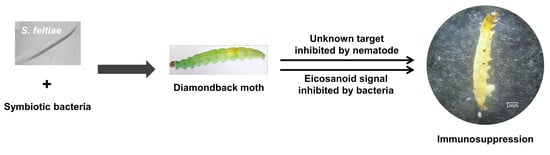
Host Immunosuppression Induced by Steinernema feltiae, an Entomopathogenic Nematode, through Inhibition of Eicosanoid Biosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Chemicals
2.3. EPN Source and Culturing
2.4. Molecular Identification of EPN Isolate
2.5. Virulence Assay of EPN
2.6. Assessment of Nodule Formation after Nematode Infection
2.7. Bioassay of P. xylostella Larvae Infected with S. feltiae with Addition of DEX or AA
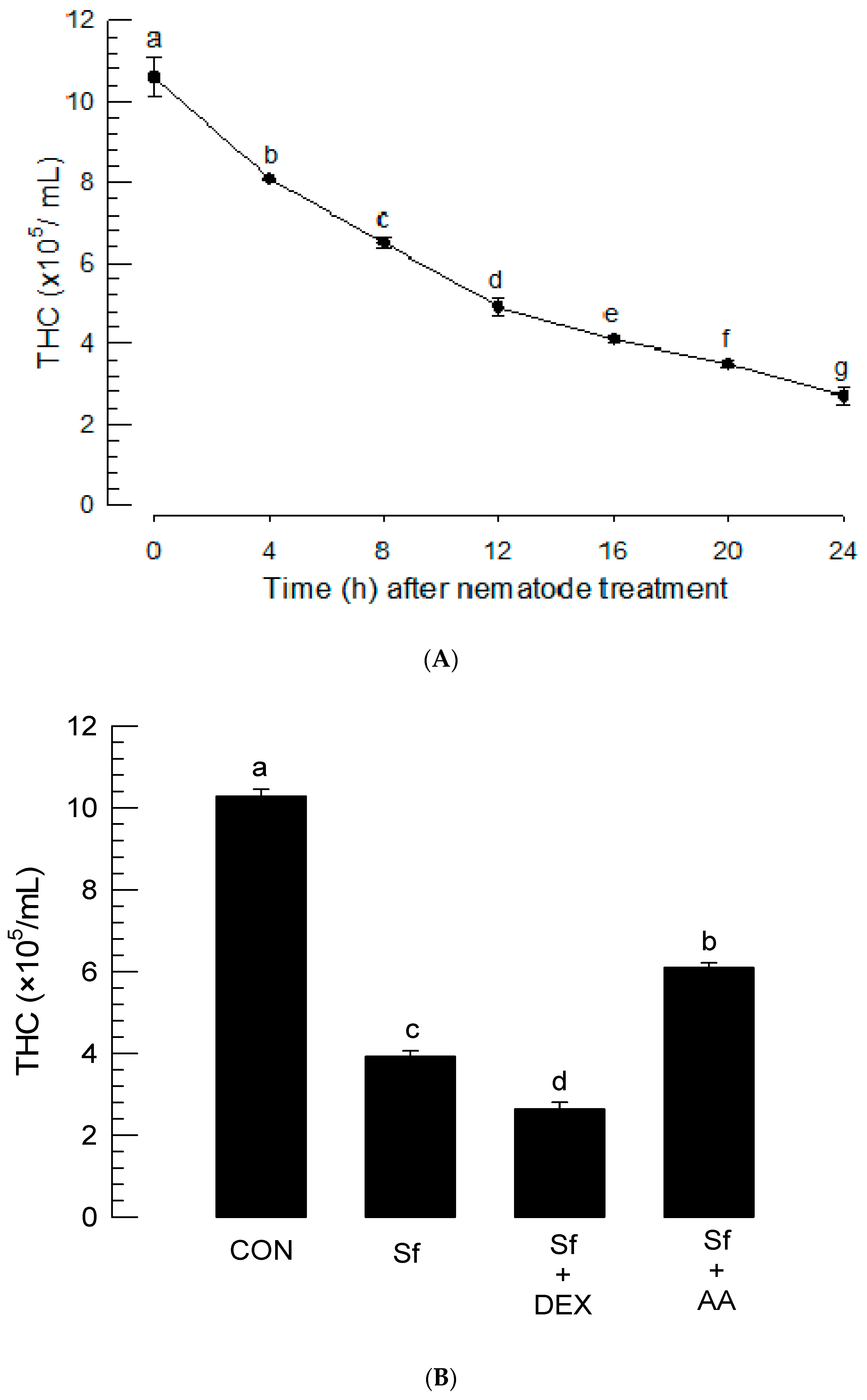
2.8. Total Hemocyte Count (THC) Analysis after Nematode Treatment
2.9. Antibiotic Screening against X. bovienii
2.10. Effect of Kanamycin in Suppressing Bacterial Growth in P. xylostella Larvae Infected with S. feltiae
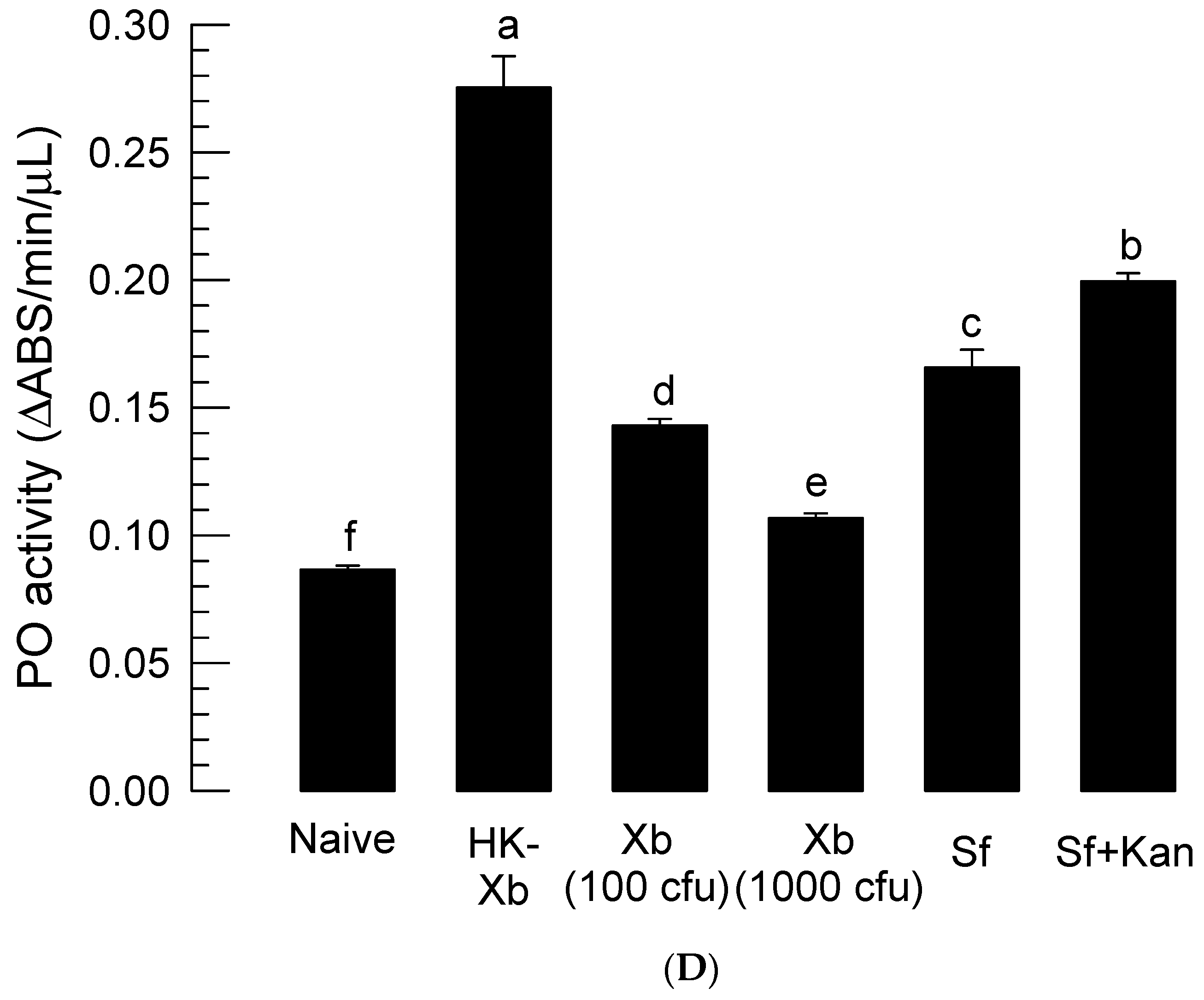
2.11. Phenol Oxidase (PO) Enzyme Activity
2.12. PLA2 Activity Measurement in Plasma after S. feltiae Infection
2.13. Statistical Analysis
3. Results
3.1. Identification of a Nematode Isolate
3.2. Insecticidal Activity of S. feltiae K1
3.3. Insecticidal Virulence of S. feltiae K1 by Down-Regulating PLA2 Activity
3.4. Infection of S. feltiae K1 Resulted in Immunosuppression and Cytotoxicity
3.5. Role of Symbiotic Bacteria in Insecticidal Activity of S. feltiae K1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Kim, Y. PGE2 mediates cytoskeletal rearrangement of hemocytes via Cdc42, a small G protein, to activate actin-remodeling factors in Spodoptera exigua (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2019, 102, e21607. [Google Scholar] [CrossRef]
- Imler, J.L.; Bulet, P. Antimicrobial peptides in Drosophila: Structures, activities and gene regulation. Chem. Immunol. Allergy. 2005, 86, 1–21. [Google Scholar]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Castillo, J.C.; Renolds, S.E.; Eleftherianos, I. Insect immune responses to nematode parasites. Trends Parasitol. 2011, 27, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, F.; Li, Q.; Zhang, J.; Li, Y.; Tang, T.; Hu, Q.; Yu, X.Q. Pattern recognition receptors in Drosophila immune responses. Dev. Comp. Immunol. 2020, 102, 103468. [Google Scholar] [CrossRef] [PubMed]
- Georgis, R.; Manweiler, S.A. Entomopathogenic nematodes: A developing biological control technology. Agric. Zool. Rev. 1994, 6, 63–94. [Google Scholar]
- Poinar, G. Nematode biopesticides. Fundam. Appl. Nematol. 1998, 21, 733–737. [Google Scholar]
- Shapiro-Ilan, D.I.; Hazir, S.; Glazer, I. Basic and applied research: Entomopathogenic nematodes. In Microbial Agents for Control of Insect Pests: From Discovery to Commercial Development and Use; Lacey, L.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2017; pp. 91–105. [Google Scholar]
