Low Genetic Variability in Bemisia tabaci MEAM1 Populations within Farmscapes of Georgia, USA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Whitefly Collections
2.2. DNA Extraction
2.3. Determination of Whitefly Species
2.4. Haplotype Analysis
2.5. Microsatellite Genotyping
2.6. Genetic Diversity of B. tabaci MEAM1
2.7. Genetic Differentiation among B. tabaci MEAM1 Populations
3. Results
3.1. Determination of Whitefly Species
3.2. Haplotype Analysis
3.3. Genetic Diversity of B. tabaci MEAM1
3.4. Genetic Differentiation among B. tabaci MEAM1 Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ehrlich, P.R.; Raven, P.H. Butterflies and Plants: A Study in Coevolution. Evolution (N. Y.) 1964, 18, 586. [Google Scholar]
- Philips, C.R.; Rogers, M.A.; Kuhar, T.P. Understanding Farmscapes and Their Potential for Improving IPM Programs. J. Integr. Pest Manag. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Morales-Hojas, R.; Sun, J.; Iraizoz, F.A.; Tan, X.; Chen, J. Contrasting population structure and demographic history of cereal aphids in different environmental and agricultural landscapes. Ecol. Evol. 2020, 10, 9647–9662. [Google Scholar] [CrossRef] [PubMed]
- Crossley, M.S.; Rondon, S.I.; Schoville, S.D. Effects of contemporary agricultural land cover on Colorado potato beetle genetic differentiation in the Columbia Basin and Central Sands. Ecol. Evol. 2019, 9, 9385–9394. [Google Scholar] [CrossRef] [Green Version]
- Frantz, A.; Plantegenest, M.; Mieuzet, L.; Simon, J.-C. Ecological specialization correlates with genotypic differentiation in sympatric host-populations of the pea aphid. J. Evol. Biol. 2006, 19, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Peccoud, J.; Ollivier, A.; Plantegenest, M.; Simon, J.-C. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl. Acad. Sci. USA 2009, 106, 7495–7500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Li, Y.; Zhang, Z. Genetic diversity of melon aphids Aphis gossypii associated with landscape features. Ecol. Evol. 2018, 8, 6308–6316. [Google Scholar] [CrossRef]
- Brown, J.K.; Frohlich, D.R.; Rosell, R.C. The Sweetpotato or Silverleaf Whiteflies: Biotypes of Bemisia tabaci or a Species Complex? Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- Schuster, D.J.; Stansly, P.A.; Polston, J.E. Expressionsofplant Damage by Bemisia; Gerling, D., Mayer, R., Eds.; Intercept Ltd.: Andover, UK, 1996. [Google Scholar]
- McCollum, T.; Stoffella, P.; Powell, C.; Cantliffe, D.; Hanif-Khan, S. Effects of silverleaf whitefly feeding on tomato fruit ripening. Postharvest Biol. Technol. 2004, 31, 183–190. [Google Scholar] [CrossRef]
- Yokomi, R.K. Relationships Between the Sweetpotato Whitefly and the Squash Silverleaf Disorder. Phytopathology 1990, 80, 895. [Google Scholar] [CrossRef]
- Summers, C.G. Chlorotic Streak of Bell Pepper: A New Toxicogenic Disorder Induced by Feeding of the Silverleaf Whitefly, Bemisia argentifolii. Plant Dis. 1996, 80, 822. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging Virus Diseases Transmitted by Whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Virus-virus interactions in a plant host and in a hemipteran vector: Implications for vector fitness and virus epidemics. Virus Res. 2020, 286, 198069. [Google Scholar] [CrossRef]
- Boykin, L.M.; Shatters, R.G., Jr.; Rosell, R.C.; McKenzie, C.L.; Bagnall, R.A.; De Barro, P.; Frohlich, D.R. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007, 44, 1306–1319. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) Mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Mugerwa, H.; Seal, S.; Wang, H.L.; Patel, M.V.; Kabaalu, R.; Omongo, C.A.; Alicai, T.; Tairo, F.; Ndunguru, J.; Sseruwagi, P.; et al. African ancestry of New World, Bemisia tabaci-whitefly species. Sci. Rep. 2018, 8, 2734. [Google Scholar] [CrossRef] [Green Version]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A Statement of Species Status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, G.; Liu, S.; Luo, C.; Chu, D.; Zhang, Y.; Zang, L.; Jiu, M.; Lü, Z.; Cui, X.; et al. Invasive mechanism and management strategy of Bemisia tabaci (Gennadius) biotype B: Progress report of 973 Program on invasive alien species in China. Sci. China Ser. C Life Sci. 2009, 52, 88–95. [Google Scholar] [CrossRef]
- Hamon, A.; Salguero, V. Bemisia tabaci, sweetpotato whitefly, in Florida (Homoptera: Aleyrodidae: Aleyrodinae). Fla. Dep. Agric. Consum. Serv. Div. Plant Ind. 1987; Entomology circular No. 292. [Google Scholar]
- Schuster, D.; Price, J.; King, J.; Everett, P. Integrated management of the sweetpotato whiteßy on commercial tomato. Univ. Fla. IFAS Bradent. GCREC Res. Rep. 1989; BRA1989-12. [Google Scholar]
- Hoelmer, K.A.; Osborne, L.S.; Yokomi, R.K. Foliage Disorders in Florida Associated with Feeding by Sweetpotato Whitefly, Bemisia tabaci. Fla. Entomol. 1991, 74, 162. [Google Scholar] [CrossRef]
- Adkins, S.; Webster, C.G.; Kousik, C.S.; Webb, S.E.; Roberts, P.D.; Stansly, P.A.; Turechek, W.W. Ecology and management of whitefly-transmitted viruses of vegetable crops in Florida. Virus Res. 2011, 159, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, T.; Degain, B.; Harpold, V.; Brown, J.; Morin, S.; Fabrick, J.; Byrne, F.; Nichols, R.L. New challenges to management of whitefly resistance to insecticides in Arizona. Univ. Ariz. Coop. Ext. Veg. Rep. 2005, T Series, 144. [Google Scholar]
