Incidence of Diaphorina citri Carrying Candidatus Liberibacter asiaticus in Brazil’s Citrus Belt
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
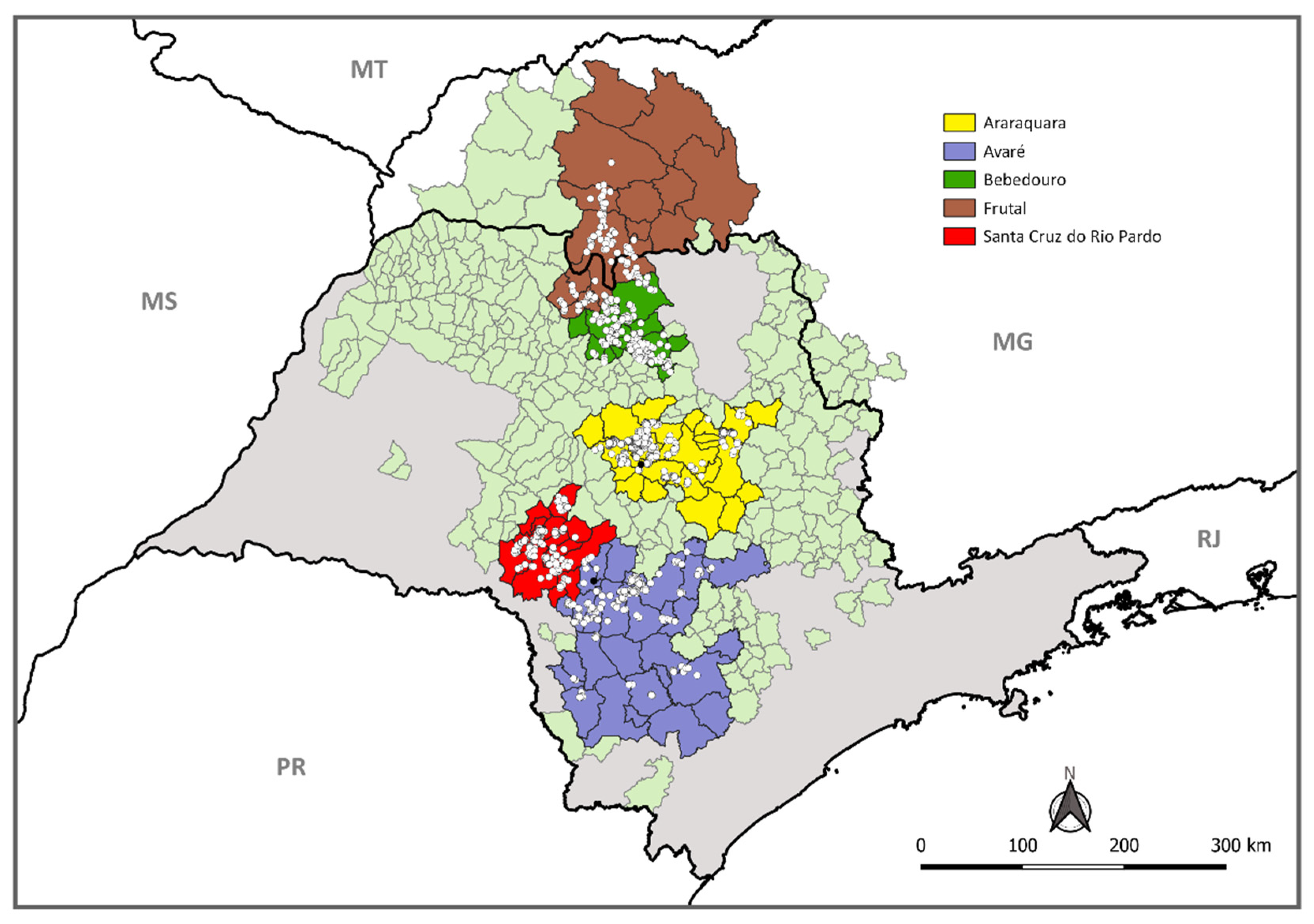
2.1. Locations, Adult Psyllid Monitoring, and Sample Collection
2.2. HLB Management and Citrus Shoot Stage Evaluation
2.3. Detection of Candidatus Liberibacter asiaticus by qPCR
2.4. Analysis of the Presence of Candidatus Liberibacter asiaticus in Diaphorina citri
3. Results
3.1. Presence of Candidatus Liberibacter asiaticus in Diaphorina citri
3.2. Seasonality of the Presence of Candidatus Liberibacter asiaticus in Psyllids
3.3. Proportion of Candidatus Liberibacter asiaticus in Psyllids
3.4. HLB Management Comparison Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Lopes, S.A.; Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Capoor, S.P.; Rao, D.G.; Viswanath, S.M. Diaphorina citri Kuway., a vector of the greening disease of citrus in India. Indian J. Agric. Sci. 1967, 37, 572–576. [Google Scholar]
- Yamamoto, P.T.; Felippe, M.R.; Garbim, L.F.; Coelho, J.H.C.; Ximenes, N.L.; Martins, E.C.; Leite, A.P.R.; Sousa, M.C.; Abrahão, D.P.; Braz, J.D. Diaphorina citri (Kuwayama) (Hemiptera: Psyllydae): Vector of the bacterium Candidatus Liberibacter americanus. In Proceedings of Huanglongbing—Greening International Workshop; Fundecitrus: Ribeirão Preto, São Paulo, Brazil, 2006; p. 96. [Google Scholar]
- Coletta-Filho, H.D.; Targon, M.L.P.N.; Takita, M.A.; De Negri, J.D.; Pompeu, J., Jr.; Machado, M.A. First report of the causal agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Dis. 2004, 88, 1382. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.C.; Danet, J.L.; Eveillard, S.; Martins, E.C.; Jesus, W.C., Jr.; Yamamoto, P.T.; Lopes, S.A.; Bassanezi, R.B.; Ayres, A.J.; Saillard, C.; et al. Citrus huanglongbing in São Paulo State, Brazil: PCR detection of the ‘Candidatus’ Liberibacter species associated with the disease. Mol. Cell. Probes 2005, 19, 173–179. [Google Scholar] [CrossRef]
- Teixeira, D.C.; Wulff, N.A.; Martins, E.C.; Kitajima, E.W.; Bassanezi, R.; Ayres, A.J.; Eveillard, S.; Saillard, C.; Bové, J.M. A phytoplasma closely related to the pigeon pea witches’-broom phytoplasma (16Sr IX) is associated with citrus huanglongbing symptoms in the state of São Paulo, Brazil. Phytopathology 2008, 98, 977–984. [Google Scholar] [CrossRef] [Green Version]