- Akhurst, R.J. Taxonomic study of Xenorhabdus, a genus of bacteria symbiotically associated with insect pathogenic nematodes. Int. J. Syst. Bacteriol. 1983, 33, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Boemare, N.E. Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI: New York, NY, USA, 2002; pp. 35–56. [Google Scholar]
- Goodrich-Blair, H.; Clarke, D.J. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: Two roads to the same destination. Mol. Microbiol. 2007, 64, 260–268. [Google Scholar] [CrossRef]
- Kaya, H.K.; Aguillera, M.M.; Alumai, A.; Choo, H.Y.; De la Torre, M.; Fodor, A.; Ganguly, S.; Hazir, S.; Lakatos, S.; Pye, A.; et al. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biol. Control 2006, 38, 134–155. [Google Scholar] [CrossRef]
- Dillman, A.R.; Chaston, J.M.; Adams, B.J.; Ciche, T.A.; Goodrich-Blair, H.; Stock, S.P.; Sternberg, P.W. An entomopathogenic nematode by any other name. PLoS Pathog. 2012, 8, e1002527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowds, B.C.A.; Peters, A. Virulence mechanisms. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI: New York, NY, USA, 2002; pp. 79–98. [Google Scholar]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.E.; Goodrich-Blair, H. Friend and foe: The two faces of Xenorhabdus nematophila. Nature Rev. Microbiol. 2007, 5, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ji, D.; Cho, S.; Park, Y. Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunodepression. J. Invertebr. Pathol. 2005, 89, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.W. Eicosanoids in Invertebrate Signal Transduction Systems; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.; Kim, Y. Prostaglandins and other eicosanoids in insects: Biosynthesis and biological actions. Front. Physiol. 2019, 9, 1927. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.; Kim, Y. Why most insects have very low proportions of C20 polyunsaturated fatty acids: The oxidative stress hypothesis. Arch. Insect Biochem. Physiol. 2020, 103, e21622. [Google Scholar] [CrossRef]
- Hasan, M.A.; Ahmed, S.; Mollah, M.M.I.; Lee, D.; Kim, Y. Variation in pathogenicity of different strains of Xenorhabdus nematophila; Differential immunosuppressive activities and secondary metabolite production. J. Invertebr. Pathol. 2019, 166, 107221. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. Biochemical characteristics of immune-associated phospholipase A2 and its inhibition by an entomopathogenic bacterium, Xenorhabdus nematophila. J. Microbiol. 2009, 47, 774–782. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm, Spodoptera exigua. Insect Biochem. Mol. Biol. 2008, 38, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Kim, Y.; Stanley, D. PGE2 induces oenocytoid cell lysis via a G protein-coupled receptor in the beet armyworm, Spodoptera exigua. J. Insect Physiol. 2011, 57, 1568–1576. [Google Scholar] [CrossRef]
