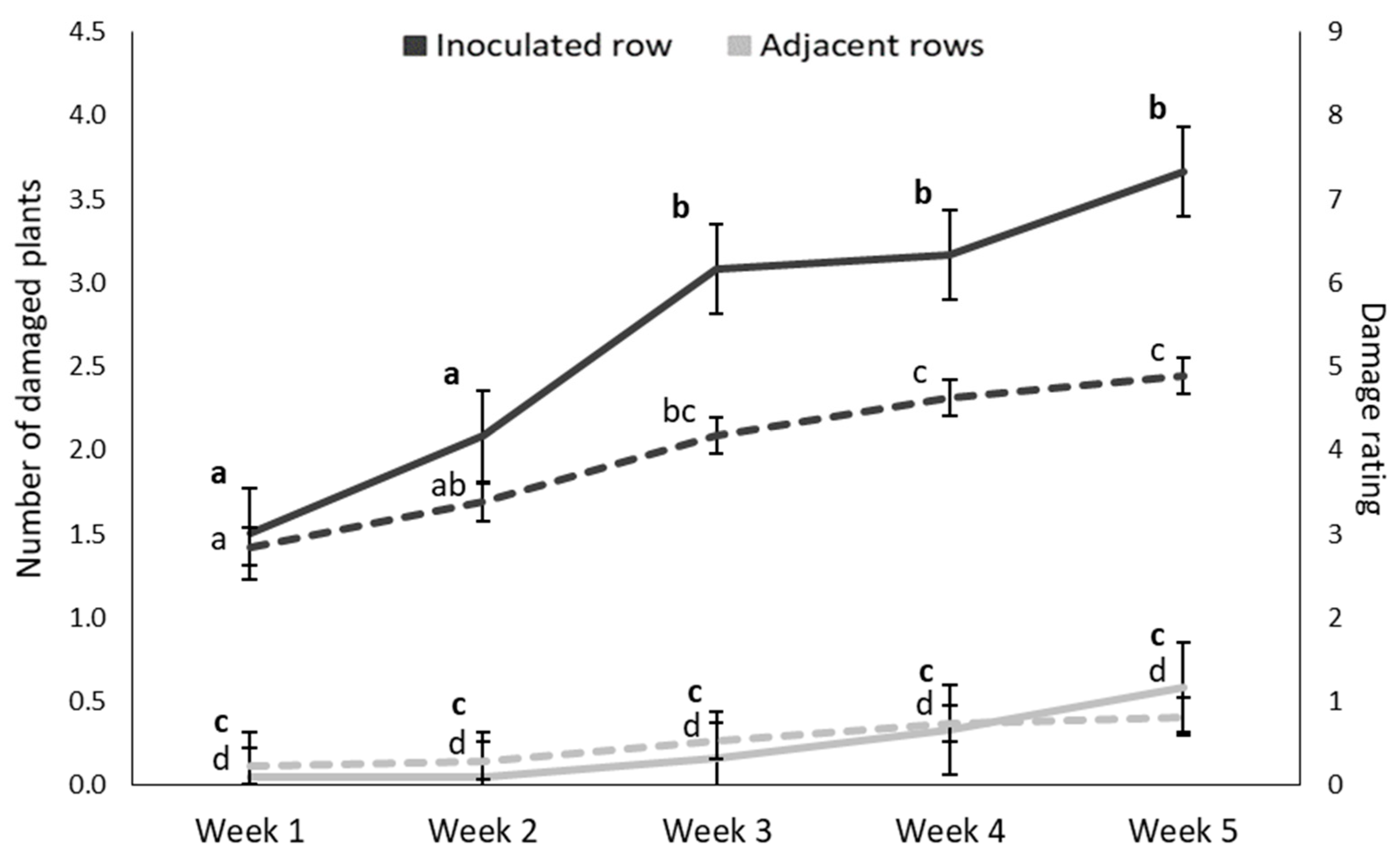
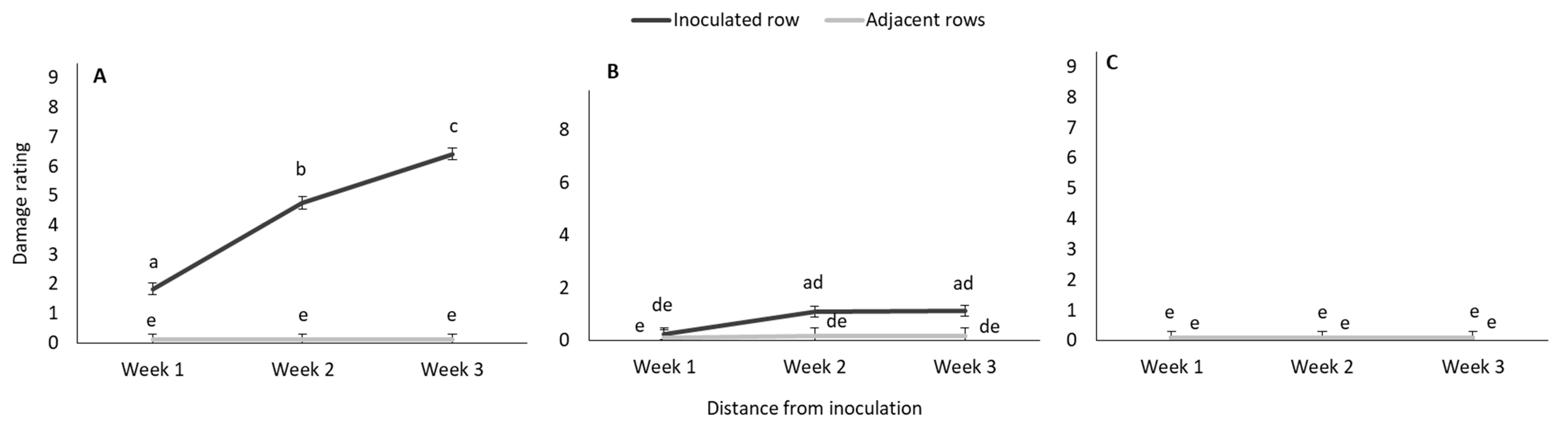
3.1.1. Weekly Monitoring of Damage Symptoms: Damage Frequency
3.1.2. Weekly Monitoring of Damage Symptoms: Damage Rating
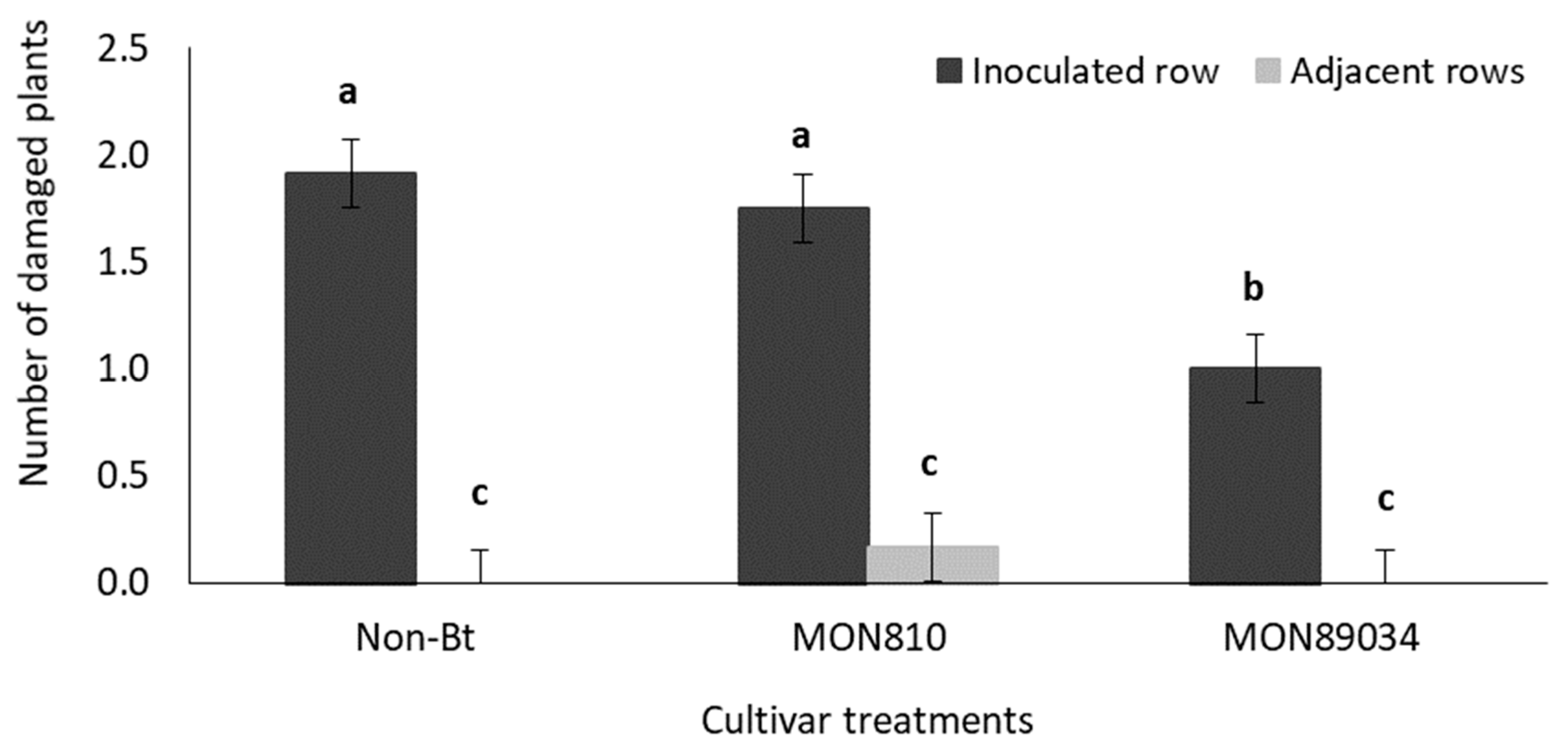
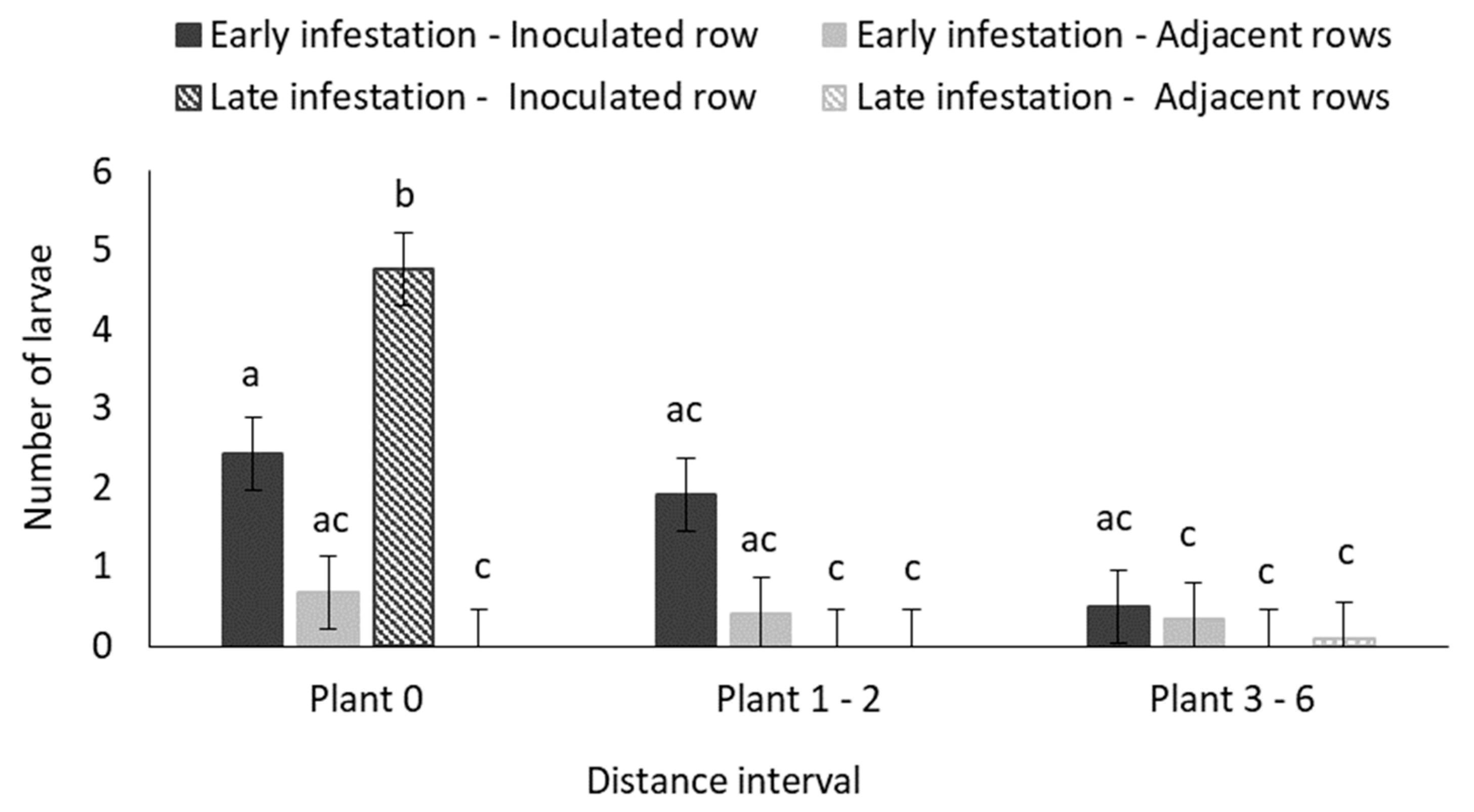
3.1.3. Destructive Sampling: Larval Survival
3.2. Field Trial: Effect of Plant Density
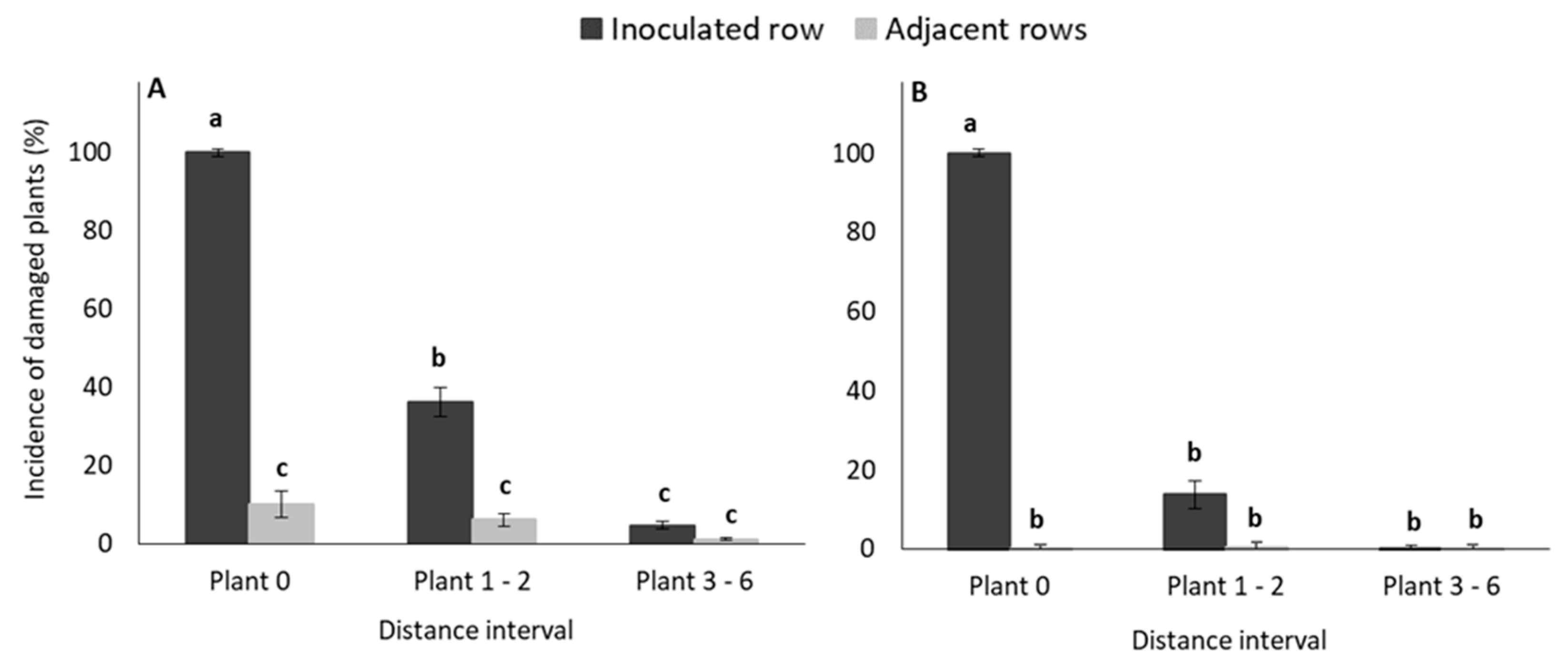
3.2.1. Weekly Monitoring of Damage Symptoms: Damage Frequency
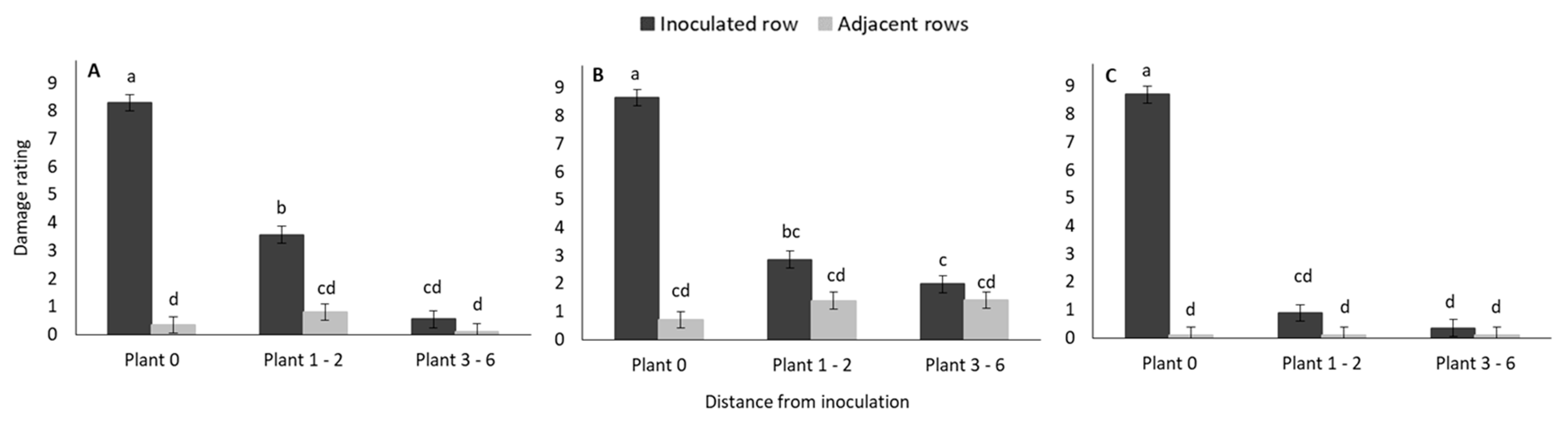
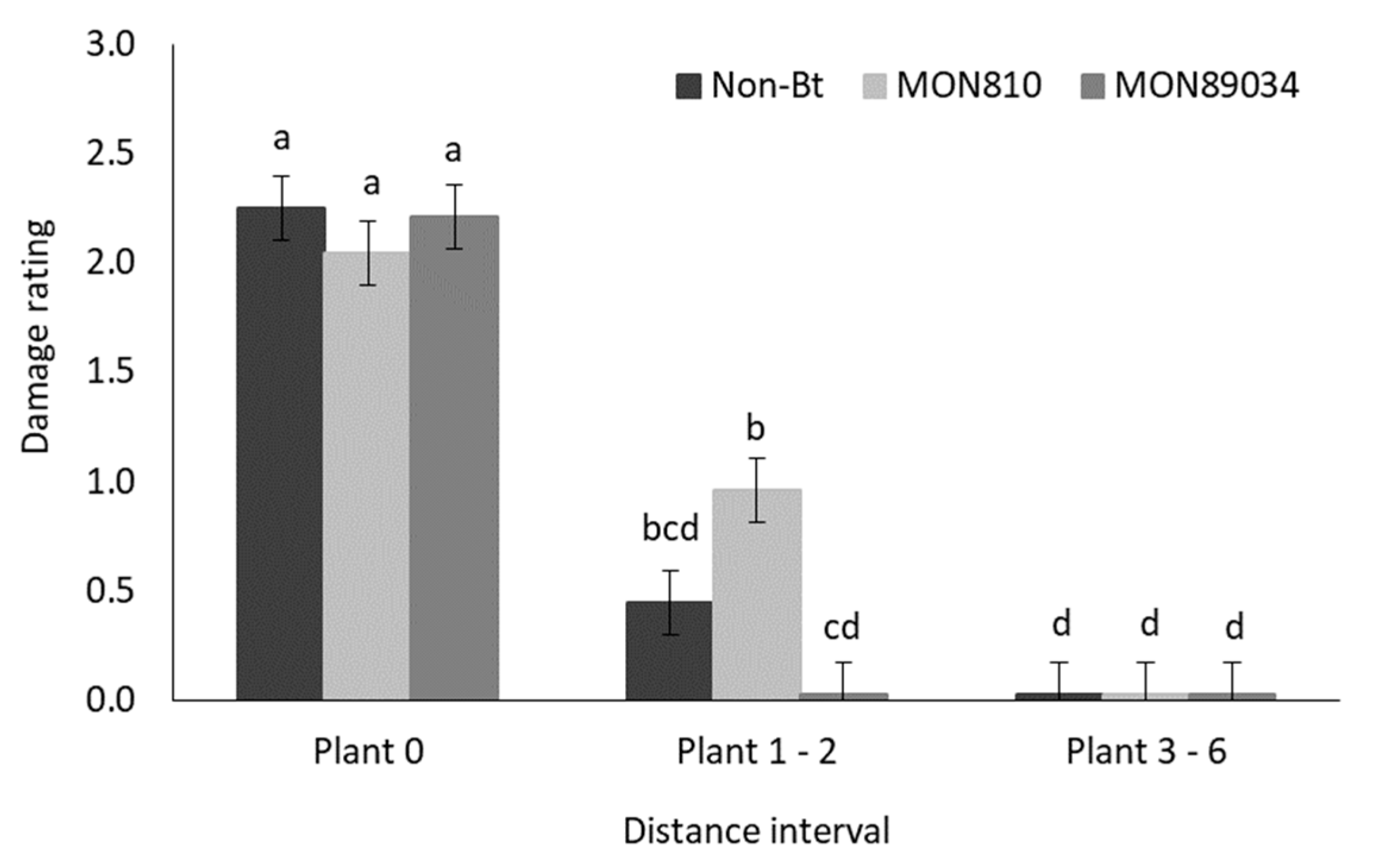
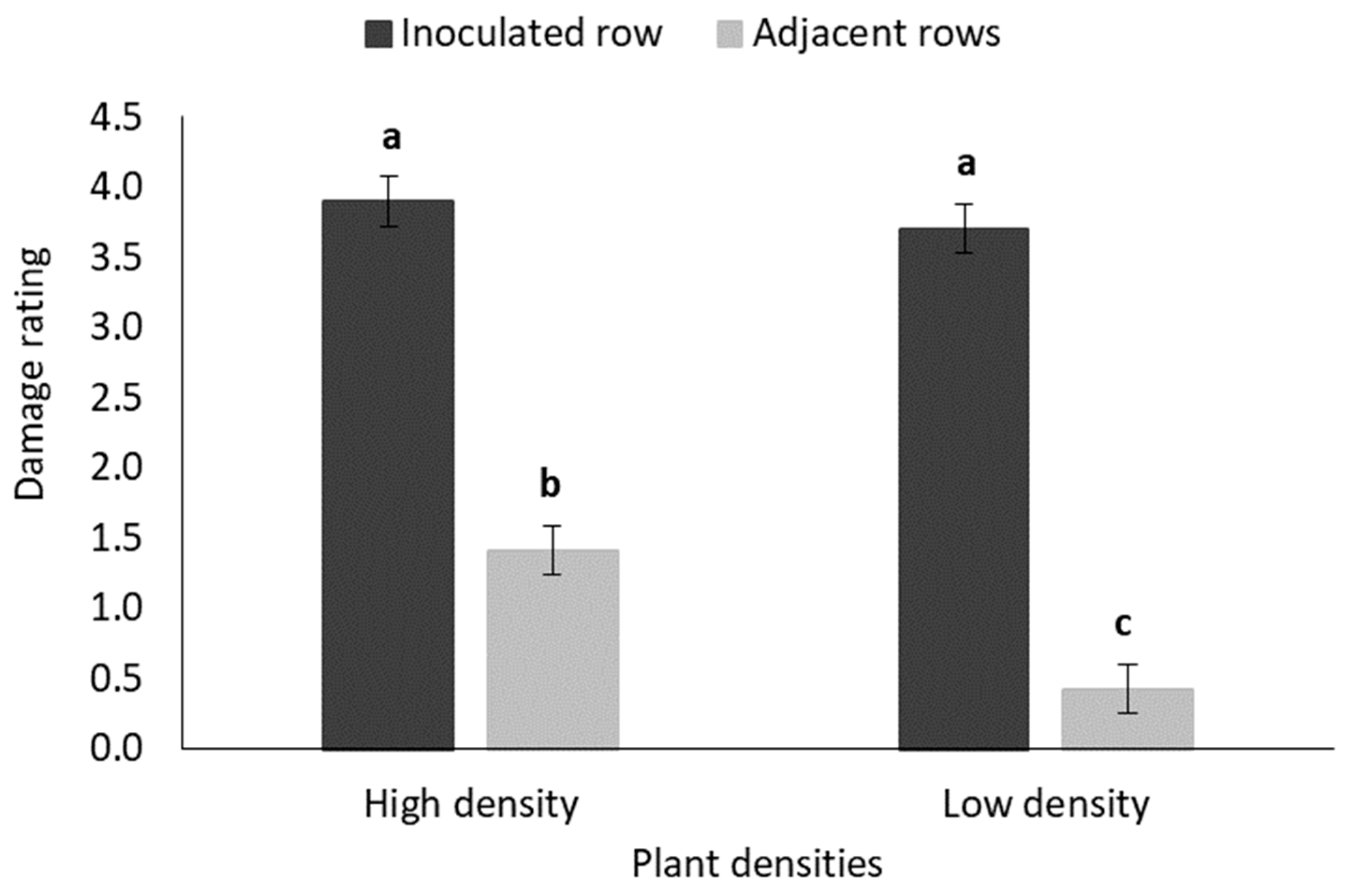
3.2.2. Weekly Monitoring of Damage Symptoms: Damage Rating
3.2.3. Destructive Sampling
4. Discussion
4.1. Importance of Laboratory, Semi-Field, and Field Experiments
