1. Introduction
Malaria is the leading cause of morbidity and mortality in sub-Saharan Africa with 212 million cases annually estimated by the WHO and about 429,000 deaths each year [
1]. Malaria prevention is mainly based on vector (mosquito) control, using insecticide indoor residual spraying (IRS) or long-lasting insecticide-impregnated mosquito bednets (LLINs) [
2]. The efficacy of these measures depends primarily on the susceptibility of the mosquito to insecticides. An estimated 663 million cases of malaria have been averted in sub-Saharan Africa since 2001 as a result of the scale-up of malaria control interventions; 69% of these reduced cases were a direct result of the use of LLINs [
3]. Unfortunately, mosquitoes have, in recent years, become resistant to insecticides including the pyrethroids, the only chemistry approved for bednets [
4,
5,
6]. This resistance is threatening the efficacy of chemical-based, vector control [
7,
8,
9]. There is an urgent need to develop new strategies to mitigate this problem.
Development of a new insecticide for bednets that is safe to human exposure every night for years, that must survive human handling each day and periodic washing, and that will not promote mosquito resistance and cross-resistance, is a challenge [
10]. One solution is the reformulation of agricultural chemicals with completely different modes of action to that of the pyrethroids. This is a challenge since adult mosquitoes have been exposed to these insecticides applied to crop plants and as larvae developing in agricultural runoff; it has been hypothesized that mosquito resistance to bednets is a result of pesticide exposure from agricultural systems more than actual bednet use [
11].
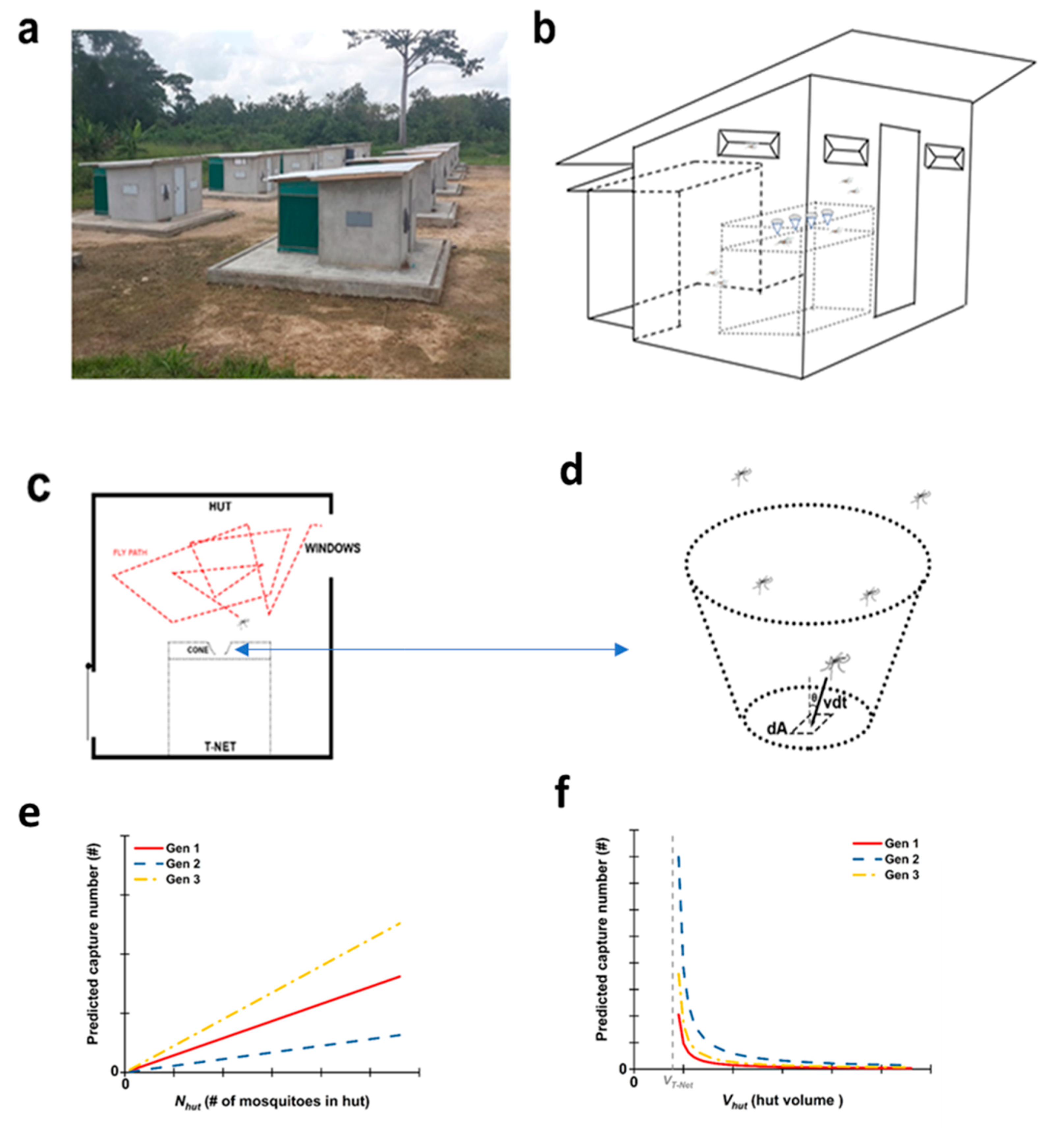
These challenges have led us to a different approach for mosquito control with bednets, a mechanical trapping and killing device. It is well known that mosquitoes are attracted to carbon dioxide gas and other human odors that are emitted from a warm body sleeping under the net, and these attractants rise through the top of the bednet [
12]. Infrared video tracking has shown that about 75% of mosquitoes follow this odorant plume and are attracted to the top surface of the bednet [
13,
14]. We have taken advantage of this natural behavior to develop a mosquito trapping bednet, the T-Net, designed to trap and kill susceptible and resistant mosquitoes. The non-insecticidal T-Net has (i) a lower sleeping compartment and (ii) an upper mosquito trap compartment with funnels on the trap roof as mosquito entry points into the trap.
To optimize trapping performance, we hypothesized that mosquito movement is random outside of the trap compartment in the absence of attractants and visual cues. If we then consider mosquitoes as ideal gas particles, we can use the Maxwell–Boltzmann distribution [
15] to develop a mathematical model to optimize the number, size, and positioning of funnels to maximize trapping efficacy that would also apply when someone is sleeping under the net. Different T-Nets derived from the model were assayed in walk-in simulated WHO huts using human subjects [
