Deciphering Novel Antimicrobial Peptides from the Transcriptome of Papilio xuthus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Insect and Immunization
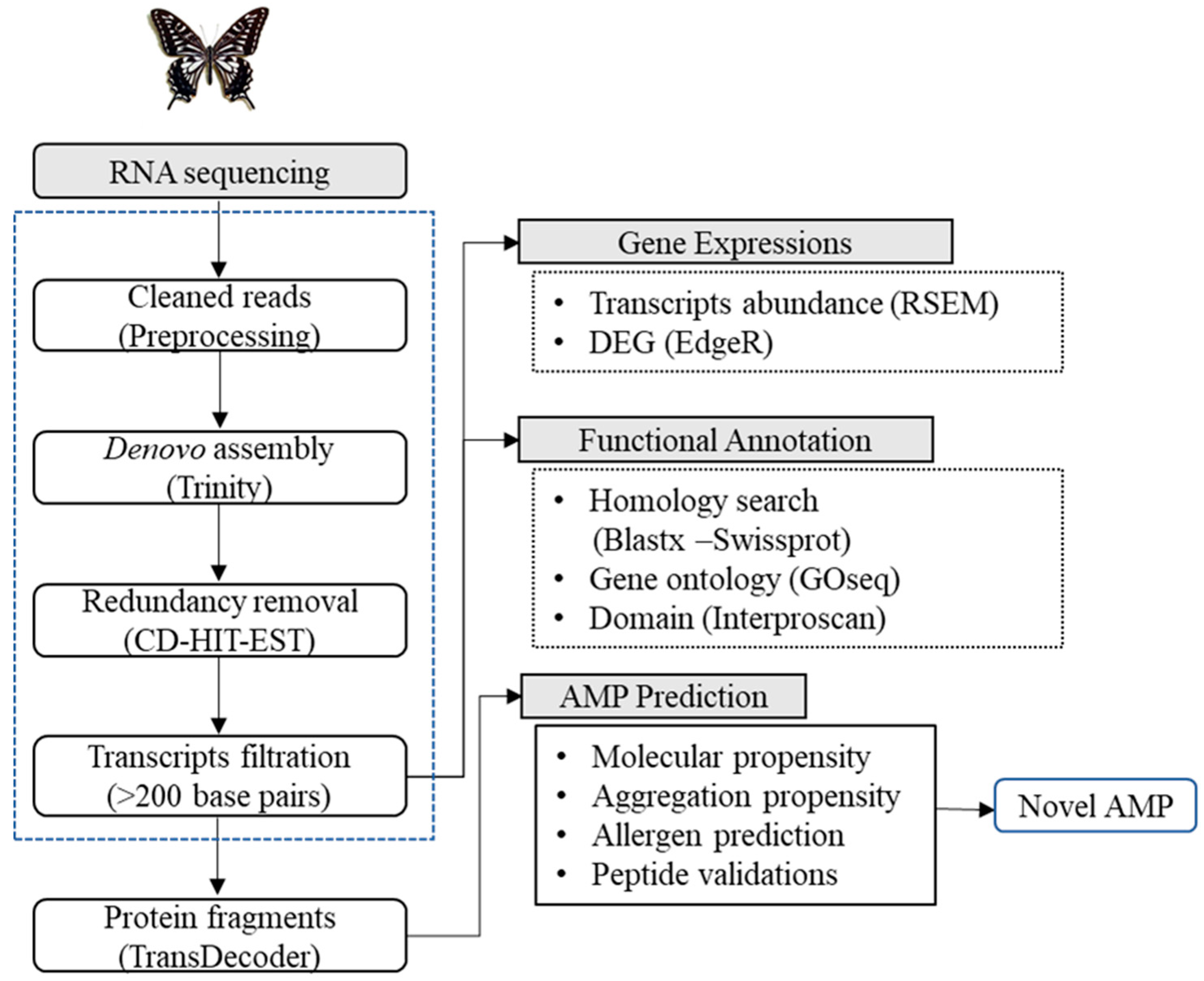
2.3. Transcriptome Sequencing, De Novo Assembly, and Functional Annotations
2.4. Expression Analysis
2.5. Antimicrobial Peptide (AMP) Prediction and Classification
2.6. Peptide Synthesis
2.7. Antimicrobial Activity Assay
2.8. Hemolytic Assay
2.9. Data Availability
3. Results and Discussion
3.1. Illumina Sequencing and De Novo Transcriptome Assembly
3.2. Functional Annotations and Differential Gene Expression Analysis
3.3. AMP Prediction
3.4. Experimental Validation of Putative and Novel AMPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Patocka, J.; Kuca, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. Int. J. Mol. Sci. 2019, 20, 5862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonk, M.; Vilcinskas, A. The Medical Potential of Antimicrobial Peptides from Insects. Curr. Top. Med. Chem. 2017, 17, 554–575. [Google Scholar] [CrossRef] [PubMed]
- Badapanda, C.; Chikara, S.K. Lepidopteran antimicrobial peptides (AMPs): Overview, regulation, modes of action, and therapeutic potentials of insect-derived AMPs. In Short Views on Insect Genomics and Proteomics; Entomology in Focus; Springer: Cham, Switzerland, 2016; Volume 4, pp. 141–163. [Google Scholar]
- Kim, S.R.; Hong, M.Y.; Park, S.W.; Choi, K.H.; Yun, E.Y.; Goo, T.W.; Kang, S.W.; Suh, H.J.; Kim, I.; Hwang, J.S. Characterization and cDNA cloning of a cecropin-like antimicrobial peptide, papiliocin, from the swallowtail butterfly, Papilio xuthus. Mol. Cells 2010, 29, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, E.; Shin, S.; Jeong, K.W.; Lee, J.Y.; Bae, S.Y.; Kim, S.H.; Lee, J.; Kim, S.R.; Lee, D.G.; et al. Structure and function of papiliocin with antimicrobial and anti-inflammatory activities isolated from the swallowtail butterfly, Papilio xuthus. J. Biol. Chem. 2011, 286, 41296–41311. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Jeong, K.W.; Lee, J.; Shin, A.; Kim, J.K.; Lee, J.; Lee, D.G.; Kim, Y. Structure-activity relationships of cecropin-like peptides and their interactions with phospholipid membrane. BMB Rep. 2013, 46, 282–287. [Google Scholar] [CrossRef]