4.2. B. fusca Larval Migration Behaviour
4.3. B. fusca Larval Migration and Plant Density
4.4. B. fusca Larval Migration and Plant Growth Stage
4.5. B. fusca Larval Migration and Bt Toxins
4.6. How Many Maize Plants Do the Larvae from a Single B. fusca Egg Batch Damage?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kfir, R.; Overholt, W.A.; Khan, Z.R.; Polaszek, A. Biology and management of economically important lepidopteran cereal stem borers in Africa. Annu. Rev. Entomol. 2002, 47, 701–731. [Google Scholar] [CrossRef] [PubMed]
- Tounou, A.K.; Gounou, S.; Borgemeister, C.; Goumedzoe, Y.M.D.; Schulthess, F. Susceptibility of Eldana saccharina (Lepidoptera: Pyralidae), Busseola fusca and Sesamia calamistis (Lepidoptera: Noctuidae) to Bacillus thuringiensis Cry toxins and potential side effects on the larval parasitoid Cotesia sesamiae (Hymenoptera: Braconidae). Biocontrol. Sci. Technol. 2010, 15, 127–137. [Google Scholar] [CrossRef]
- Van den Berg, J.; Van Rensburg, J.B.J. Effect of various directional insecticide sprays against Busseola fusca (Lepidoptera: Noctuidae) and Chilo partellus (Lepidoptera: Pyralidae) in maize and sorghum. S. Afr. J. Plant Soil 1996, 13, 51–54. [Google Scholar] [CrossRef]
- Van den Berg, J.; Nur, A.F. Chemical control. In Cereal Stem Borers in Africa: Economic Importance, Taxonomy, Natural Enemies and Control; Polaszek, A., Ed.; CABI: Wallingford, UK, 1998; ISBN 085-199-175-0. [Google Scholar]
- Van den Berg, J.; Van Wyk, A. The effect of Bt maize on Sesamia calamistis in South Africa. Entomol. Exp. Appl. 2007, 122, 45–51. [Google Scholar] [CrossRef]