16] and once proof of principle was demonstrated, further validated with wild-type, insecticide-resistant
Anopheles gambiae mosquitoes under field conditions in Tiassale, Côte d’Ivoire, Africa. From the field data, we developed a second model to predict community-level mosquito control by the T-Net versus the most common bednet deployed in Africa.
2. Materials and Methods
2.1. T-Net Construction
The non-insecticidal T-Net has (i) a lower sleeping compartment and (ii) an upper mosquito trap compartment with funnels on the trap roof (with the small opening of the cone(s) pointing down into the trap compartment). The funnels serve as mosquito entry points into the trap. The sleeping compartment of the T-Net was a standard (single-sleeper), non-insecticidal bednet (Huzhou Shuanglu Knitting Mill Co., Ltd., Jinhua, Zhejiang, China). The trap compartment was constructed from a second non-insecticidal bednet that was sewn on the original bednet roof (open end down) but where the height of the sleeping compartment (which is now the trap compartment) is reduced. Then knitted cones described later were sewn into holes made in the roof of the trap compartment. The detailed architecture of the T-Net and different iterations used in our research are discussed later. Once mosquitoes enter into the trap compartment through the funnels, they are blocked from reaching the sleeper by the roof of the first bednet.
2.2. Cone Knitting
T-Net prototyping was conducted using different knitted cones sewn into the roof of the trap compartment. Knitted cones can easily be constructed in any shape and size, are easily sewn into our T-Net, and can be folded into a flat, compact package for storing and shipping of bednets. Cones were knitted on a flat, computerized knitting machine, SWGN2 (Shima Seiki, Sakata Wakayama, Japan), where their dimensions for prototyping are easily changed by standard program options. Details on cone sizes are discussed later. The gauge of knitting machine was selected so that the resulting cone fabric would have openings between loops similar to those of traditional bednets. Like the construction of stockings or socks, cones were knitted in-the-round to make a 3D shape. Use of this construction method allowed us to alter the shape, depth, width, and height in all sections of the cone to meet our design demands. The knitted cones had three main parts—a large opening at the mouth (which was designed to allow for passage of mosquitoes into the cone), tapered cone walls (which were designed to direct the movement of mosquitoes into the trapping compartment), and a neck opening into the trapping compartment (the size of the neck was optimized to allow passage of mosquitoes and minimize egression from the trap). To prevent collapsing and flattening of the knitted cones, they were constructed with thermal bond fibers. This yarn is a polyester binder-spun yarn in which each fiber is composed of a sheath part (co-polyester) and a core (regular polyester). The bursting strength and durability of these cones described later were compared to conventional bednet material to make sure they would stand up to years of use.