- McKenzie, C.L.; Bethke, J.A.; Byrne, F.J.; Chamberlin, J.R.; Dennehy, T.J.; Dickey, A.M.; Gilrein, D.; Hall, P.M.; Ludwig, S.; Oetting, R.D.; et al. Distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) Biotypes in North America After the Q Invasion. J. Econ. Entomol. 2012, 105, 753–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickey, A.M.; Osborne, L.S.; Shatters, R.G.; Hall, P.M.; Mckenzie, C.L. Population Genetics of Invasive Bemisia tabaci (Hemiptera: Aleyrodidae) Cryptic Species in the United States Based on Microsatellite Markers. J. Econ. Entomol. 2013, 106, 1355–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Abdelkrim, A.; Hattab, T.; Fakhfakh, H.; Belkadhi, M.S.; Gorsane, F. A landscape genetic analysis of important agricultural pest species in Tunisia: The whitefly Bemisia tabaci. PLoS ONE 2017, 12, e0185724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Nam, H.Y.; Baek, S.; Lee, S.H.; Lee, J.-H. Population genetic structure of Bemisia tabaci MED (Hemiptera: Aleyrodidae) in Korea. PLoS ONE 2019, 14, e0220327. [Google Scholar] [CrossRef]
- Li, H.-R.; Pan, H.-P.; Tao, Y.-L.; Zhang, Y.-J.; Chu, D. Population genetics of an alien whitefly in China: Implications for its dispersal and invasion success. Sci. Rep. 2017, 7, 2228. [Google Scholar] [CrossRef] [Green Version]
- Díaz, F.; Endersby, N.M.; Hoffmann, A.A. Genetic structure of the whitefly Bemisia tabaci populations in Colombia following a recent invasion. Insect Sci. 2015, 22, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Delatte, H.; David, P.; Granier, M.; Lett, J.M.; Goldbach, R.; Peterschmitt, M.; Reynaud, B. Microsatellites reveal extensive geographical, ecological and genetic contacts between invasive and indigenous whitefly biotypes in an insular environment. Genet. Res. 2006, 87, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Vanlerberghe-Masutti, F.; Mistral, P.; Loiseau, A.; Boissot, N. Insight into the durability of plant resistance to aphids from a demo-genetic study of Aphis gossypii in melon crops. Evol. Appl. 2016, 9, 756–768. [Google Scholar] [CrossRef]
- Brévault, T.; Carletto, J.; Tribot, J.; Vanlerberghe-Masutti, F. Insecticide use and competition shape the genetic diversity of the aphid Aphis gossypii in a cotton-growing landscape. Bull. Entomol. Res. 2011, 101, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannell, J.R.; Charlesworth, B. Effects of metapopulation processes on measures of genetic diversity. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1851–1864. [Google Scholar] [CrossRef]
- Manani, D.; Ateka, E.; Nyanjom, S.; Boykin, L. Phylogenetic Relationships among Whiteflies in the Bemisia tabaci (Gennadius) Species Complex from Major Cassava Growing Areas in Kenya. Insects 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaei Shoorcheh, H.; Kazemi, B.; Manzari, S.; Brown, J.K.; Sarafrazi, A. Genetic variation and mtCOI phylogeny for Bemisia tabaci (Hemiptera, Aleyrodidae) indicate that the ‘B’ biotype predominates in Iran. J. Pest Sci. 2008, 81, 199–206. [Google Scholar] [CrossRef]
- Baoli, Q.; Coats, S.A.; Shunxiang, R.; Idris, A.M.; Caixia, X.; Brown, J.K. Phylogenetic relationship of native and introduced Bemisia tabaci (Homoptera: Aleyrodidae) from China and India based on mtCOI DNA sequencing and host plant comparisons. Prog. Nat. Sci. 2007, 17, 645–654. [Google Scholar] [CrossRef]
- Brown, W.M.; George, M.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frohlich, D.R.; Torres-Jerez, I.; Bedford, I.D.; Markham, P.G.; Brown, J.K. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999, 8, 1593–1602. [Google Scholar] [CrossRef]
- Schlötterer, C. Genealogical inference of closely related species based on microsatellites. Genet. Res. 2001, 78, 209–212. [Google Scholar] [CrossRef]
- Chu, D.; Guo, D.; Tao, Y.; Jiang, D.; Li, J.; Zhang, Y. Evidence For Rapid Spatiotemporal Changes in Genetic Structure of an Alien Whitefly During Initial Invasion. Sci. Rep. 2015, 4, 4396. [Google Scholar] [CrossRef] [Green Version]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Bayhan, E.; Ulusoy, M.R.; Brown, J.K. Host range, distribution, and natural enemies of Bemisia tabaci ‘B biotype’ (Hemiptera: Aleyrodidae) in Turkey. J. Pest Sci. 2006, 79, 233–240. [Google Scholar] [CrossRef]
- Athar, H.-R.; Bhatti, A.R.; Bashir, N.; Zafar, Z.U.; Abida; Farooq, A. Modulating infestation rate of white fly (Bemicia tabaci) on okra (Hibiscus esculentus L.) by nitrogen application. Acta Physiol. Plant. 2011, 33, 843–850. [Google Scholar] [CrossRef]
- Hodges, G.S.; Gregory, A.E. An identification guide to the whiteflies (Hemiptera: Aleyrodidae) of the southeastern united states. Fla. Entomol. 2005, 88, 518–534. [Google Scholar] [CrossRef]
- Ovalle, T.M.; Parsa, S.; Hernández, M.P.; Becerra Lopez-Lavalle, L.A. Reliable molecular identification of nine tropical whitefly species. Ecol. Evol. 2014, 4, 3778–3787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rozas, J.; Sanchez-DelBarrio, J.C.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- De Barro, P.J.; Scott, K.D.; Graham, G.C.; Lange, C.L.; Schutze, M.K. Isolation and characterization of microsatellite loci in Bemisia tabaci. Mol. Ecol. Notes 2002, 3, 40–43. [Google Scholar] [CrossRef]