- Tende, R.M.; Mugo, S.N.; Nderitu, J.H.; Olubayo, F.M.; Songa, J.M.; Bergvinson, D.J. Evaluation of Chilo partellus and Busseola fusca susceptibility to d-endotoxins in Bt-maize. Crop Prot. 2010, 29, 115–120. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 1994, 39, 47–79. [Google Scholar] [CrossRef]
- Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 1998, 43, 701–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassmann, A.J.; Carrière, Y.; Tabashnik, B.E. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2009, 54, 147–163. [Google Scholar] [CrossRef]
- Carrière, Y.; Crowder, D.W.; Tabashnik, B.E. Evolutionary ecology of insect adaptation to Bt crops. Evol. Appl. 2010, 3, 561–573. [Google Scholar] [CrossRef]
- Bourguet, D.; Desquilbet, M.; Lemarié, S. Regulating insect resistance management: The case of non-Bt corn refuges in the US. J. Environ. Manag. 2005, 76, 210–220. [Google Scholar] [CrossRef]
- Siegfried, B.; Jurat-Fuentes, J.L. Editorial overview: Pests and resistance: Resistance to Bt toxins in transgenic crops. Curr. Opin. Insect Sci. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, J.B.J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil 2007, 24, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Tabashnik, B.E.; Brevault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). The Environmental Protection Agency’s White Paper on Bt Plant-Pesticide Resistance Management; United States Environmental Protection Agency: Washington, DC, USA, 1998; ISBN 978-124-406-634-2.