2.3. Cone Bursting Strength and Durability Measurements
A burst test (James H. Heal TruBurst 2, ASTM D3786, James H. Heal & Co. Ltd., Halifax, UK) was used to measure the durability of the cones constructed versus bednet fabric; this is achieved by increasing the hydrostatic pressure across the textiles until they ruptured (
Figure S1). The pressure was applied to a circular region of the fabric via an elastic diaphragm. The fabric sample was firmly held around the circular edge by pneumatic clamping. When the pressure was applied, the fabric deformed together with the diaphragm. The bursting strength corresponded to the maximum pressure supported by the fabric before failure.
The abrasion test (
Figure S2) was used to assess the cone textile durability by friction compared to the bednet fabric. Abrasion resistance refers to the resistance of a fabric in the process of repeated friction with a defined, rough surface. To run the test, the fabric was loaded onto the lower plates of the abrasion tester (Maxi Martindale 1609, ASTM D4966, James H. Heal & Co. Ltd., Halifax, UK), and then abraded using oscillating circles. The assay end point was the duration and number of circles until the first hole appeared.
2.4. Tunnel Test for Assessment of Cone Efficiency
A modified WHO-tunnel test [
17] was conducted to assess the rate at which mosquitoes would move through knitted cones and their potential use as part of a trap mechanism. The tunnel test was modified to include three compartments—a top compartment which operated as a mosquito release chamber, a middle compartment which operated as a trapping container, and a lower compartment that contained the arm of a human subject to attract host-seeking mosquitoes. The top and middle compartments were separated by the knitted cone, and the middle and lower compartments were separated by a polyester net fabric to prevent the human subject from receiving mosquito bites (NCSU IRB approved protocol 16897).
The tunnel test was placed vertically to mimic the position of a cone on the top of the trap compartment, with the small opening of the cone pointing down. A human arm cleaned with distilled water and then air dried was inserted into the bottom compartment. Two replicates (the first one of 106 mosquitoes and the s using 50 depending on availability) of unfed five to eight-day-old, host seeking
An. gambiae Kisumu-strain female adult mosquitoes were released into the top compartment. Mosquitoes used were reared in the Dearstyne Laboratory at North Carolina State University (Raleigh, NC) according to MR4 rearing protocols [
18]. After 2 h from the time of release, the mosquitoes that remained in the top chamber were removed using a mouth aspirator and counted. The human arm was removed from the bottom compartment, and trapped mosquitoes were observed for an additional 2 h in the middle compartment. Mosquitoes moving from the middle to the top compartment was scored as egression.
2.5. Laboratory Walk-In Hut Trials
Laboratory walk-in hut trials were conducted in the Dearstyne Entomology Building. The rooms were 1.58 × 2.18 × 2.03 m (depth × width × height) and the tests conducted at 27 ± 1 °C and 65 ± 4% relative humidity. The T-Net was set up above a portable bed with the bottom of the sleeping compartment of the net on the floor of the room. Trapping was assessed with a human subject in the sleeping position under the net (NCSU IRB approved protocol 9067). Five- to eight-day old, unfed An. gambiae Kisumu-strain, adult female mosquitoes (described earlier) were used for these studies. For each test, the mosquitoes were released into the room but outside of the bednet. The number of mosquitoes released varied between 40 and 100 to determine if population density in the room had any effects on trap efficacy. The total number of replicates was six and these were conducted on different days. Trapping was conducted for 3 h during the photophase, since this mosquito strain has, for years, been fed with lights on. At the end of the experiment, mosquitoes were vacuum-collected from outside of the trap compartment (in the room) and counted immediately. To assess egression, trapped mosquitoes were not removed from the trap compartment. The number remaining in the trap were then counted after 30 min, 1 h, 2 h, 6 h, and overnight.