- Wulff, N.A.; Fassini, C.G.; Marques, V.V.; Martins, E.C.; Coletti, D.A.B.; Teixeira, D.C.; Sanches, M.M.; Bové, J.M. Molecular characterization and detection of 16SrIII group phytoplasma associated with Huanglongbing symptoms. Phytopathology 2019, 109, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.N.; Teixeira, D.C.; Yamamoto, P.T.; Lopes, J.R.S. Weedy hosts and prevalence of potential leafhopper vectors (Hemiptera: Cicadellidae) of a phytoplasma (16SIX group) associated with Huanglongbing symptoms in citrus groves. J. Econ. Entomol. 2012, 105, 329–337. [Google Scholar] [CrossRef]
- Teixeira, D.C.; Wulff, N.A.; Lopes, S.A.; Yamamoto, P.T.; Miranda, M.P.; Sposito, M.B.; Belasque, J., Jr.; Bassanezi, R.B. Caracterização e etiologia das bactérias associadas ao Huanglongbing dos citros. Citrus Res. Tech. 2010, 31, 115–128. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing and the future of citrus in São Paulo State, Brazil. J. Plant Pathol. 2012, 94, 465–467. [Google Scholar] [CrossRef]
- Belasque, J., Jr.; Bassanezi, R.B.; Yamamoto, P.T.; Ayres, A.J.; Tachibana, A.; Violante, A.R.; Tank, A., Jr.; Di Giorgi, F.; Tersi, F.E.A.; Menezes, G.M.; et al. Lessons from huanglongbing management in São Paulo state, Brazil. J. Plant Pathol. 2010, 92, 285–302. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Montesino, L.H.; Gimenes-Fernandes, N.; Yamamoto, P.T.; Gottwald, T.R.; Amorim, L.; Bergamin Filho, A. Efficacy of area-wide inoculum reduction and vector control on temporal progress of huanglongbing in young sweet orange plantings. Plant Dis. 2013, 97, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.P.; Santos, F.L.; Bassanezi, R.B.; Montesino, L.H.; Barbosa, J.C.; Sétamou, M. Monitoring methods for Diaphorina citri Kuwayama (Hemiptera: Liviidae) on citrus groves with different insecticide application programmes. J. Appl. Entomol. 2018, 142, 89–96. [Google Scholar] [CrossRef]
- Fundo de Defesa da Citricultura. Levantamento de Doenças. Available online: https://www.fundecitrus.com.br/levantamentos (accessed on 15 August 2020).
