Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Experimental Design
2.3. Data Analysis
3. Results
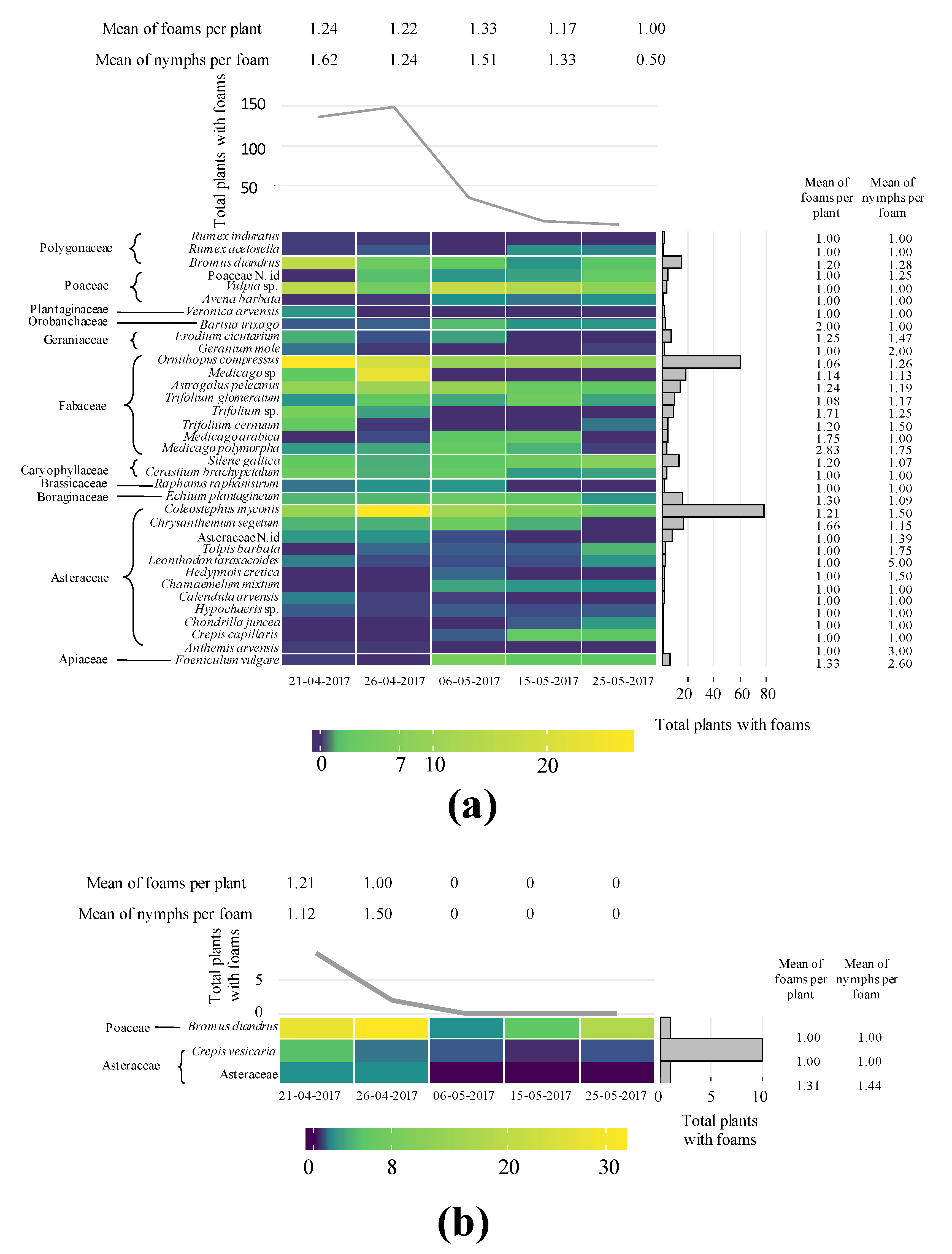
3.1. Plant Preference by Aphrophoridae Nymphs
3.2. Distribution of Foams Along Plant Stems
3.3. Dynamics of Nymphal Instars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almeida, R.P.P. Can Apulia’s olive trees be saved? Science 2016, 353, 346–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Serio, F.D.; Bodino, N.; Cavalieri, V.; Demichelis, S.; Di Carolo, M.; Dongiovanni, C.; Fumarola, G.; Gilioli, G.; Guerrieri, E.; Picciotti, U.; et al. Collection of data and information on biology and control of vectors of Xylella fastidiosa. EFSA Supporting Publ. 2019, 16. [Google Scholar] [CrossRef]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P.P. Xylella fastidiosa: Insights into an emerging plant pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef] [Green Version]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, V.; Dongiovanni, C.; Tauro, D.; Altamura, G.; Di Carolo, M.; Fumarola, G.; Saponari, M.; Bosco, D. Transmission of the CoDiRO strain of Xylella fastidiosa by different insect species. In Proceedings of the XI European Congress of Entomology (ECE 2018), Naples, Italy, 2–6 July 2018; pp. 144–145. [Google Scholar]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by—Naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- Martelli, G.P.; Boscia, D.; Porcelli, F.; Saponari, M. The olive quick decline syndrome in south-east Italy: A threatening phytosanitary emergency. Eur. J. Plant Pathol. 2016, 144, 235–243. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, D.; Di Carolo, M.; Fumarola, G.; Ltamura, G.; Lasorella, C.; Bosco, D. Plant selection and population trend of spittlebug immatures (Hemiptera: Aphrophoridae) in olive groves of the Apulia region of Italy. J. Econ. Entomol. 2019, 112, 67–74. [Google Scholar] [CrossRef] [Green Version]
- EPPO. PM 7/141 (1) Philaenus spumarius, Philaenus italosignus and Neophilaenus campestris. Bull. OEPP/EPPO Bull. 2019, 50, 1–9. [Google Scholar]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance 1 become a new threat to European agriculture. J. Pest. Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and relative abundance of insect vectors of Xylella fastidiosa in Olive Groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauru, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean olive groves: Host-plant exploitation throughout the year. Insects 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, C.R.; King, D.R. Meadow Spittlebug, Philaenus leucophthalmus (L.); Research Bulletin; Ohio Agricultural Experiment Station: Wooster, OH, USA, 1954. [Google Scholar]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Simonetto, A.; Fumarola, G.; Di Carolo, M.; Porcelli, F.; et al. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 17725. [Google Scholar] [CrossRef] [PubMed]