2.6. Field Trials in Africa
The study was conducted in experimental huts according to WHO protocols [
17] in the municipality of Tiassalé (5°53′54” N et 4°49′42” W) in November 2018. The site is located in the south of Côte d’Ivoire, about 110 km north of the country’s major city, Abidjan. The climate is tropical and characterized by four seasons—a long rainy season (March–July) during which two thirds of the annual rainfall occurs, a short dry season (July–August), a short rainy season (September–November), and a long dry season (December–March). The average annual rainfall is 1739 mm with an average annual temperature of 26.6 °C. The annual average relative humidity is around 70%. Rice production occurs in the lowlands of Tiassalé which facilitates the proliferation of mosquitoes throughout the year, and malaria is the leading cause of morbidity in the local population. The transmission of the disease is mainly due to
Anopheles coluzzii (80%) and
An. gambiae (20%) which have developed multiple resistances to insecticides [
19,
20].
The experimental field station of Tiassalé is made up of 18 standardized experimental huts (described in more detail later) situated close to the rice plantations. Each hut is 2.5 m long, 1.75 m wide, and 2 m high. The walls are made of concrete bricks and plastered with cement, while the floor is made of cement and the roof of corrugated iron sheets. A plastic cover is mounted underneath the roof as a ceiling to facilitate manual collection of mosquitoes. Each hut is built on a platform made of concrete surrounded by a water-filled moat that prevents entry of foraging ants. Entry of mosquitoes is facilitated through four window slits located on three sides of the hut. The slits are designed in such a way as to prevent mosquitoes from escaping once they are inside the hut. Each hut is equipped with a veranda trap located on the fourth side, made of sheeting and screening to capture mosquitoes that would otherwise escape.
Four sleepers were trained and paid to sleep under the bednets. Each day, both sleepers and bednets were randomly positioned between four different huts. Trapping was conducted from 21:00 to 5:00, and the mosquitoes collected each morning after the sleep cycle was completed. Sleepers and bednets were rotated on a daily basis according to two Latin-square tables (
Figure S3). The trial was conducted for 14 d. Each day, after mosquito collections, the huts were cleaned and the bednets rotated appropriately. Prior to the trial, sleepers were all vaccinated against yellow fever and were taken to the hospital to check for malaria parasites. All sleepers gave informed consent prior to enrolment in the study (CSRS IRB protocol #02-2011/MSLS/CNER-P). Within the hut, resting and dead non-trapped mosquitoes were collected from the room and in the veranda trap using 5 mL (12 mm × 75 mm) glass hemolysis tubes. Trapped mosquitoes were removed with mouth aspirators from the T-Net trap compartment by the field technicians. All mosquitoes were taken to the field laboratory and identified to genus level and scored as trapped-dead, trapped-alive, not trapped-dead, or not trapped-alive. Live mosquitoes were placed in cups and given access to a sugar solution for 24 h in the insectary at 25–27 °C and 70–80% relative humidity to assess delayed mortality.
Three different insecticide-free T-Nets (described in detail later) were evaluated in comparison to the WHO-recommended and widely-used Permanet 2.0-LLIN (PN2.0), a positive control. The PN2.0 was brand new and provided by the National Malaria Control program in Cote d’Ivoire.
The Kruskal–Wallis non-parametric test with the Conover–Iman multiple pairwise comparisons and the Bonferroni correction were used to analyze the data (alpha = 0.05). The analyses were conducted using the XLSTAT software package version 2019.4.1. [
21] The overall blood-feeding rate was less than 3%.
4. Discussion
Malaria is a devastating disease, and vector control is a crucial factor in saving lives. However, the situation has become critical because mosquitoes have become resistant to core intervention tools such as LLINs and IRS. Tackling insecticide resistance by developing new approaches for vector control is imperative to controlling malaria, and we need to think “out of the box”.
The current model-driven insecticide-free trapping bednet is a paradigm shift in malaria vector control and could be a simple solution to the problem. Its functionality is based on the attraction and trapping of mosquitoes regardless of their insecticide-resistance status. This proof-of-concept study describes, for the first time, the efficacy of a trapping bednet as a malaria vector control strategy, that addresses the problem of insecticide resistance without the need to develop new chemistry. The advantage of the model developed is that we can predict the mosquito trapping rate before going into the field for testing; this facilitates additional innovation in the future.