- Lee, E.; Kim, J.K.; Jeon, D.; Jeong, K.W.; Shin, A.; Kim, Y. Functional Roles of Aromatic Residues and Helices of Papiliocin in its Antimicrobial and Anti-inflammatory Activities. Sci. Rep. 2015, 5, 12048. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Yoo, W.G.; Lee, J.H.; Shin, Y.; Shim, J.Y.; Jung, M.; Kang, B.C.; Oh, J.; Seong, J.; Lee, H.K.; Kong, H.S.; et al. Antimicrobial peptides in the centipede Scolopendra subspinipes mutilans. Funct. Integr. Genom. 2014, 14, 275–283. [Google Scholar] [CrossRef]
- Waghu, F.H.; Gopi, L.; Barai, R.S.; Ramteke, P.; Nizami, B.; Idicula-Thomas, S. CAMP: Collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res. 2014, 42, D1154–D1158. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Yoo, D.; Berardini, T.Z.; Mueller, L.A.; Weems, D.C.; Weng, S.; Cherry, J.M.; Rhee, S.Y. PatMatch: A program for finding patterns in peptide and nucleotide sequences. Nucleic Acids Res. 2005, 33, W262–W266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, D.A.; Lehrer, R.I. Designer assays for antimicrobial peptides. Disputing the “one-size-fits-all” theory. Methods Mol. Biol. 1997, 78, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Lee, J.H.; Subramaniyam, S.; Yun, E.Y.; Kim, I.; Park, J.; Hwang, J.S. De Novo Transcriptome Analysis and Detection of Antimicrobial Peptides of the American Cockroach Periplaneta americana (Linnaeus). PLoS ONE 2016, 11, e0155304. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.W.; Markkandan, K.; Lee, J.H.; Subramaniyam, S.; Yoo, S.; Park, J.; Hwang, J.S. Transcriptome Profiling and In Silico Analysis of the Antimicrobial Peptides of the Grasshopper Oxya chinensis sinuosa. J. Microbiol. Biotechnol. 2016, 26, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Elashry, A.; Guz, N.; Grundler, F.M.; Vilcinskas, A.; Dehne, H.W. Next generation sequencing based transcriptome analysis of septic-injury responsive genes in the beetle Tribolium castaneum. PLoS ONE 2013, 8, e52004. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.; Hwang, S.; Lee, J.; Cho, S. Identification of immunity-related genes in the larvae of Protaetia brevitarsis seulensis (Coleoptera: Cetoniidae) by a next-generation sequencing-based transcriptome analysis. J. Insect Sci. 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Garnier, J.; Gibrat, J.-F.; Robson, B. GOR method for predicting protein secondary structure from amino acid sequence. Methods. Enzymol. 1996, 266, 540–553. [Google Scholar] [CrossRef] [PubMed]