- Nickel, H. The Leafhoppers and Planthoppers of Germany (Hemiptera Auchenorrhyncha): Patterns and Strategies in A Highly Diverse Group of Phytophagous Insects; Pensoft Publishers: Sofia, Bulgaria; Moscow Goecke & Evers: Keltern, Germany, 2003; 460p. [Google Scholar]
- Chen, X.; Meyer-Rochow, B.; Fereres, A.; Morente, M.; Liang, A.-P. The role of biofoam in shielding spittlebug nymphs (Insecta, Hemiptera, Cercopidae) against bright Light. Ecol. Entomol. 2018, 43, 273–281. [Google Scholar] [CrossRef]
- Whittaker, J.B. Cercopid spittle as a microhabitat. Oikos 1970, 21, 59–64. [Google Scholar] [CrossRef]
- Dader, B.; Viñuela, E.; Moreno, A.; Plaza, M.; Garzo, E.; del Estal, P.; Fereres, A. Sulfoxaflor and natural pyrethrin with piperonyl butoxide are efective alternatives to neonicotinoids against juveniles of Philaenus spumarius, the european vector of Xylella fastidiosa. Insects 2019, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Cornara, D.; Saponari, M.; Zeilinger, A.; De Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.P.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Antonatos, S.; Papachristos, D.P.; Kapantaidaki, D.E.; Lytra, I.C.; Varikou, K.; Evangelou, V.I.; Panagiotis, M. Presence of Cicadomorpha in olive orchards of Greece with special reference to Xylella fastidiosa vectors. J. Appl. Entomol. 2020, 144, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Halkka, O.; Raatikainen, M.; Vasarainen, A.; Heinonen, L. Ecology and ecological genetics of Philaenus spumarius (L.) (Homoptera). Ann. Zool. Fenn. 1967, 4, 1–18. [Google Scholar]
- Morente, M.; Fereres, A. Vectores de Xylella fastidiosa. In Enfermedades causadas por la bactéria Xylella Fastidiosa; Cajamar Caja Rural: Almería, Spain, 2017; pp. 73–93. [Google Scholar]
- Goidanich, A. Fileno. In Enciclopedia Agraria Italiana; REDA: Roma, Italy, 1957; Volume I, pp. 185–186. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica Part 2: Cicadidae, Cercopidae, Membracidae, Cicadellidae; Brill: Leiden, The Netherlands, 1981. [Google Scholar]
- Giustina, W.D. La faune de France des Cercopinae (Ho. Cicadomorpha). Bull. Soc. Entomol. Fr. 1983, 88, 192–196. [Google Scholar]