- Mckenzie, C.L.; Hodges, G.; Osborne, L.S.; Byrne, F.J.; Shatters, R.G. Distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) Biotypes in Florida–Investigating the Q Invasion. J. Econ. Entomol. 2009, 102, 670–676. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piry, S.; Luikart, G.; Cornuet, J.-M. Computer note. BOTTLENECK: A computer program for detecting recent reductions in the effective size using allele frequency data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Luikart, G. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 1998, 89, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes. 2005, 5, 184–186. [Google Scholar] [CrossRef] [Green Version]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [Green Version]
- Bagley, R.K.; Sousa, V.C.; Niemiller, M.L.; Linnen, C.R. History, geography and host use shape genomewide patterns of genetic variation in the redheaded pine sawfly (Neodiprion lecontei). Mol. Ecol. 2017, 26, 1022–1044. [Google Scholar] [CrossRef]
- Fasulo, T.R.; Weems, H.V. Citrus Whitefly, Dialeurodes citri (Ashmead) (Insecta: Hemiptera: Aleyrodidae). IFAS Ext. 1996, 128, 1–6. [Google Scholar] [CrossRef]
- BI, J.L.; Toscano, N.C.; Ballmer, G.R. Seasonal Population Dynamics of the Greenhouse Whitefly Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on Strawberries in Southern California. J. Econ. Entomol. 2002, 95, 1179–1184. [Google Scholar] [CrossRef]
- Wintermantel, W.M. Emergence of Greenhouse Whitefly (Trialeurodes vaporariorum) Transmitted Criniviruses as Threats to Vegetable and Fruit Production in North America. APSnet Featur. Artic. 2004. [Google Scholar] [CrossRef] [Green Version]
- Navas-Castillo, J.; López-Moya, J.J.; Aranda, M.A. Whitefly-transmitted RNA viruses that affect intensive vegetable production. Ann. Appl. Biol. 2014, 165, 155–171. [Google Scholar] [CrossRef]
- McKenzie, C.L.; Osborne, L.S. Bemisia tabaci MED (Q biotype) (Hemiptera: Aleyrodidae) in Florida is on the Move to Residential Landscapes and May Impact Open-Field Agriculture. Fla. Entomol. 2017, 100, 481–484. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Wan, F.H.; Zhang, Y.J.; Brown, J.K. Change in the Biotype Composition of Bemisia tabaci in Shandong Province of China From 2005 to 2008. Environ. Entomol. 2010, 39, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, S.; Zhou, J.; Du, Y.; Zhang, Y.; Wang, J. Status of insecticide resistance and associated mutations in Q-biotype of whitefly, Bemisia tabaci, from eastern China. Crop Prot. 2012, 31, 67–71. [Google Scholar] [CrossRef]
- Perring, T.M. Biological differences of two species of Bemisia that contribute to adaptive advantage. In Bemisia 1995: Tax- Onomy, Biology, Damage Control and Management; Gerling, D., Mayer, R.T., Eds.; Intercept Scientific, Medical and Technical Publications: Andover, UK, 1996; pp. 3–16. [Google Scholar]
- Brewster, C.; Allen, J.; Schuster, D.; Stansly, P.A. Simulating the dynamics of Bemisia argentifolii (Homoptera: Aleyrodidae) in an organic cropping system with a spatiotemporal model. Environ. Entomol. 1997, 26, 603–616. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Castle, S.J.; De Barro, P.J.; Liu, S.-S. Population Dynamics, Demography, Dispersal and Spread of Bemisia tabaci. In Bemisia: Bionomics and Management of a Global Pest; Springer: Dordrecht, The Netherlands, 2009; pp. 185–226. [Google Scholar]
- Puillandre, N.; Dupas, S.; Dangles, O.; Zeddam, J.-L.; Capdevielle-Dulac, C.; Barbin, K.; Torres-Leguizamon, M.; Silvain, J.-F. Genetic bottleneck in invasive species: The potato tuber moth adds to the list. Biol. Invasions 2008, 10, 319–333. [Google Scholar] [CrossRef]
- Orantes, L.C.; Zhang, W.; Mian, M.A.R.; Michel, A.P. Maintaining genetic diversity and population panmixia through dispersal and not gene flow in a holocyclic heteroecious aphid species. Heredity 2012, 109, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, N.D.; Suarez, A.V.; Holway, D.A.; Case, T.J. Reduced genetic variation and the success of an invasive species. Proc. Natl. Acad. Sci. USA 2000, 97, 5948–5953. [Google Scholar] [CrossRef] [Green Version]
- Luikart, G.; Cornuet, J.-M. Empirical Evaluation of a Test for Identifying Recently Bottlenecked Populations from Allele Frequency Data. Conserv. Biol. 2008, 12, 228–237. [Google Scholar] [CrossRef]
- Dalmon, A.; Halkett, F.; Granier, M.; Delatte, H.; Peterschmitt, M. Genetic structure of the invasive pest Bemisia tabaci: Evidence of limited but persistent genetic differentiation in glasshouse populations. Heredity 2008, 100, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, A.; Halkett, F.; Granier, M.; Gueguen, G.; Peterschmitt, M. Evidence of gene flow between sympatric populations of the Middle East-Asia Minor 1 and Mediterranean putative species of Bemisia tabaci. Ecol. Evol. 2013, 3, 2619–2633. [Google Scholar] [CrossRef]
- Simón, B.; Cenis, J.L.; De La Rúa, P. Distribution patterns of the Q and B biotypes of Bemisia tabaci in the Mediterranean Basin based on microsatellite variation. Entomol. Exp. Appl. 2007, 124, 327–336. [Google Scholar] [CrossRef]
- Westerfield, R. Vegetable Planting Chart. UGA Ext. 2019; Circular 963. [Google Scholar]
- Lee, R.D.; Johnson, J.T. Planting Guide for Row Crops in Georgia. UGA Ext. 2014; Circular 813. [Google Scholar]
- Georgia Cotton Commisison. Available online: http://www.georgiacottoncommission.org/index.cfm?show=10&mid=5 (accessed on 10 August 2020).