- Boina, D.R.; Meyer, W.; Onagbola, E.O.; Stelinski, L.L. Quantifying dispersal of Diaphorina citri (Hemiptera: Psyllidae) by immunomarking and potential impact of unmanaged groves on commercial citrus management. Environ. Entomol. 2009, 38, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Tomaseto, A.F.; Krugner, R.; Lopes, J.R.S. Effect of plant barriers and citrus leaf age on dispersal of Diaphorina citri (Hemiptera: Liviidae). J. Appl. Entomol. 2016, 140, 91–102. [Google Scholar] [CrossRef]
- Tomaseto, A.F.; Miranda, M.P.; Moral, R.A.; Lara, I.A.R.; Fereres, A.; Lopes, J.R.S. Environmental conditions for Diaphorina citri Kuwayama (Hemiptera: Liviidae) take-off. J. Appl. Entomol. 2016, 142, 104–113. [Google Scholar] [CrossRef]
- Cifuentes-Arenas, J.C.; Goes, A.; Miranda, M.P.; Beattie, G.A.C.; Lopes, S.A. Citrus flush ontogeny modulates biotic potential of Diaphorina citri. PLoS ONE 2018, 13, e0190563. [Google Scholar] [CrossRef]
- Inoue, H.; Ohnishi, J.; Ito, T.; Tomimura, K.; Miyata, S.; Iwanami, T.; Ashihara, W. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann. Appl. Biol. 2009, 155, 29–36. [Google Scholar] [CrossRef]
- Lee, J.A.; Halbert, S.A.; Dawson, W.O.; Robertson, C.J.; Keesling, J.E.; Singer, B.H. Asymptomatic spread of huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. USA 2015, 112, 7605–7610. [Google Scholar] [CrossRef] [Green Version]
- Li, W.B.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Manjunath, K.L.; Halbert, S.E.; Ramadugu, C.; Webb, S.; Lee, R.F. Detection of ‘Candidatus Liberibacter asiaticus’ in Diaphorina citri and its importance in the management of citrus huanglongbing in Florida. Phytopathology 2008, 98, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, E.-D.; Shatters, R.G.; Hall, D.G. Localization of Candidatus Liberibacter asiaticus, associated with citrus huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 2011, 159, 726–734. [Google Scholar] [CrossRef]
- Ammar, E.-D.; Achor, D.; Levy, A. Immuno-ultrastructural localization and putative multiplication sites of huanglongbing bacterium in Asian citrus psyllid Diaphorina citri. Insects 2019, 10, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suaste-Dzul, A.; Gallou, A.; Félix-Portillo, M.; Moreno-Carrillo, G.; Sánchez-González, J.; Palomares-Pérez, M.; Arredondo-Bernal, H. Seasonal incidence of ‘Candidatus Liberibacter asiaticus’ (Rhizobiales: Rhizobiaceae) in Diaphorina citri (Hemiptera: Liviidae) in Colima, Mexico. Trop. Plant Pathol. 2017, 42, 410–415. [Google Scholar] [CrossRef]
- Hall, D.G. Incidence of “Candidatus Liberibacter asiaticus” in a Florida population of Asian citrus psyllid. J. Appl. Entomol. 2018, 142, 97–103. [Google Scholar] [CrossRef]
- Fundo de Defesa da Citricultura. Alerta Fitossanitário. Available online: https://www.fundecitrus.com.br/alerta-fitossanitario (accessed on 15 August 2020).