- Onstad, D.W.; Mitchell, P.D.; Hurley, T.M.; Lundgren, J.G.; Porter, R.P.; Krupke, C.H.; Spencer, J.L.; DiFonzo, C.D.; Baute, T.S.; Hellmich, R.L. Seeds of change: Corn seed mixtures for resistance management and integrated pest management. J. Econ. Entomol. 2011, 104, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Razze, J.M.; Mason, C.E. Dispersal behavior of neonate European corn borer (Lepidoptera: Crambidae) on Bt corn. J. Econ. Entomol. 2012, 105, 1214–1223. [Google Scholar] [CrossRef] [Green Version]
- Pannuti, L.E.R.; Paula-Moraes, S.V.; Hunt, T.E.; Baldin, E.L.L.; Dana, L.; Malaquias, J.V. Plant-to-plant movement of Striacosta albicosta (Lepidoptera: Noctuidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize (Zea mays). J. Econ. Entomol. 2016, 109, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.M.; Onstad, D.W. Seed mixtures as resistance management strategy for European corn borers (Lepidoptera: Crambidae) infesting transgenic corn expressing Cry1Ab protein. J. Econ. Entomol. 2000, 93, 937–984. [Google Scholar] [CrossRef]
- Heuberger, S.; Crowder, D.W.; Brévault, T.; Tabashnik, B.E.; Carrière, Y. Modeling the effects of plant-to-plant gene flow, larval behavior, and refuge size on pest resistance to Bt cotton. Environ. Entomol. 2011, 40, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Ives, A.R.; Glaum, P.R.; Ziebarth, N.L.; Andow, D.A. The evolution of resistance to two-toxin pyramid transgenic crops. Ecol. Appl. 2011, 21, 503–515. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.F. Host-Plant Selection by Phytophagous Insects; Chapman and Hall: London, UK, 1994; ISBN 0-412-0311-I-6. [Google Scholar]
- Schoonhoven, L.M.; Van Loon, J.J.A. An inventory of taste in caterpillars: Each species its own key. Acta Zool. Acad. Sci. Hung. 2002, 48, 215–263. [Google Scholar]
- Calatayud, P.A.; Juma, G.; Njagi, P.G.N.; Faure, N.; Calatayud, S.; Dupas, S.; le Ru, B.P.; Magoma, G.; Silvain, J.-F.; Frérot, B. Differences in mate acceptance and host plant recognition between wild and laboratory-reared Busseola fusca (Fuller). J. Appl. Entomol. 2008, 132, 255–264. [Google Scholar] [CrossRef]
- Petit, C.; Ahuya, P.; Le Rü, B.; Kaiser-Arnauld, L.; Harry, M.; Calatayud, P.A. Odour and feeding preference of noctuid moth larvae conditioned to vanillin diet and non-vanillin diet. Phytoparasitica 2018, 46, 223–232. [Google Scholar] [CrossRef]
- Visser, A.; Du Plessis, H.; Erasmus, A.; Van den Berg, J. Preference of Bt-resistant and susceptible Busseola fusca moths and larvae for Bt and non-Bt maize. Entomol. Exp. Appl. 2019, 167, 849–867. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. Ecology and behaviour of first instar larval Lepidoptera. Annu. Rev. Entomol. 2002, 47, 361–391. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.; van den Berg, J. Genetically modified maize: Adoption practices of small-scale farmers in South Africa and implications for resource-poor farmers on the continent. Asp. Appl. Biol. 2010, 96, 215–223. [Google Scholar]
- Jacobson, K.; Myhr, A.I. GM crops and smallholders: Biosafety and local practice. J. Environ. Dev. 2012, 22, 104–124. [Google Scholar] [CrossRef]
- Aheto, D.W.; Bøhn, T.; Breckling, B.; Van den Berg, J.; Ching, L.L.; Wikmark, O. Implications of GM crops in subsistence-based agricultural systems in Africa. In GM Crop Cultivation-Ecological Effects in a Landscape Scale. Theorie in der Ökologie, 17; Breckling, B., Verhoeven, R., Eds.; Peter Lang: Frankfurt, Germany, 2013; pp. 93–103. [Google Scholar]
- Kotey, D.A.; Assefa, Y.; Van den Berg, J. Enhancing smallholder farmers’ awareness of GM maize technology, management practices and compliance to stewardship requirements in the Eastern Cape Province of South Africa: The role of public extension and advisory services. S. Afr. J. Agric. Ext. 2017, 45, 49–63. [Google Scholar] [CrossRef]
- Calatayud, P.A.; Le Rü, B.; Van den Berg, J.; Schulthess, F. Ecology of the African maize stalk borer, Busseola fusca (Lepidoptera: Noctuidae) with special reference to insect-plant interactions. Insects 2014, 5, 539–563. [Google Scholar] [CrossRef] [Green Version]
- Van Rensburg, J.B.J.; Van Rensburg, G.D.J. Laboratory production of Busseola fusca (Fuller) (Lepidoptera: Noctuidae) and techniques for the detection of resistance in maize plants. Afr. Entomol. 1993, 1, 25–28. [Google Scholar]
- Kotey, D.A.; Obi, A.; Assefa, Y.; Erasmus, A.; Van den Berg, J. Monitoring resistance to Bt maize in field populations of Busseola fusca (Fuller) (Lepidoptera: Noctuidae) from smallholder farms in the Eastern Cape Province of South Africa. Afr. Entomol. 2017, 25, 200–209. [Google Scholar] [CrossRef]
- Strydom, E.; Erasmus, A.; Du Plessis, H.; Van den Berg, J. Resistance status of Busseola fusca (Lepidoptera: Noctuidae) populations to single- and stacked-gene Bt maize in South Africa. J. Econ. Entomol. 2018, 112, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.; Dlamini, T. Determining genetic variations in B. fusca Fuller (Lepidoptera: Noctuidae) and Chilo partellus Swinhoe (Lepidoptera: Crambidae) from Swaziland and South Africa through sequences of the mtDNA cytochrome oxidase sub-unit 1 gene. Int. J. Adv. Res. Biol. Sci. 2016, 3, 208–213. [Google Scholar]
- Solomon, M.E. Control of humidity with potassium hydroxide, sulphuric acid or other solutions. Bull. Entomol. Res. 1951, 42, 543–554. [Google Scholar] [CrossRef]
- Kruger, M.; Van Rensburg, J.B.J.; Van den Berg, J. Resistance to Bt Maize in Busseola fusca (Lepidoptera: Noctuidae) From Vaalharts, South Africa. Environ. Entomol. 2011, 40, 477–483. [Google Scholar] [CrossRef]
- Kruger, M.; Van Rensburg, J.B.J.; Van den Berg, J. No fitness costs associated with resistance of Busseola fusca (Lepidoptera: Noctuidae) to genetically modified Bt maize. Crop Prot. 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Davis, F.M.; NG, S.S.; Williams, W.P. Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Miss. Agric. For. Exp. Stn. Res. Bull. 1992, 186, 1–9. [Google Scholar]
- Ndjomatchoua, F.T.; Tonnang, H.E.Z.; Plantamp, C.; Campagne, P.; Tchawoua, C.; Le Rü, B.P. Spatial and temporal spread of maize stem borer Busseola fusca (Fuller) (Lepidoptera: Noctuidae) damage in smallholder farms. Agric. Ecosyst. Environ. 2016, 235, 105–118. [Google Scholar] [CrossRef]
- Du Plessis, J. Maize Production; Department of Agriculture, Republic of South Africa: Pretoria, South Africa, 2003.