- Nauen, R.; Stumpf, N.; Elbert, A. Toxicological and mechanistic studies on neonicotinoid cross resistance in Q-type Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2002, 58, 868–875. [Google Scholar] [CrossRef]
- Roditakis, E.; Roditakis, N.E.; Tsagkarakou, A. Insecticide resistance in Bemisia tabaci (Homoptera: Aleyrodidae) populations from Crete. Pest Manag. Sci. 2005, 61, 577–582. [Google Scholar] [CrossRef]
- Cahill, M.; Byrne, F.J.; Gorman, K.; Denholm, I.; Devonshire, A.L. Pyrethroid and organophosphate resistance in the tobacco whitefly Bemisia tabaci (Homoptera: Aleyrodidae). Bull. Entomol. Res. 1995, 85, 181–187. [Google Scholar] [CrossRef]
- Naveen, N.C.; Chaubey, R.; Kumar, D.; Rebijith, K.B.; Rajagopal, R.; Subrahmanyam, B.; Subramanian, S. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 2017, 7, 40634. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, P.C.; Martinez-Carrillo, J.L. IPM for Bemisia tabaci: A case study from North America. Crop Prot. 2001, 20, 853–869. [Google Scholar] [CrossRef]
- Palumbo, J.; Horowitz, A.; Prabhaker, N. Insecticidal control and resistance management for Bemisia tabaci. Crop Prot. 2001, 20, 739–765. [Google Scholar] [CrossRef]
- Caballero, R.; Cyman, S.; Schuster, D.J.; Portillo, H.E.; Slater, R. Baseline susceptibility of Bemisia tabaci (Genn.) biotype B in southern Florida to cyantraniliprole. Crop Prot. 2013, 44, 104–108. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
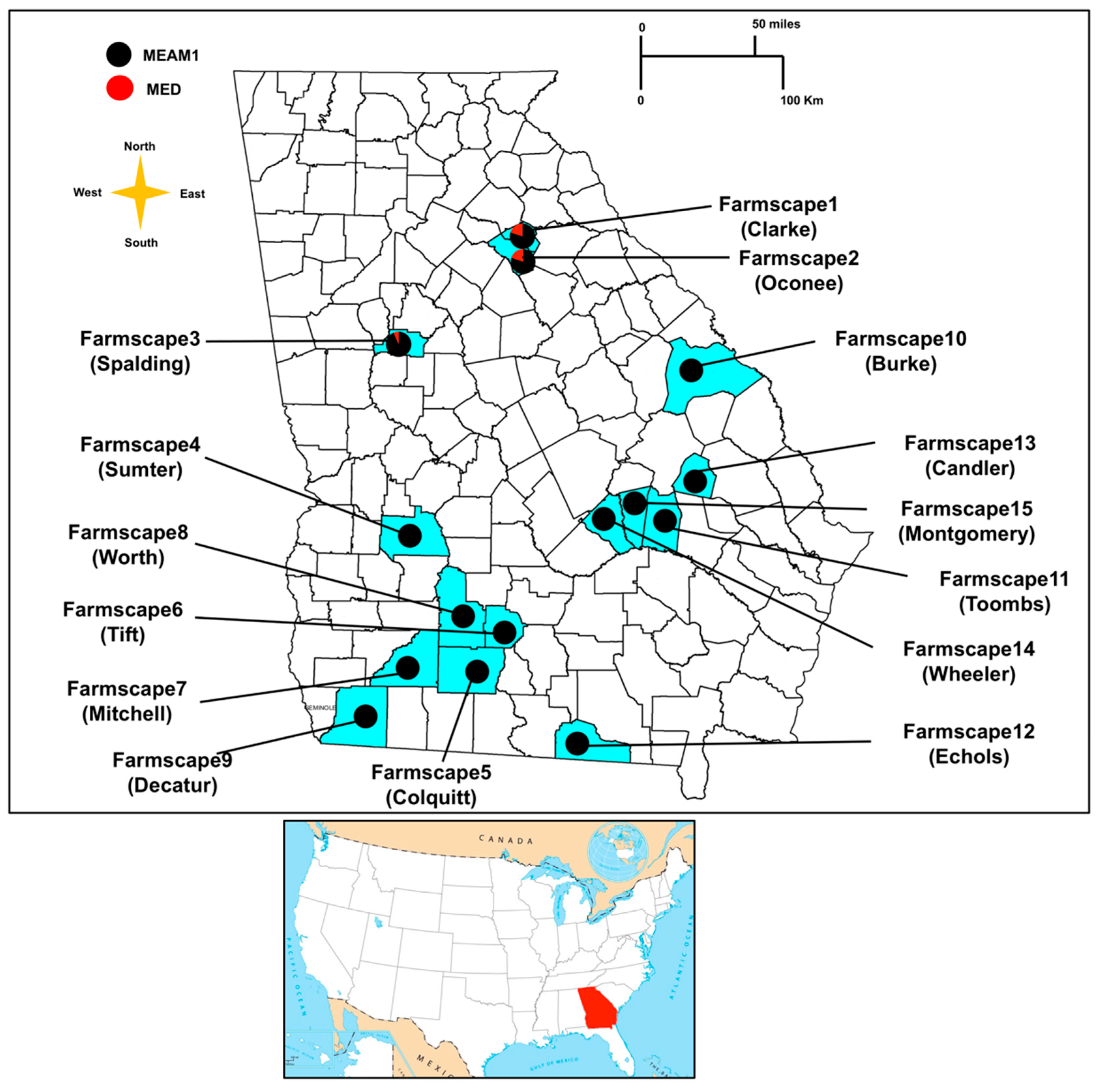


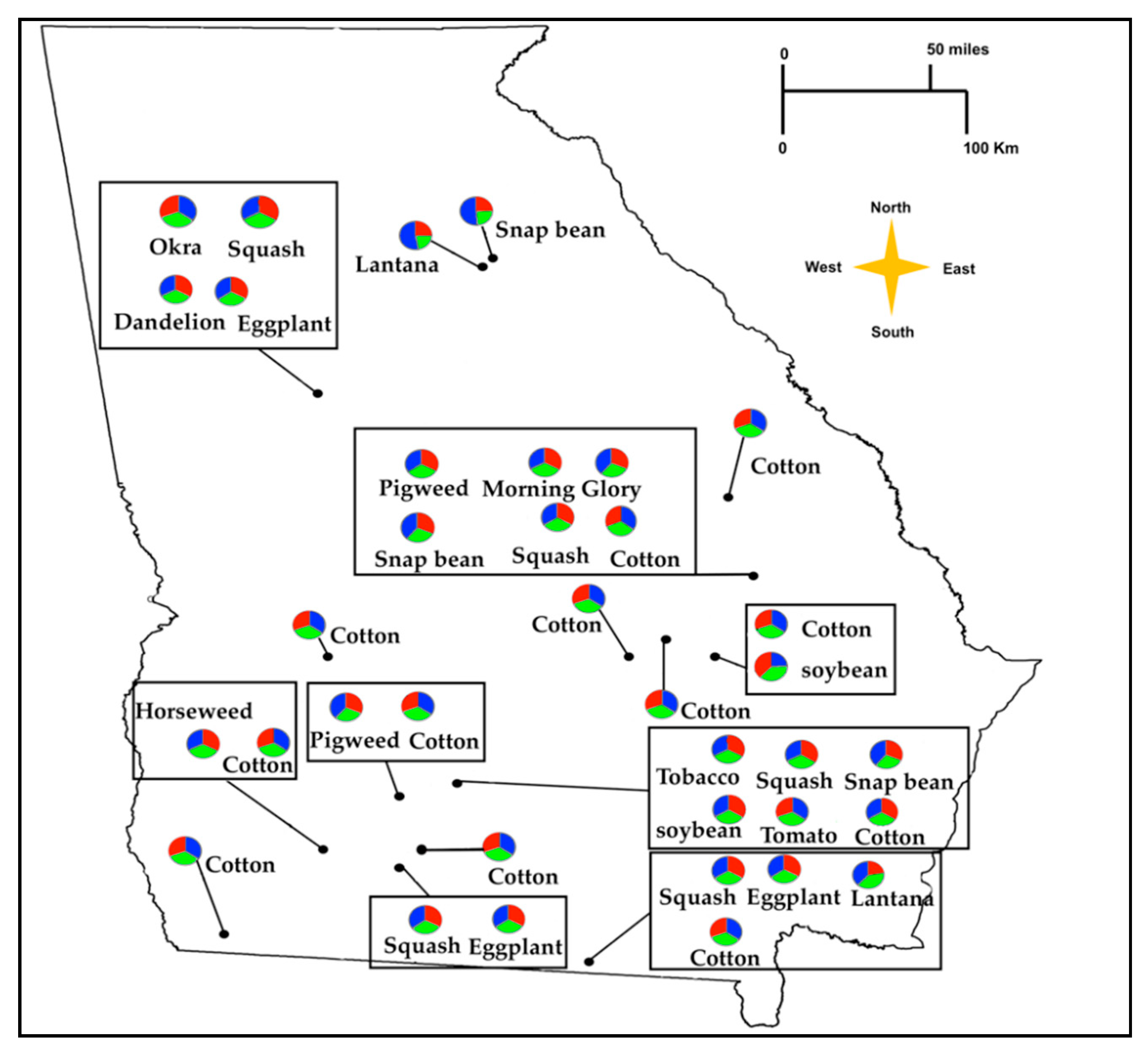

| Population Number | Farmscape | County | Host Plant | Collection Date | GPS Coordinates (DMS) a |
|---|---|---|---|---|---|
| 1 | Farmscape 1 | Oconee | Snap bean (Phaseolus vulgaris L.) | 08/23/2019 | 33°43’26.5″ N 83°19’41.5″ W |
| 2 | Farmscape 2 | Clarke | Lantana (Lantana camara L.) | 08/23/2019 | 33°54’02.9″ N 83°22’56.6″ W |
| 3 | Farmscape 3 | Spalding | Okra (Abelmoschus esculentus (L.) | 07/19/2019 | 33°15’48.0″ N 84°18’25.5″ W |
| 4 | Farmscape 3 | Spalding | Dandelion (Taraxacum officinale Weber) | 07/20/2019 | 33°15’57.1″ N 84°18’22.4″ W |
| 5 | Farmscape 3 | Spalding | Eggplant (Solanum melongena L.) | 07/20/2019 | 33°15’46.7″ N 84°17’30.6″ W |
| 6 | Farmscape 3 | Spalding | Squash (Cucurbita pepo L.) | 07/21/2019 | 33°15’45.2″ N 84°17’06.8″ W |
| 7 | Farmscape 4 | Sumter | Cotton (Gossypium hirsutum L.) | 08/19/2019 | 32°02’35.2″ N 84°22’13.4″ W |
| 8 | Farmscape 5 | Colquitt | Cotton (Gossypium hirsutum L.) | 08/01/2019 | 31°11’32.0″ N 83°40’18.6″ W |
| 9 | Farmscape 5 | Colquitt | Squash (Cucurbita pepo L.) | 08/01/2019 | 31°12’07.6″ N 83°40’10.8″ W |
| 10 | Farmscape 5 | Colquitt | Eggplant (Solanum melongena L.) | 08/01/2019 | 31°11’23.5″ N 83°43’41.2″ W |