- Carmo-Souza, M.; Garcia, R.B.; Wulff, N.A.; Fereres, A.; Miranda, M.P. Drench application of systemic insecticides disrupts probing behavior of Diaphorina citri (Hemiptera: Liviidae) and inoculation of Candidatus Liberibacter asiaticus. Insects 2020, 11, 314. [Google Scholar] [CrossRef]
- Lopes, S.A.; Luiz, F.Q.B.F.; Martins, E.C.; Fassini, C.G.; Sousa, M.C.; Barbosa, J.C.; Beattie, G.A.C. ‘Candidatus Liberibacter asiaticus’ titers in citrus and acquisition rates by Diaphorina citri are decreased by higher temperature. Plant Dis. 2013, 97, 1563–1570. [Google Scholar] [CrossRef] [Green Version]
- Sprinthall, R.C. Basic Statistical Analysis, 9th ed.; Pearson Education: London, UK, 2011; 672p. [Google Scholar]
- Chatfield. The Analysis of Time Series: An Introduction (Chapman &); Chapman & Hall: New York, NY, USA, 2004. [Google Scholar]
- Sala, I.; Martins, E.C.; Coletti, D.A.B.; Montesino, L.H.; Bassanezi, R.B.; Wulff, N.A.; Teixeira, D.C. Detection of Candidatus Liberibacter asiaticus in Diaphorina citri caught on yellow sticky traps during winter and summer of São Paulo State, Brazil. In Proceedings of the Third International Research Conference on Huanglongbing, Orlando, FL, USA, 4–8th February 2013; UC Riverside: Riverside, CA, USA, 2014; Volume 1, p. 108. [Google Scholar]
- Canale, M.C.; Tomaseto, A.F.; Haddad, M.L.; Coletta-Filho, H.D.; Lopes, J.R.S. Latency and persistence of ´Candidatus Liberibacter asiaticus´ in its psyllid vector, Diaphorina citri (Hemiptera: Liviidae). Phytopathology 2017, 107, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Ukuda-Hosokawa, R.; Sodayama, Y.; Kishaba, M.; Kuriwada, T.; Anbutsu, H.; Fukatsu, T. Infection density dynamics of the citrus greening bacterium “Candidatus Liberibacter asiaticus” in field populations of the psyllid Diaphorina citri and its relevance to the efficiency of pathogen transmission to citrus plants. Appl. Environ. Microbiol. 2015, 81, 3728–3736. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Zhou, L.; Hall, D.G.; Li, W.; Doddapaneni, H.V.; Lin, H.; Liu, L.; Vahling, C.M.; Gabriel, D.W.; Williams, K.P.; et al. Complete genome sequence of citrus huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Mol. Plant-Microbe Interact. 2009, 22, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, V.; Maheshwari, Y.; Hajeri, S.; Chen, J.; McCollum, T.G.; Yokomi, R. Development of a duplex droplet digital PCR assay for absolute quantitative detection of ‘Candidatus Liberibacter asiaticus’. PLoS ONE 2018, 13, e0197184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.V.; Machado, E.C.; Brunini, E. Ocorrência de condições ambientais para a indução do florescimento de laranjeiras no estado de São Paulo. Rev. Bras. Frutic. 2006, 28, 247–253. [Google Scholar] [CrossRef]
- Yamamoto, P.T.; Paiva, P.E.B.; Gravena, S. Flutuação populacional de Diaphorina citri Kuwayama (Hemiptera: Psyllidae) em pomares de citros na região norte do estado de São Paulo. Neotropical Entomol. 2001, 30, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Lopes, S.A.; Bertolini, E.; Frare, G.F.; Martins, E.C.; Wulff, N.A.; Teixeira, D.C.; Fernandes, N.G.; Cambra, M. Graft transmission efficiencies and multiplication of ‘Candidatus Liberibacter americanus’ and ‘Candidatus Liberibacter asiaticus’ in citrus plants. Phytopathology 2009, 99, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, S.A.; Luiz, F.Q.B.F.; Oliveira, H.T.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L. Seasonal variation of ‘Candidatus Liberibacter asiaticus’ titers in new shoots of citrus in distinct environments. Plant Dis. 2017, 101, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi, M.F.; Keremane, M.L.; Ramadugu, C.; Roose, M.; Khan, I.A.; Lee, R.F. Detection of citrus huanglongbing-associated ‘Candidatus Liberibacter asiaticus’ in citrus and Diaphorina citri in Pakistan, seasonal variability, and implications for disease management. Phytopathology 2014, 104, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.V.; Belasque, J., Jr.; Yamamoto, P.T.; Paiva, P.E.B. Influência do tipo de controle de Huanglongbing em áreas citrícolas na dispersão de Diaphorina citri e na disseminação da doença para pomares próximos. In Proceedings of the Anais do III Simpósio MasterCitrus, Araraquara, Brazil, 4 September 2020; Bassanezi, R.B., Behlau, F., Silva, G.J., Jr., Miranda, M.P., Eds.; Fundecitrus: Araraquara, Brazil, 2015; Volume 1, p. 19. Available online: https://www.fundecitrus.com.br/pdf/simposio/III_Simposio_MasterCitrus_WEB.pdf (accessed on 15 August 2020).