| Peptide | Sequence | Length | Mw (Da) |
|---|---|---|---|
| Px 3 | R to L * | 15 | 1797.3 |
| Px 4 | V to S * | 18 | 2213.6 |
| Px 5 | Y to L * | 17 | 2216.7 |
| Px 6 | H to K * | 16 | 1838.3 |
| Px 7 | R to Y * | 20 | 2501.1 |
| Px 11 | F to R * | 15 | 1878.2 |
| Px 13 | S to K * | 14 | 1685.1 |
| Peptide (μg/mL) | Melittin | Px 3 | Px 4 | Px 5 | Px 6 | Px 7 | Px 11 | Px 13 |
|---|---|---|---|---|---|---|---|---|
| 200 | 70.3 | 111.3 | 90.4 | 90.2 | 108.8 | 80 | 106.5 | 118.7 |
| 100 | 35.1 | 55.6 | 45.2 | 45.1 | 54.4 | 40 | 53.2 | 59.3 |
| 50 | 17.6 | 27.8 | 22.6 | 22.6 | 27.2 | 20 | 26.6 | 29.7 |
| 25 | 8.8 | 13.9 | 11.3 | 11.3 | 13.6 | 10 | 13.3 | 14.8 |
| 12.5 | 4.4 | 7.0 | 5.6 | 5.6 | 6.8 | 5 | 6.7 | 7.4 |
| 6.25 | 2.2 | 3.5 | 2.8 | 2.8 | 3.4 | 2.5 | 3.3 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Chung, H.; Shin, Y.P.; Kim, M.-A.; Natarajan, S.; Veerappan, K.; Kim, S.H.; Park, J.; Hwang, J.S. Deciphering Novel Antimicrobial Peptides from the Transcriptome of Papilio xuthus. Insects 2020, 11, 776. https://doi.org/10.3390/insects11110776
Lee JH, Chung H, Shin YP, Kim M-A, Natarajan S, Veerappan K, Kim SH, Park J, Hwang JS. Deciphering Novel Antimicrobial Peptides from the Transcriptome of Papilio xuthus. Insects. 2020; 11(11):776. https://doi.org/10.3390/insects11110776
Chicago/Turabian StyleLee, Joon Ha, Hoyong Chung, Yong Pyo Shin, Mi-Ae Kim, Sathishkumar Natarajan, Karpagam Veerappan, Seong Hyun Kim, Junhyung Park, and Jae Sam Hwang. 2020. "Deciphering Novel Antimicrobial Peptides from the Transcriptome of Papilio xuthus" Insects 11, no. 11: 776. https://doi.org/10.3390/insects11110776
APA StyleLee, J. H., Chung, H., Shin, Y. P., Kim, M.-A., Natarajan, S., Veerappan, K., Kim, S. H., Park, J., & Hwang, J. S. (2020). Deciphering Novel Antimicrobial Peptides from the Transcriptome of Papilio xuthus. Insects, 11(11), 776. https://doi.org/10.3390/insects11110776