| 11 | Farmscape 6 | Tift | Snap bean (Phaseolus vulgaris L.) | 07/23/2019 | 31°28’17.7″ N 83°31’47.7″ W |
| 12 | Farmscape 6 | Tift | Squash (Cucurbita pepo L.) | 07/23/2019 | 31°29’01.3″ N 83°31’18.3″ W |
| 13 | Farmscape 6 | Tift | Tomato (Solanum lycopersicum Mill) | 07/23/2019 | 31°29’01.3″ N 83°31’18.3″ W |
| 14 | Farmscape 6 | Tift | Soybean (Glycine max Merrill) | 07/23/2019 | 31°29’01.3″ N 83°31’18.3″ W |
| 15 | Farmscape 6 | Tift | Tobacco (Nicotiana tabacum L.) | 07/23/2019 | 31°28’13.0″ N 83°31’54.1″ W |
| 16 | Farmscape 6 | Tift | Cotton (Gossypium hirsutum L.) | 08/13/2019 | 31°30’07.5″ N 83°32’43.0″ W |
| 17 | Farmscape 7 | Mitchell | Cotton (Gossypium hirsutum L.) | 08/16/2019 | 31°16’49.0″ N 84°17’38.1″ W |
| 18 | Farmscape 7 | Mitchell | Horseweed (Conyza canadensis L.) | 08/16/2019 | 31°16’39.8″ N 84°17’54.5″ W |
| 19 | Farmscape 8 | Worth | Cotton (Gossypium hirsutum L.) | 08/06/2019 | 31°35’19.6″ N 83°49’38.4″ W |
| 20 | Farmscape 8 | Worth | Redroot pigweed (Amaranthus retroflexus L.) | 08/06/2019 | 31°35’08.4″ N 83°50’03.3″ W |
| 21 | Farmscape 9 | Decatur | Cotton (Gossypium hirsutum L.) | 08/16/2019 | 30°45’49.7″ N 84°29’09.7″ W |
| 22 | Farmscape 10 | Burke | Cotton (Gossypium hirsutum L.) | 08/15/2019 | 32°52’35.1″ N 82°13’05.2″ W |
| 23 | Farmscape 11 | Toombs | Soybean (Glycine max Merrill) | 08/15/2019 | 32°01’03.2″ N 82°13’15.5″ W |
| 24 | Farmscape 11 | Toombs | Cotton (Gossypium hirsutum L.) | 08/15/2019 | 32°00’55.2″ N 82°13’19.8″ W |
| 25 | Farmscape 12 | Echols | Cotton (Gossypium hirsutum L.) | 08/01/2019 | 30°38’47.1″ N 83°01’42.8″ W |
| 26 | Farmscape 12 | Echols | Squash (Cucurbita pepo L.) | 08/01/2019 | 30°37’43.1″ N 83°02’20.0″ W |
| 27 | Farmscape 12 | Echols | Eggplant (Solanum melongena L.) | 08/01/2019 | 30°39’56.9″ N 83°01’54.9″ W |
| 28 | Farmscape 12 | Echols | Lantana (Lantana camara L.) | 08/01/2019 | 30°39’56.9″ N 83°01’54.9″ W |
| 29 | Farmscape 13 | Candler | Cotton (Gossypium hirsutum L.) | 09/02/2019 | 32°25’44.0″ N 82°04’50.7″ W |
| 30 | Farmscape 13 | Candler | Snap bean (Phaseolus vulgaris L.) | 09/02/2019 | 32°25’52.5″ N 82°04’27.5″ W |
| 31 | Farmscape 13 | Candler | Squash (Cucurbita pepo L.) | 09/02/2019 | 32°26’03.6″ N 82°03’59.1″ W |
| 32 | Farmscape13 | Candler | Redroot pigweed (Amaranthus retroflexus L.) | 09/02/2019 | 32°26’03.6″ N 82°03’59.1″ W |
| 33 | Farmscape 13 | Candler | Purple morning glory (Ipomoea purpurea L.) | 09/02/2019 | 32°26’15.7″ N 82°03’52.4″ W |
| 34 | Farmscape 14 | Wheeler | Cotton (Gossypium hirsutum L.) | 08/21/2019 | 32°06’12.3″ N 82°48’21.6″ W |
| 35 | Farmscape 15 | Montgomery | Cotton (Gossypium hirsutum L.) | 08/21/2019 | 32°11’59.7″ N 82°30’12.6″ W |