- Sétamou, M.; Bartels, D.W. Living on the edges: Spatial niche occupation of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in citrus groves. PLoS ONE 2015, 10, e0131917. [Google Scholar] [CrossRef] [Green Version]



| Region (Name) a | Sampling Period | Traps as Source of Psyllids b | Psyllids Analyzed/Sampled c | Psyllids with Las (%) d |
|---|---|---|---|---|
| Southwestern (Avaré) | February 2014 to March 2017 | Fundecitrus | 1910/2025 | 1425 (74.6) |
| Southwestern (Santa Cruz do Rio Pardo) | February 2014 to February 2016 | Fundecitrus | 1940/2000 | 1438 (74.1) |
| Central (Araraquara) | February 2014 to March 2017 | Fundecitrus | 3117/3210 | 2092 (67.1) |
| Northern (Bebedouro) | February 2014 to February 2016 | Fundecitrus | 1427/1459 | 796 (55.8) |
| Northern (Frutal) | March 2016 to March 2017 | Fundecitrus | 749/766 | 247 (33.0) |
| Southwestern (Iaras) | March 2016 to March 2017 | Grower | 324/338 | 213 (65.7) |
| Central (Gavião Peixoto) | April 2016 to March 2017 | Grower | 406/414 | 238 (58.6) |
| Total (average %) | 9873/10,212 | 6449 (65.3) |
| Ct Range (HLBaspr) Range (Titer) a | Southwestern (Avaré) b | Southwestern (S. C. R Pardo) | Central (Araraquara) | Northern (Bebedouro) | Northern (Frutal) | Southwestern (Iaras) | Central (Gavião Peixoto) | Overall |
|---|---|---|---|---|---|---|---|---|
| <15.6 (>6.1) | 22 (1.5%) | 20 (1.4%) | 14 (0.7%) | 5 (0.6%) | 2 (0.8%) | - | - | 63 (1.0%) |
| 15.7 to 19.1 (5.1 to 6.0) | 109 (7.6%) | 157 (10.9%) | 148 (7.1%) | 64 (8.0%) | 10 (4.0%) | 2 (0.9%) | - | 490 (6.4%) |
| 19.2 to 22.6 (4.1 to 5.0) | 202 (14.2%) | 207 (14.4%) | 307 (14.7%) | 103 (12.9%) | 33 (13.4%) | 17 (8.0%) | 31 (13.0%) | 900 (12.9%) |
| 22.7 to 26.0 (3.1 to 4.0) | 227 (15.9%) | 261 (18.2%) | 384 (18.4%) | 116 (14.6%) | 54 (21.9%) | 50 (23.5%) | 59 (24.8%) | 1151 (19.6%) |
| 26.1 to 29.4 (2.1 to 3.0) | 286 (20.1%) | 301 (20.9%) | 405 (19.4%) | 132 (16.6%) | 34 (13.8%) | 42 (19.7%) | 49 (20.6%) | 1249 (18.7%) |
| 29.5 to 32.9 (1.1 to 2.0) | 341 (23.9%) | 303 (21.1%) | 469 (22.4%) | 192 (24.1%) | 53 (21.5%) | 62 (29.1%) | 52 (21.8%) | 1472 (23.4%) |
| 33.0 to 35.0 (0.4 to 1.0) | 238 (16.7%) | 189 (13.1%) | 365 (17.4%) | 184 (23.1%) | 61 (24.7%) | 40 (18.8%) | 47 (19.7%) | 1124 (19.1%) |
| Sum | 1425 | 1438 | 2092 | 796 | 247 | 213 | 238 | 6449 |
| Period/Region | Southwestern (Avaré) a | Southwestern (Santa Cruz do Rio Pardo) | Central (Araraquara) | Northern (Bebedouro) | ||||
|---|---|---|---|---|---|---|---|---|
| Prop. Las+ | p-Value | Prop. Las+ | p-Value | Prop. Las+ | p-Value | Prop. Las+ | p-Value | |