- Visser, A.; Du Plessis, H.; Erasmus, A.; Van den Berg, J. Plant abandonment by Busseola fusca (Lepidoptera: Noctuidae) larvae: Do Bt toxins have an effect? Insects. Under review.
- Han, P.; Velasco-Hernández, M.C.; Ramirez-Romero, R.; Desneux, N. Behavioral effects of insect-resistant genetically modified crops on phytophagous and beneficial arthropods: A review. J. Pest. Sci. 2016, 89, 859–883. [Google Scholar] [CrossRef]
- Calatayud, P.A.; Ahuya, P.O.; Groutte, S.; Le Rü, B. The first hours in the life of a Busseola fusca (Lepidoptera: Noctuidae) larva. Entomol. Ornithol. Herpetol. 2015, 4. [Google Scholar] [CrossRef]
- Ntiri, E.S.; Calatayud, P.A.; Van den Berg, J.; Schulthess, F.; Le Rü, B.P. Influence of temperature on intra- and interspecific resource utilization within a community of lepidopteran maize stemborers. PLoS ONE 2016, 11, e0148735. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.; Van Rensburg, J.B.J.; Van der Westhuizen, M.C. The effect of single and mixed populations of Busseola fusca (Lepidoptera: Noctuidae) and Chilo partellus (Lepidoptera: Pyralidae) on damage to grain sorghum. J. Entomol. Soc. S. Afr. 1991, 54, 231–242. [Google Scholar]
- Goldstein, J.A.; Mason, C.E.; Pesek, J. Dispersal and movement behavior of neonate European corn borer (Lepidoptera: Crambidae) on non-Bt and transgenic Bt corn. J. Econ. Entomol. 2010, 103, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Razze, J.M.; Mason, C.E.; Pizzolato, T.D. Feeding behavior of neonate Ostrinia nubilalis (Lepidoptera: Crambidae) on Cry1Ab Bt corn: Implications for resistance management. J. Econ. Entomol. 2011, 104, 806–813. [Google Scholar] [CrossRef]
- Chapman, R.F.; Woodhead, S.; Bernays, E.A. Survival and dispersal of young larvae of Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) in two cultivars of sorghum. Bull. Entomol. Res. 1983, 73, 65–74. [Google Scholar] [CrossRef]
- Berger, A. Larval movements of Chilo partellus (Lepidoptera: Pyralidae) within and between plants: Timing, density responses, and survival. Bull. Entomol. Res. 1992, 82, 441–448. [Google Scholar] [CrossRef]
- Ross, S.E.; Ostlie, K.R. Dispersal and survival of early instars of European corn borer (Lepidoptera: Pyralidae) in field corn. J. Econ. Entomol. 1990, 83, 831–836. [Google Scholar] [CrossRef]
- Van den Berg, J.; Van Rensburg, J.B.J.; Pringle, K.L. Comparative injuriousness of Busseola fusca (Lepidoptera: Noctuidae) and Chilo partellus (Lepidoptera: Pyralidae) on grain sorghum. Bull. Entomol. Res. 1991, 82, 137–143. [Google Scholar] [CrossRef]
- Cohen, M.B.; Romena, A.M.; Gould, F. Dispersal by larvae of the stem borers Scirpophaga incertulas (Lepidoptera: Pyralidae) and Chilo suppressalis (Lepidoptera: Crambidae) in plots of transplanted rice. Environ. Entomol. 2000, 29, 958–971. [Google Scholar] [CrossRef] [Green Version]
- Van Rensburg, J.B.J.; Walters, M.C.; Giliomee, J.H. Ecology of the maize stalk borer, Busseola fusca (Fuller) (Lepidoptera: Noctuidae). Bull. Entomol. Res. 1987, 77, 255–269. [Google Scholar] [CrossRef]
- Halcomb, J.L.; Benedict, J.H.; Cook, B.; Ring, D.R.; Correa, J.C. Feeding behavior of bollworm and tobacco budworm (Lepidoptera: Noctuidae) larvae in mixed stands of non-transgenic and transgenic cotton expressing an insecticidal protein. J. Econ. Entomol. 2000, 93, 1300–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rensburg, J.B.J.; Walters, M.C.; Giliomee, J.H. Plant population and cultivar effects on yield losses caused by the maize stalk borer, Busseola fusca (Lepidoptera: Noctuidae). S. Afr. J. Plant Soil 1988, 5, 215–218. [Google Scholar] [CrossRef]
- Ogunwolu, E.O.; Nwosu, K.; Ogunyebi, S.O. Stem borer damage in maize as affected by host plant density. J. Agric. Sci. 1981, 96, 695–697. [Google Scholar] [CrossRef]
- Van den Berg, J.; Van Rensburg, J.B.J. The effect of plant density on the injuriousness of Busseola fusca (Fuller) (Lepidoptera: Noctuidae) in grain sorghum. S. Afr. J. Plant Soil 1991, 8, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Ntambo, M.S.; Manyangarirwa, W.; Tuarira, M.; Onesime, M.K. Effect of Lepidopterous stemborers, Busseola fusca (Fuller) and Chilo partellus (Swinhoe) on maize (Zea mays L) yield: A review. Int. J. Innov. Res. Dev. 2015, 4, 181–188. [Google Scholar]
- Harris, K.M. Lepidopterous stem borers of cereals in Nigeria. Bull. Entomol. Res. 1962, 53, 139–171. [Google Scholar] [CrossRef]
- Van Rensburg, J.B.J. Evaluation of Bt.-Transgenic maize for resistance to the stem borers Busseola fusca (Fuller) and Chilo partellus (Swinhoe) in South Africa. S. Afr. J. Plant Soil 1999, 16, 38–43. [Google Scholar] [CrossRef]