| Locus | Number of Alleles | Evenness | Expected Heterozygosity (Hexp) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FIS) | Fixation Index (FST) |
|---|---|---|---|---|---|---|
| BEM6 | 5.00 | 0.43 | 0.14 | 0.08 | 0.36 | 0.19 |
| BEM11 | 7.00 | 0.74 | 0.55 | 0.61 | −0.16 | 0.08 |
| BEM15 | 7.00 | 0.83 | 0.73 | 0.59 | 0.14 | 0.05 |
| BEM23 | 5.00 | 0.44 | 0.12 | 0.05 | 0.51 | 0.10 |
| BEM25 | 7.00 | 0.72 | 0.60 | 0.63 | 0.03 | 0.04 |
| BEM31 | 4.00 | 0.43 | 0.15 | 0.10 | 0.33 | 0.18 |
| Mean | 5.80 | 0.60 | 0.38 | 0.34 | 0.12 | 0.11 |
| Population | Sample Size | Mean number of Alleles | Shannon’s Information Index (I) | Expected Heterozygosity (Hexp) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FIS) 1 |
|---|---|---|---|---|---|---|
| Cotton | 144 | 4.83 | 0.80 | 0.41 | 0.36 | 0.23 |
| Soybean | 24 | 3.17 | 0.72 | 0.35 | 0.37 | −0.08 |
| Squash | 60 | 4.33 | 0.72 | 0.37 | 0.38 | −0.04 |
| Tomato | 12 | 2.83 | 0.66 | 0.33 | 0.29 | 0.08 |
| Snap bean | 36 | 3.50 | 0.66 | 0.35 | 0.41 | 0.10 |
| Lantana | 24 | 3.33 | 0.74 | 0.38 | 0.35 | 0.25 |
| Horseweed | 10 | 2.83 | 0.70 | 0.35 | 0.40 | −0.15 |
| Pigweed | 24 | 3.17 | 0.71 | 0.40 | 0.31 | 0.22 |
| Okra | 12 | 2.00 | 0.53 | 0.39 | 0.32 | 0.21 |
| Dandelion | 12 | 2.50 | 0.61 | 0.38 | 0.44 | 0.01 |
| Eggplant | 36 | 4.17 | 0.86 | 0.42 | 0.32 | 0.29 |
| Tobacco | 12 | 2.67 | 0.57 | 0.31 | 0.37 | −0.17 |
| Morning glory | 12 | 2.50 | 0.55 | 0.30 | 0.29 | 0.01 |
| Mean | 32.15 | 3.22 | 0.68 | 0.37 | 0.30 | 0.10 |
| Population | Sample Size | Mean No. of Alleles | Shannon’s Information Index (I) | Expected Heterozygosity (Hexp) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FIS) 1 |
|---|---|---|---|---|---|---|
| Farmscape1 | 12 | 2.83 | 0.70 | 0.39 | 0.36 | 0.23 |
| Farmscape2 | 12 | 2.83 | 0.72 | 0.39 | 0.38 | 0.09 |
| Farmscape3 | 48 | 3.33 | 0.80 | 0.41 | 0.32 | 0.33 |
| Farmscape4 | 12 | 2.67 | 0.70 | 0.34 | 0.14 | 0.55 |
| Farmscape5 | 36 | 4.00 | 0.88 | 0.49 | 0.47 | 0.07 |
| Farmscape6 | 72 | 3.83 | 0.82 | 0.37 | 0.36 | −0.02 |
| Farmscape7 | 22 | 3.17 | 0.79 | 0.34 | 0.36 | −0.06 |
| Farmscape8 | 24 | 2.83 | 0.64 | 0.37 | 0.37 | 0.24 |
| Farmscape9 | 12 | 2.83 | 0.74 | 0.35 | 0.39 | 0.08 |
| Farmscape10 | 12 | 2.83 | 0.61 | 0.32 | 0.33 | −0.08 |
| Farmscape11 | 24 | 2.83 | 0.57 | 0.35 | 0.35 | 0.13 |
| Farmscape12 | 48 | 3.33 | 0.65 | 0.34 | 0.35 | 0.03 |
| Farmscape13 | 60 | 3.83 | 0.68 | 0.35 | 0.35 | 0.11 |
| Farmscape14 | 12 | 2.33 | 0.54 | 0.33 | 0.43 | −0.29 |
| Farmscape15 | 12 | 2.33 | 0.61 | 0.33 | 0.42 | −0.26 |
| Mean | 27.87 | 3.05 | 0.69 | 0.39 | 0.36 | 0.23 |
| Wilcoxon Test p-Values 1 | ||||
|---|---|---|---|---|
| Infinite Alleles Model IAM | Two-Phase Model TPM | Stepwise Mutation Model SMM | ||