- Barro, P.; Pavan, F. Life cycle and host plants of Lepyronia coleoptrata (L.) (Auchenorrhyncha Cercopidae) in northern Italy. Redia 2000, 82, 145–154. [Google Scholar]
- Markheiser, A.; Cornara, D.; Fereres, A.; Maixner, M. Analysis of vector behavior as a tool to Xylella fastidiosa patterns of spread. Entomol. Gen. 2020, 40, 1–13. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; ·Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.; Santos, S.A.P.; Marrão, R.; Pinheiro, L.A.; López-Saez, J.A.; Mexia, A.; Bento, A.; Pereira, J.A. Syrphids feed on multiple patches in heterogeneous agricultural landscapes during the autumn season, a period of food scarcity. Agric. Ecosyst. Environ. 2016, 233, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.; Somavilla, I.; Santos, S.A.P.; López-Sáez, J.A.; Pereira, J.A. Pollen feeding habits of Chrysoperla carnea s.l. adults in the olive grove agroecosystem. Agric. Ecosyst. Environ. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sastre, B.; Marques, M.J.; García-Díaz, A.; Bienes, R. Three years of management with cover crops protecting sloping olive groves soils, carbon and water effects on gypsiferous soil. Catena 2018, 171, 115–124. [Google Scholar] [CrossRef]
- Guzmán, G.; Cabezas, J.M.; Sánchez-Cuesta, R.; Lora, A.; Bauer, T.; Strauss, P.; Winter, S.; Zaller, J.G.; Gómez, J.A. A field evaluation of the impact of temporary cover crops on soil properties and vegetation communities in southern Spain vineyards. Agric. Ecosyst. Environ. 2019, 272, 135–145. [Google Scholar]
- Godefroid, M.; Cruaud, A.; Streito, J.-C.; Rasplus, J.-Y.; Rossi, J.-P. Xylella fastidiosa: Climate suitability of European continent. Sci. Rep. UK 2019, 9, 8844. [Google Scholar] [CrossRef] [Green Version]
- Castroviejo, S. Flora ibérica, Real Jardín Botánico. CSIC Madrid 2005, 2, 8. [Google Scholar]
- Aizpuru, I.; Aseguinolaza, C.; Uribe-Echebarría, P.M.; Urrutia, P.; Zorrakín, I. Claves Ilustradas de la Flora de País Vasco y Territorios Limítrofe; Servicio Central de Publicaciones del Gobierno Vasco: Vitoria-Gasteiz, Spain, 2007; p. 832. [Google Scholar]
- Vilbaste, J. Preliminary key for the identification of the nymphs of North European Homoptera Cicadinea. II. Cicadelloidea. Ann. Zool. Fennici. 1982, 19, 1–20. [Google Scholar]
- Bates, B.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 23 September 2020).
- Patil, I. ggstatsplot: ‘ggplot2’ Based Plots with Statistical Details. CRAN. 2018. Available online: https://cran.r-project.org/web/packages/ggstatsplot/index.html (accessed on 23 September 2020).