| 15 January to 15 June | 0.79a | 0.2858 | 0.79a | 0.0041 | 0.67a | 0.0003 | 0.56a | 0.6192 |
| 15 July to 15 December | 0.75a | 0.71b | 0.56b | 0.54a | ||||
| 16 January to 16 June | 0.82a | 0.7674 | - | - | 0.70a | 0.3169 | - | - |
| 16 July to 16 December | 0.83a | - | 0.73a | - | ||||
| July to December/2014 b | ||||
| Southwestern-Avaré (0.66) | Southwestern-Santa Cruz (0.74) | Central-Araraquara (0.68) | Northern-Bebedouro (0.48) | |
| Southwestern-Avaré (0.66) | - | |||
| Southwestern-Santa Cruz (0.74) | 0.0066 | - | ||
| Central-Araraquara (0.68) | 0.5056 | 0.0294 | - | |
| Northern-Bebedouro (0.48) | 0.0001 | 0.0001 | 0.0001 | - |
| January to June/2015 | ||||
| Southwestern-Avaré (0.79) | Southwestern-Santa Cruz (0.79) | Central-Araraquara (0.67) | Northern-Bebedouro (0.56) | |
| Southwestern-Avaré (0.79) | - | |||
| Southwestern-Santa Cruz (0.79) | 1 | - | ||
| Central-Araraquara (0.67) | 0.0007 | 0.0001 | - | |
| Northern-Bebedouro (0.56) | 0.0001 | 0.0001 | 0.0020 | - |
| July to December/2015 | ||||
| Southwestern-Avaré (0.75) | Southwestern-Santa Cruz (0.71) | Central-Araraquara (0.56) | Northern-Bebedouro (0.54) | |
| Southwestern-Avaré (0.75) | - | |||
| Southwestern-Santa Cruz (0.71) | 0.2535 | - | ||
| Central-Araraquara (0.56) | 0.0001 | 0.0001 | - | |
| Northern-Bebedouro (0.54) | 0.0001 | 0.0001 | 0.5715 | - |
| January to June/2016 c | ||||
| Southwestern-Avaré (0.82) | Central-Araraquara (0.70) | |||
| Southwestern-Avaré (0.82) | - | |||
| Central-Araraquara (0.70) | 0.0034 | - | ||
| July to December/2016 c | ||||
| Southwestern-Avaré (0.83) | Central-Araraquara (0.73) | |||
| Southwestern-Avaré (0.83) | - | |||
| Central-Araraquara (0.73) | 0.0001 | - | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulff, N.A.; Daniel, B.; Sassi, R.S.; Moreira, A.S.; Bassanezi, R.B.; Sala, I.; Coletti, D.A.B.; Rodrigues, J.C. Incidence of Diaphorina citri Carrying Candidatus Liberibacter asiaticus in Brazil’s Citrus Belt. Insects 2020, 11, 672. https://doi.org/10.3390/insects11100672
Wulff NA, Daniel B, Sassi RS, Moreira AS, Bassanezi RB, Sala I, Coletti DAB, Rodrigues JC. Incidence of Diaphorina citri Carrying Candidatus Liberibacter asiaticus in Brazil’s Citrus Belt. Insects. 2020; 11(10):672. https://doi.org/10.3390/insects11100672
Chicago/Turabian StyleWulff, Nelson A., Bruno Daniel, Rodrigo S. Sassi, Alécio S. Moreira, Renato B. Bassanezi, Ivaldo Sala, Daniela A. B. Coletti, and Júlio C. Rodrigues. 2020. "Incidence of Diaphorina citri Carrying Candidatus Liberibacter asiaticus in Brazil’s Citrus Belt" Insects 11, no. 10: 672. https://doi.org/10.3390/insects11100672