| Host Plants | Heterozygosity Excess | Heterozygosity Excess | Heterozygosity Excess | Mode Shift |
| Cotton | 0.50 | 0.78 | 0.99 | L |
| Soybean | 0.31 | 0.50 | 0.68 | L |
| Squash | 0.71 | 0.98 | 1.00 | L |
| Tomato | 0.56 | 0.93 | 0.96 | L |
| Snap bean | 0.68 | 0.68 | 0.96 | L |
| Lantana | 0.31 | 0.50 | 0.89 | L |
| Horseweed | 0.06 | 0.06 | 0.06 | S |
| Pigweed | 0.31 | 0.31 | 0.40 | L |
| Okra | 0.03 | 0.03 | 0.03 | L |
| Dandelion | 0.31 | 0.68 | 1.00 | L |
| Eggplant | 0.40 | 0.68 | 1.00 | L |
| Tobacco | 0.06 | 0.12 | 0.81 | L |
| Morning glory | 0.13 | 0.13 | 0.81 | L |
| Wilcoxon Test p-Values 1 | ||||
|---|---|---|---|---|
| Infinite Alleles Model IAM | Two-Phase Model TPM | Stepwise Mutation Model SMM | ||
| Farmscapes | Heterozygosity Excess | Heterozygosity Excess | Heterozygosity Excess | Mode Shift |
| Farmscape1 | 0.92 | 0.40 | 0.89 | S |
| Farmscape2 | 0.31 | 0.41 | 0.41 | S |
| Farmscape3 | 0.05 | 0.31 | 0.41 | L |
| Farmscape4 | 0.56 | 0.84 | 0.94 | L |
| Farmscape5 | 0.04 | 0.42 | 0.96 | L |
| Farmscape6 | 0.78 | 0.57 | 0.98 | L |
| Farmscape7 | 0.63 | 0.63 | 0.63 | L |
| Farmscape8 | 0.05 | 0.40 | 0.59 | L |
| Farmscape9 | 0.84 | 0.84 | 1.00 | L |
| Farmscape10 | 0.16 | 0.16 | 0.56 | L |
| Farmscape11 | 0.41 | 0.69 | 0.92 | L |
| Farmscape12 | 0.58 | 0.92 | 0.98 | L |
| Farmscape13 | 0.59 | 0.92 | 0.98 | L |
| Farmscape14 | 0.06 | 0.06 | 0.06 | S |
| Farmscape15 | 0.06 | 0.06 | 0.06 | S |
| Cotton | Soybean | Squash | Tomato | Snapbean | Lantana | Horseweed | Pigweed | Okra | Dandelion | Eggplant | Tobacco | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean | 0.01 | |||||||||||
| Squash | 0.01 | 0.02 | ||||||||||
| Tomato | 0.01 | 0.03 | 0.02 | |||||||||
| Snapbean | 0.01 | 0.02 | 0.02 | 0.01 | ||||||||
| Lantana | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | |||||||
| Horseweed | 0.01 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | ||||||
| Pigweed | 0.01 | 0.03 | 0.02 | 0.03 | 0.01 | 0.02 | 0.03 | |||||
| Okra | 0.01 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.04 | 0.01 | ||||
| Dandelion | 0.00 | 0.02 | 0.01 | 0.04 | 0.02 | 0.01 | 0.03 | 0.02 | 0.03 | |||
| Eggplant | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | ||
| Tobacco | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | 0.02 | 0.05 | 0.04 | 0.02 | |
| Morning Glory | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.04 | 0.02 | 0.04 | 0.03 | 0.01 | 0.01 |
| Farmscape 1 | Farmscape 2 | Farmscape 3 | Farmscape 4 | Farmscape 5 | Farmscape 6 | Farmscape 7 | Farmscape 8 | Farmscape 9 | Farmscape 10 | Farmscape 11 | Farmscape 12 | Farmscape 13 | Farmscape 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farmscape 2 | 0.04 | |||||||||||||