- Hwang, J.; Park, Y.; Kim, Y.; Hwang, J.; Lee, D. An entomopathogenic bacterium, Xenorhabdus nematophila, suppresses expression of antimicrobial peptides controlled by Toll and Imd pathways by blocking eicosanoid biosynthesis. Arch. Insect Biochem. Physiol. 2013, 83, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Morisseau, C.; Yang, J.; Lee, K.S.; Kamita, S.G.; Hammock, B.D. Ingestion of the epoxide hydrolase inhibitor AUDA modulates immune responses of the mosquito, Culex quinquefasciatus during blood feeding. Insect Biochem. Mol. Biol. 2016, 76, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef]
- Goh, H.G.; Lee, S.G.; Lee, B.P.; Choi, K.M.; Kim, J.H. Simple mass-rearing of beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), on an artificial diet. Korean J. Appl. Entomol. 1990, 29, 180–183. [Google Scholar]
- Jung, J.K.; Seo, B.Y.; Cho, C.R.; Kwon, Y.H.; Kim, G.H. Occurrence of lepidopteran insect pests and injury aspects in Adzuki bean fields. Korean J. Appl. Entomol. 2009, 48, 29–35. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Han, S. An improved collecting method of the infective juveniles of the entomopathogenic nematode, Steinernema carpocapsae Weiser. Korean J. Soil Zool. 2000, 5, 97–100. [Google Scholar]
- Kang, S.; Han, S.; Kim, Y. Identification and pathogenic characteristics of two Korean isolates of Heterorhabditis megidis. J. Asia Pac. Entomol. 2005, 8, 411–418. [Google Scholar] [CrossRef]
- Vrain, T.C.; Wakarchuk, D.A.; Levesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fund. Appl. Nematol. 1992, 15, 563–574. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye finding. Anal. Biochem. 1972, 72, 248–254. [Google Scholar] [CrossRef]
- SAS Institute, Inc. SAS/STAT User’s Guide; SAS Institute: Cary, NC, USA, 1989. [Google Scholar]
- Ellis, H. Genetic control of programmed cell death in the nematode C. Elegans. Cell 1986, 44, 817–829. [Google Scholar] [CrossRef]
- Puza, V.; Chundelova, D.; Nermut, J.; Zurovcova, M.; Mracek, Z. Intra-individual variability of ITS region in entomopathogenic nematodes (Steinernematidae: Nematoda): Implications for their taxonomy. Biocontrol 2015, 60, 547–554. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Smart, G.C., Jr. Identification of entomopathogenic nematodes in the Steinernematidae and Heterorhabditidae (Nemata: Rhabditida). J. Nematol. 1996, 28, 286–300. [Google Scholar] [PubMed]