- Purcell, A.H. Almond Leaf Scorch: Leafhopper and spittlebug vectors. Entomol. Soc. Am. 1980, 73, 834–838. [Google Scholar] [CrossRef]
- Wise, M.J.; Kieffer, D.L.; Abrahamson, W.G. Costs and benefits of gregarious feeding in the meadow spittlebug, Philaenus spumarius. Ecol. Entomol. 2006, 31, 548–555. [Google Scholar] [CrossRef]
- Bierdermann, R. Aggregation and survival of Neophilaenus albipennis (Hemiptera: Cernopidae) spittlebug nymphs. Eur. J. Entomol. 2003, 100, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.F.; Lambdin, P.L.; Follum, R.A. Infestation levels and seasonal incidence of the meadow spittlebug (Homoptera: Cercopidae) on musk thistle in Tennessee. J. Agric. Entomol. 1998, 15, 83–91. [Google Scholar]
- McEvoy, P.B. Niche partitioning in spittlebugs (Homoptera: Cercopidae) sharing shelters on host plants. Ecology 1986, 67, 465–478. [Google Scholar] [CrossRef]
- Hoffman, G.D.; McEvoy, P.B. Mechanical limitations on feeding by meadow spittlebugs Philaenus spumarius (Homoptera: Cercopidae) on wild and cultivated host plants. Ecol. Entomol. 1985, 10, 415–426. [Google Scholar] [CrossRef]
- European Commission. Available online: https://ec.europa.eu/info/food-farming-fisheries/key-policies/common-agricultural-policy/income-support/greening_en (accessed on 17 September 2019).
- Harper, G.; Whittaker, J.B. The role of natural enemies in the colour polymorphism of Philaenus spumarius (L.). J. Anim. Ecol. 1976, 45, 91–104. [Google Scholar] [CrossRef]
- Huber, J.T. Revision of Ooctonus (Hymenoptera: Mymaridae) in the neartic region. J. Ent. Soc. Ont. 2012, 143, 15–105. [Google Scholar]
- Mesmin, X.; Chartois, M.; Genson, G.; Rossi, J.P.; Cruaud, A.; Rasplus, J.Y. Ooctonus vulgatus (Hymenoptera, Mymaridae), a potential biocontrol agent to reduce populations of Philaenus spumarius (Hemiptera, Aphrophoridae) the main vector of Xylella fastidiosa in Europe. PeerJPreprints. 2019. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.; Villa, M.; Rodrigues, I.; Camerirão, C.; Baptista, P.; Pereira, J.A. Potential natural biocontrol agents of Aphrophoridae eggs. European Research on Emerging Plant Diseases. In Proceedings of the 2nd Joint Annual Meeting of the Contributions of the H2020 projects PonTE and XF-ACTORS, Valencia, Spain, 23–26 October 2018; p. 80. [Google Scholar]
- Hartley, S.E.; Garner, S.M. The response of Philaenus spumarius (Homoptera: Cercopidae) to fertilizing and shading its moorland host-plant (Calluna vulgaris). Ecol. Entomol. 1995, 20, 396–399. [Google Scholar] [CrossRef]
- Thompson, V. Spittlebug indicators of nitrogen-fixing plants. Ecol. Entomol. 1994, 19, 391–398. [Google Scholar] [CrossRef]
- Ranieri, E.; Ruschioni, S.; Riolo, P.; Isidoro, N.; Roman, R. Fine structure of antennal sensilla of the spittlebug Philaenus spumarius L. (Insecta: Hemiptera: Aphrophoridae). I. Chemoreceptors and thermo-/hygroreceptors. Arthropod Struct. Devel. 2016, 45, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLoS ONE 2017, 12, e0190454. [Google Scholar] [CrossRef] [Green Version]
- Cornara, D.; Ripamonti, M.; Morente, M.; Garzo, E.; Bosco, D.; Moreno, A.; Fereres, A. Artificial diet delivery system for Philaenus spumarius, the European vector of Xylella fastidiosa. J. Appl. Entomol. 2019, 143, 882–892. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, M.; Rodrigues, I.; Baptista, P.; Fereres, A.; Pereira, J.A. Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal. Insects 2020, 11, 720. https://doi.org/10.3390/insects11100720
Villa M, Rodrigues I, Baptista P, Fereres A, Pereira JA. Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal. Insects. 2020; 11(10):720. https://doi.org/10.3390/insects11100720
Chicago/Turabian StyleVilla, María, Isabel Rodrigues, Paula Baptista, Alberto Fereres, and José Alberto Pereira. 2020. "Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal" Insects 11, no. 10: 720. https://doi.org/10.3390/insects11100720
APA StyleVilla, M., Rodrigues, I., Baptista, P., Fereres, A., & Pereira, J. A. (2020). Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal. Insects, 11(10), 720. https://doi.org/10.3390/insects11100720