| Farmscape 3 | 0.02 | 0.01 | ||||||||||||
| Farmscape 4 | 0.04 | 0.04 | 0.02 | |||||||||||
| Farmscape 5 | 0.02 | 0.03 | 0.02 | 0.03 | ||||||||||
| Farmscape 6 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | |||||||||
| Farmscape 7 | 0.03 | 0.03 | 0.01 | 0.03 | 0.02 | 0.03 | ||||||||
| Farmscape 8 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | |||||||
| Farmscape 9 | 0.03 | 0.04 | 0.02 | 0.05 | 0.02 | 0.01 | 0.01 | 0.01 | ||||||
| Farmscape 10 | 0.03 | 0.05 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.03 | |||||
| Farmscape 11 | 0.03 | 0.03 | 0.01 | 0.03 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | ||||
| Farmscape 12 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| Farmscape 13 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| Farmscape 14 | 0.05 | 0.05 | 0.02 | 0.05 | 0.02 | 0.01 | 0.01 | 0.03 | 0.03 | 0.03 | 0.02 | 0.01 | 0.01 | |
| Farmscape 15 | 0.06 | 0.06 | 0.03 | 0.07 | 0.02 | 0.01 | 0.02 | 0.04 | 0.04 | 0.06 | 0.05 | 0.03 | 0.02 | 0.02 |
| Source of Variation | Degrees of Freedom | Sums of Squares | Mean Sums of Squares | % Variation | p-Value |
|---|---|---|---|---|---|
| A, Host Plants | |||||
| Among host plants | 12 | 34.35 | 2.86 | 2.00 | 0.39 |
| Among populations within a host plant | 22 | 36.51 | 1.66 | 1.00 | 0.32 |
| Among individuals within a population | 409 | 708.27 | 1.64 | 23.00 | <0.001 |
| Within individuals | 444 | 442.00 | 1.00 | 74.00 | <0.001 |
| Total | 887 | 1184.63 | 1.34 | 100.00 | |
| B, Farmscapes | |||||
| Among farmscapes | 14 | 45.88 | 3.28 | 2.00 | 0.43 |
| Among populations within a farmscape | 20 | 40.42 | 2.01 | 0.00 | 0.63 |
| Among individuals within a population | 409 | 680.32 | 1.59 | 22.00 | <0.001 |
| Within individuals | 444 | 446.50 | 1.01 | 76.00 | <0.001 |
| Total | 887 | 1172.69 | 1.32 | 100.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, S.; Crossley, M.S.; Dutta, B.; Coolong, T.; Simmons, A.M.; da Silva, A.; Snyder, W.E.; Srinivasan, R. Low Genetic Variability in Bemisia tabaci MEAM1 Populations within Farmscapes of Georgia, USA. Insects 2020, 11, 834. https://doi.org/10.3390/insects11120834
Gautam S, Crossley MS, Dutta B, Coolong T, Simmons AM, da Silva A, Snyder WE, Srinivasan R. Low Genetic Variability in Bemisia tabaci MEAM1 Populations within Farmscapes of Georgia, USA. Insects. 2020; 11(12):834. https://doi.org/10.3390/insects11120834
Chicago/Turabian StyleGautam, Saurabh, Michael S. Crossley, Bhabesh Dutta, Timothy Coolong, Alvin M. Simmons, Andre da Silva, William E. Snyder, and Rajagopalbabu Srinivasan. 2020. "Low Genetic Variability in Bemisia tabaci MEAM1 Populations within Farmscapes of Georgia, USA" Insects 11, no. 12: 834. https://doi.org/10.3390/insects11120834