- Murfin, K.E.; Whooley, A.C.; Klassen, J.L.; Goodrich-Blair, H. Comparison of Xenorhabdus bovienii bacterial strain genomes reveals diversity in symbiotic functions. BMC Genomics 2015, 16, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yooyangket, T.; Muangpat, P.; Polseela, R.; Tandhavanant, S.; Thanwisai, A.; Vitta, A. Identification of entomopathogenic nematodes and symbiotic bacteria from Nam Nao National Park in Thailand and larvicidal activity of symbiotic bacteria against Aedes aegypti and Aedes albopictus. PLoS ONE 2018, 13, e0195681. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, Y. Potentiating effect of Bacillus thuringiensis subsp. kurstaki on pathogenicity of entomopathogenic bacterium Xenorhabdus nematophila K1 against diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2007, 100, 246–250. [Google Scholar]
- Vatanparast, M.; Ahmed, S.; Herrero, S.; Kim, Y. A non-venomous sPLA2 of a lepidopteran insect: Its physiological functions in development and immunity. Dev. Comp. Immunol. 2018, 89, 83–92. [Google Scholar] [CrossRef]
- Cho, S.; Kim, Y. Hemocyte Apoptosis Induced by Entomopathogenic Bacteria, Xenorhabdus and Photorhabdus, in Bombyx mori. J. Asia Pac. Entomol. 2004, 72, 195–200. [Google Scholar] [CrossRef]
- Proschak, A.; Zhou, Q.; Schöner, T.; Thanwisai, A.; Kresovic, D.; Dowling, A.; ffrench-Constant, R.; Proschak, E.; Bode, H.B. Biosynthesis of the insecticidal xenocyloins in Xenorhabdus bovienii. Chembiochem 2014, 15, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Bisch, G.; Ogier, J.C.; Médigue, C.; Rouy, Z.; Vincent, S.; Tailliez, P.; Givaudan, A.; Gaudriault, S. Comparative genomics between two Xenorhabdus bovienii strains highlights differential evolutionary scenarios within an entomopathogenic bacterial species. Genome Biol. Evol. 2016, 8, 148–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisch, G.; Pagès, S.; McMullen, J.G.; Stock, S.P.; Duvic, B.; Givaudan, A.; Gaudriault, S. Xenorhabdus bovienii CS03, the bacterial symbiont of the entomopathogenic nematode Steinernema weiseri, is a non-virulent strain against lepidopteran insects. J. Invertebr. Pathol. 2015, 124, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Z.; Serra, L.; Lu, D.; Mortazavi, A.; Dillman, A.R. A core set of venom proteins is released by entomopathogenic nematodes in the genus Steinernema. PLoS Pathog. 2019, 15, e1007626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brivio, M.F.; Mastore, M. Nematobacterial complexes and insect hosts: Different weapons for the same war. Insects 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Species Blasted in NCBI-GenBank | GenBank Accession Number | Total Score | E Value | Identity (%) |