- Van den Berg, J.; Hilbeck, A.; Bøhn, T. Pest resistance to Cry1Ab Bt maize: Field resistance, contributing factors and lessons from South Africa. J. Crop Prot. 2013, 54, 154–160. [Google Scholar] [CrossRef]
- Lawani, S.M. A review of the effects of various agronomic practices on cereal stem borer populations. Trop. Pest Manag. 1982, 28, 266–276. [Google Scholar] [CrossRef]
- Van Rensburg, J.B.J.; Walters, M.C.; Giliomee, J.H. The influence of rainfall on seasonal abundance and flight activity of the maize stalk borer, Busseola fusca in South Africa. S. Afr. J. Plant Soil 1987, 4, 183–187. [Google Scholar]
- Archer, T.L.; Schuster, G.; Patrick, C.; Cronholm, G.; Bynum, E.D.; Morrison, W.P. Whorl and stalk damage by European and Southwestern corn borers to four events of Bacillus thuringiensis transgenic maize. J. Crop Prot. 2000, 19, 181–190. [Google Scholar] [CrossRef]
- Walker, K.A.; Hellmich, R.L.; Lewis, L.C. Late-instar European corn borer (Lepidoptera: Crambidae) tunneling and survival in transgenic corn hybrids. J. Econ. Entomol. 2000, 93, 1276–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obopile, M.; Hammond, R.B.; Paul, A.P. Interaction between maize phenology and trangenic maize hybrids on stalk rots following European corn borer infestation. Afr. J. Plant Sci. 2011, 5, 878–886. [Google Scholar]
- Carroll, M.W.; Head, G.; Caprio, M.; Stork, L. Theoretical and empirical assessment of a seed mix refuge in corn for southwestern corn borer. J. Crop Prot. 2013, 49, 58–65. [Google Scholar] [CrossRef]
- Erasmus, A.; Marais, J.; Van den Berg, J. Movement and survival of Busseola fusca (Lepidoptera: Noctuidae) larvae within maize plantings with different ratios of non-Bt and Bt seed. Pest Manag. Sci. 2016. [Google Scholar] [CrossRef]
- Berdegué, M.; Trumble, J.T.; Moar, W.J. Effect of CryIC toxin from Bacillus thuringiensis on larval feeding behaviour of Spodoptera exigua. Entomol. Exp. Appl. 1996, 80, 389–401. [Google Scholar] [CrossRef]
- Pannuti, L.E.R.; Baldin, E.L.L.; Hunt, T.E.; Paula-Moraes, S.V. On-plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environ. Entomol. 2016, 45, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Paula-Moraes, S.; Hunt, T.E.; Wright, R.J.; Hein, G.L.; Blankenship, E.E. Western bean cutworm survival and the development of economic injury levels and economic thresholds in field corn. J. Econ. Entomol. 2013, 106, 1274–1285. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, T. Behavioural biology, feeding habits and ecology of three species of maize stemborers: Eldana saccharina (Lepidoptera: Pyralidae), Sesamia calamistis and Busseola fusca (Noctuidae) in Ibadan, Nigeria, West Africa. J. Ga. Entomol. Soc. 1983, 18, 259–272. [Google Scholar]
- Glatz, J.; Du Plessis, H.; Van den Berg, J. The effect of temperature on the development and reproduction of Busseola fusca (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2017, 107, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, P.A.; Ahuya, P.O.; Wanjoya, A.; Le Rü, B.; Silvain, J.; Frérot, B. Importance of plant physical cues in host acceptance for oviposition by Busseola fusca. Entomol. Exp. Appl. 2008, 126, 233–242. [Google Scholar] [CrossRef]
- Van Rensburg, J.B.J.; Pringle, K.L. Sequential sampling technique for surveys of eggs laid by the maize stalk borer, Busseola fusca (Fuller) (Lepidoptera: Noctuidae). J. Entomol. Soc. Sth. Afr. 1989, 52, 223–228. [Google Scholar]





| Treatment | Number of Plants in Treatment Plot | Number of Plants Damaged at 21 Days | % of Plants Damaged at 21 Days | |
|---|---|---|---|---|
| Season 1 | 100% Non-Bt | 113 | 18.08 | 16 |
| 5% Non-Bt, 95% MON810 | 123 | 8.61 | 7 | |
| 100% Non-Bt | 180 | 28.80 | 16 | |
| 5% Non-Bt, 95% MON89034 | 174 | 17.40 | 10 | |
| Season 2 | 100% Non-Bt | 125 | 20.00 | 16 |
| 5% Non-Bt, 95% MON810 | 125 | 1.25 | 1 | |
| 100% Non-Bt | 190 | 28.50 | 15 | |
| 5% Non-Bt, 95% MON89034 | 180 | 1.80 | 1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visser, A.; Du Plessis, H.; Erasmus, A.; van den Berg, J. Larval Migration Behaviour of Busseola fusca (Lepidoptera: Noctuidae) on Bt and Non-Bt Maize under Semi-Field and Field Conditions. Insects 2020, 11, 16. https://doi.org/10.3390/insects11010016
Visser A, Du Plessis H, Erasmus A, van den Berg J. Larval Migration Behaviour of Busseola fusca (Lepidoptera: Noctuidae) on Bt and Non-Bt Maize under Semi-Field and Field Conditions. Insects. 2020; 11(1):16. https://doi.org/10.3390/insects11010016
Chicago/Turabian StyleVisser, Andri, Hannalene Du Plessis, Annemie Erasmus, and Johnnie van den Berg. 2020. "Larval Migration Behaviour of Busseola fusca (Lepidoptera: Noctuidae) on Bt and Non-Bt Maize under Semi-Field and Field Conditions" Insects 11, no. 1: 16. https://doi.org/10.3390/insects11010016