As commonly found in experimental hut trials in which non-insecticide and insecticide-treated products are compared [
22,
23], we found higher mosquito entry rates in huts with T-Nets (free of insecticides) than that observed with the PN2.0-LLIN. Similarly, the exit rate was higher for the PN2.0-LLIN than that of the T-Nets. This is likely due to the excito-repellency property of the insecticide in the nets. The excito-repellency effect can be perceived as beneficial to the extent that it keeps some mosquitoes away from the sleeper, and thus provides personal protection to the net user. However, at the community level, the benefit of the excito-repellency effect can be nullified or even contribute to behavioral resistance and increase outdoor malaria transmission as mosquitoes are repelled outdoors and are not killed [
24,
25]. Direct observation of our field results showed a 2.7 to 4.3-fold increase mortality for the T-Nets compared to the PN 2.0-LLIN. However, due to mosquito deterrence and repellency for the insecticide-treated bednet, community protection was estimated by our new model to be 13-fold greater for the Gen3 T-Net compared to PN2.0. Thus, mass mosquito trapping from the long-term use of T-Nets could lead to a decline in the vector population where transmission is no longer stable. This population decrease is even more likely in the context of insecticide resistance where mosquito mortality is lower than expected by LLINs.
The T-Net exploits the natural behavior of mosquitoes, which principally interact with the roof of the mosquito net [
13,
14]. Therefore, the positioning of the traps on the top of the net promotes entrapment of mosquitoes. This is especially true for insecticide-free T-Nets which do not cause repellent effects. Untreated T-Nets can therefore be an asset for vector control as they trap and kill mosquitoes irrespective of their insecticide-resistance status. Nevertheless, the addition of a trap compartment to an existing LLIN can also enhance the killing performance of the net (data now shown). An insecticide-free bednet for malaria vector control is far from being adopted because of regulatory requirements that focus on the use of insecticides in bednets and the possible requirement for epidemiological data to prove the public health value. Although the idea of an insecticide-free bednet for malaria vector control was recently mentioned [
26], an interim solution could be a hybrid version of the T-Net consisting of an insecticide-free trap compartment mounted on currently-used LLINs. In this case, community protection would be provided both by the insecticidal effect and the mass trapping of mosquitoes.
Studies have shown that the escalation in resistance to insecticides closely matches the introduction of insecticide-treated nets. To combat resistance to insecticides, the trend today is to use LLINs combined with the synergist piperonyl-butoxide (PBO) [
27]. The PBO inhibits enzymatic activity of mosquito insecticide detoxification enzymes, and therefore enhances the insecticidal effect of the treated bednet. Unfortunately, decreases in performance of these mosquito nets are starting to be observed in some regions of Africa where mosquitoes are resistant [
22,
27]. Because the T-Net is insecticide-free, it does not exert any insecticide-resistance selection pressure on mosquitoes and is not impacted by insecticide resistance. The exclusive and prolonged use of the T-Net in areas that are endemic for insecticide resistance should eventually lead to a decrease in the level of resistance and thus facilitate the reintroduction of insecticides.
Though we have not analyzed the escape rate for trapped mosquitoes in the field, our lab assays suggest the egression rate will be low or zero. We also have found, in laboratory conditions, that trapping elicits a mosquito escape response that rapidly leads to exhaustion and death from dehydration and starvation. The dead mosquitoes in the trap compartment are not easily sighted by someone sleeping under the net, and trapped dead mosquitoes are easily removed by traditional washing methods in Africa. This finding suggests that removing mosquitoes from the T-Net trap compartment is not an issue. They could also be removed by net shaking when the funnels are pulled out. However, we believe that the view of mosquitoes in the trap compartment can be an asset to the extent that it could encourage the use of bednets.
Sampling of mosquito populations for transmission studies or evaluation of other vector-control tools such as IRS could also be conducted with insecticide-free T-Nets. Various anthropophagous mosquito sampling tools exist but are complex to build [
28,
29,
30]. If compared to these methods, the simple construction mode of the T-Net, which uses inexpensive materials, makes it an easy-to-assemble and a less expensive sampling tool. It would be interesting to consider a comparative study of its effectiveness as a sampling method vis-à-vis other methods.
We have not conducted in depth manufacturing cost studies, yet improvements for mass production at low cost are underway. For example, the cones on the roof of the T-Net can simply be mass produced by heat stamping. We anticipate a satisfactory cost range of USD 2–4 [
31] per T-Net, which is the current range for LLINs on the market. Furthermore, because there is no new chemistry to develop, the route to market should be rapid compared to an insecticide-treated bednet where new chemistry must be proven safe. It would also be useful to survey public opinion about the use of an insecticide-free bednet versus an LLIN where efficacies for both in a worst-case scenario are equal.