|---|---|---|---|---|
| S. feltiae strain 626 | KM016348.1 | 1724 | 0.0 | 99.68 |
| S. feltiae isolate WG-01 | MK294325.1 | 1718 | 0.0 | 99.58 |
| S. feltiae strain SN | AF121050.2 | 1718 | 0.0 | 99.58 |
| S. feltiae strain T 92 | AY230185.1 | 1714 | 0.0 | 99.47 |
| S. feltiae strain 626 | KM016351.1 | 1714 | 0.0 | 99.47 |
| Morphometric Characters | S. feltae Isolate Mean ± SE (n = 20) | S. feltae1 |
|---|---|---|
| TBL (total body length), µm | 805 ± 10.6 | 849 (736–950) |
| MBW (maximum body width), µm | 26.8 ± 0.6 | 26 (22–29) |
| EP (excretory pore), µm | 62.0 ± 1.5 | 62 (53–67) |
| ES (esophagus length), µm | 138 ± 2.4 | 136 (115–150) |
| TL (tail length), µm | 83.9 ± 1.7 | 81 (70–92) |
| TBL/MBW | 30.3 ± 0.8 | 31 (29–33) |
| TBL/ES | 5.9 ± 0.1 | 6.0 (5.3–6.4) |
| TBL/TL | 9.6 ± 0.2 | 10.4 (9.2–12.6) |
| EP/ES × 100 | 45.1 ± 1.3 | 45 (42–51) |
| EP/TL × 100 | 74.2 ± 2.2 | 78 (69–86) |
| Target Insects | N | LC50 (IJs/Larva) | Slope ± SE | LT50 (h) | Slope ± SE |
|---|---|---|---|---|---|
| S. exigua | 30 | 24.85 (11.9 ± 51.6) | 1.08 ± 0.16 | 46.36 (39.0 ± 55.1) | 4.15 ± 0.04 |
| T. molitor | 30 | 39.33 (23.7 ± 64.3) | 1.67 ± 0.11 | 46.99 (38.3 ± 57.7) | 3.36 ± 0.05 |
| P. xylostella | 30 | 23.67 (10.9 ± 51.2) | 1.02 ± 0.17 | 49.04 (40.7 ± 58.1) | 3.82 ± 0.04 |
| M. vitrata | 30 | 25.04 (13.9 ± 45.3) | 1.39 ± 0.13 | 50.80 (41.5 ± 62.2) | 3.43 ± 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra Roy, M.; Lee, D.; Kim, Y. Host Immunosuppression Induced by Steinernema feltiae, an Entomopathogenic Nematode, through Inhibition of Eicosanoid Biosynthesis. Insects 2020, 11, 33. https://doi.org/10.3390/insects11010033
Chandra Roy M, Lee D, Kim Y. Host Immunosuppression Induced by Steinernema feltiae, an Entomopathogenic Nematode, through Inhibition of Eicosanoid Biosynthesis. Insects. 2020; 11(1):33. https://doi.org/10.3390/insects11010033
Chicago/Turabian StyleChandra Roy, Miltan, Dongwoon Lee, and Yonggyun Kim. 2020. "Host Immunosuppression Induced by Steinernema feltiae, an Entomopathogenic Nematode, through Inhibition of Eicosanoid Biosynthesis" Insects 11, no. 1: 33. https://doi.org/10.3390/insects11010033
APA StyleChandra Roy, M., Lee, D., & Kim, Y. (2020). Host Immunosuppression Induced by Steinernema feltiae, an Entomopathogenic Nematode, through Inhibition of Eicosanoid Biosynthesis. Insects, 11(1), 33. https://doi.org/10.3390/insects11010033