Antioxidant Enzymes and Heat Shock Protein Genes from Liposcelis bostrychophila Are Involved in Stress Defense upon Heat Shock
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Insect Collection, Thermal Stress and Protein Extraction
2.3. Enzyme Activity and MDA Assay
2.4. RNA Isolation and cDNA Cloning of LbHsps
2.5. Gene Characterization and Phylogenetic Analysis
2.6. Quantitative Real-Time PCR (qPCR)
2.7. Statistical Analysis
3. Results
3.1. Changes in MDA
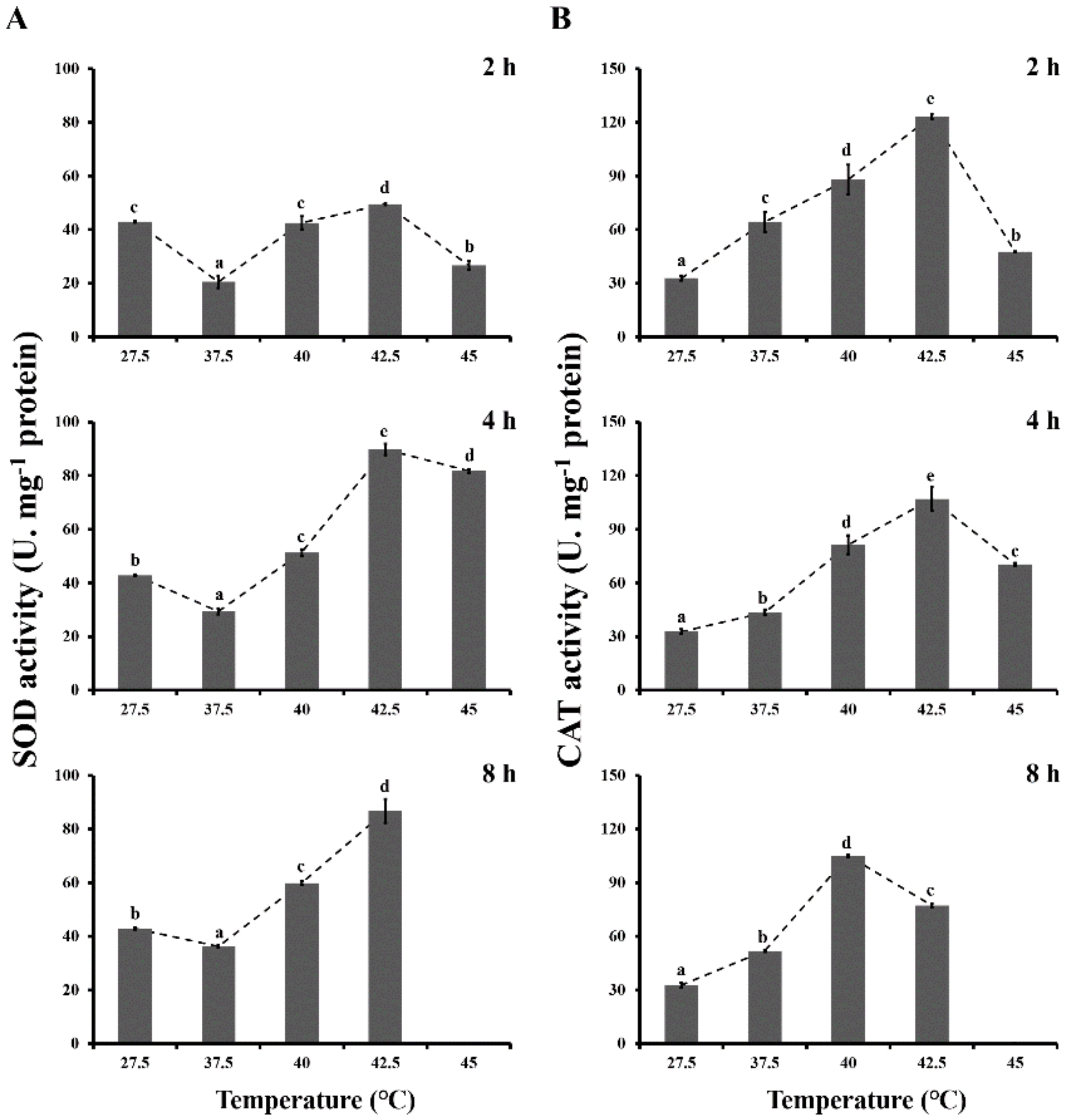
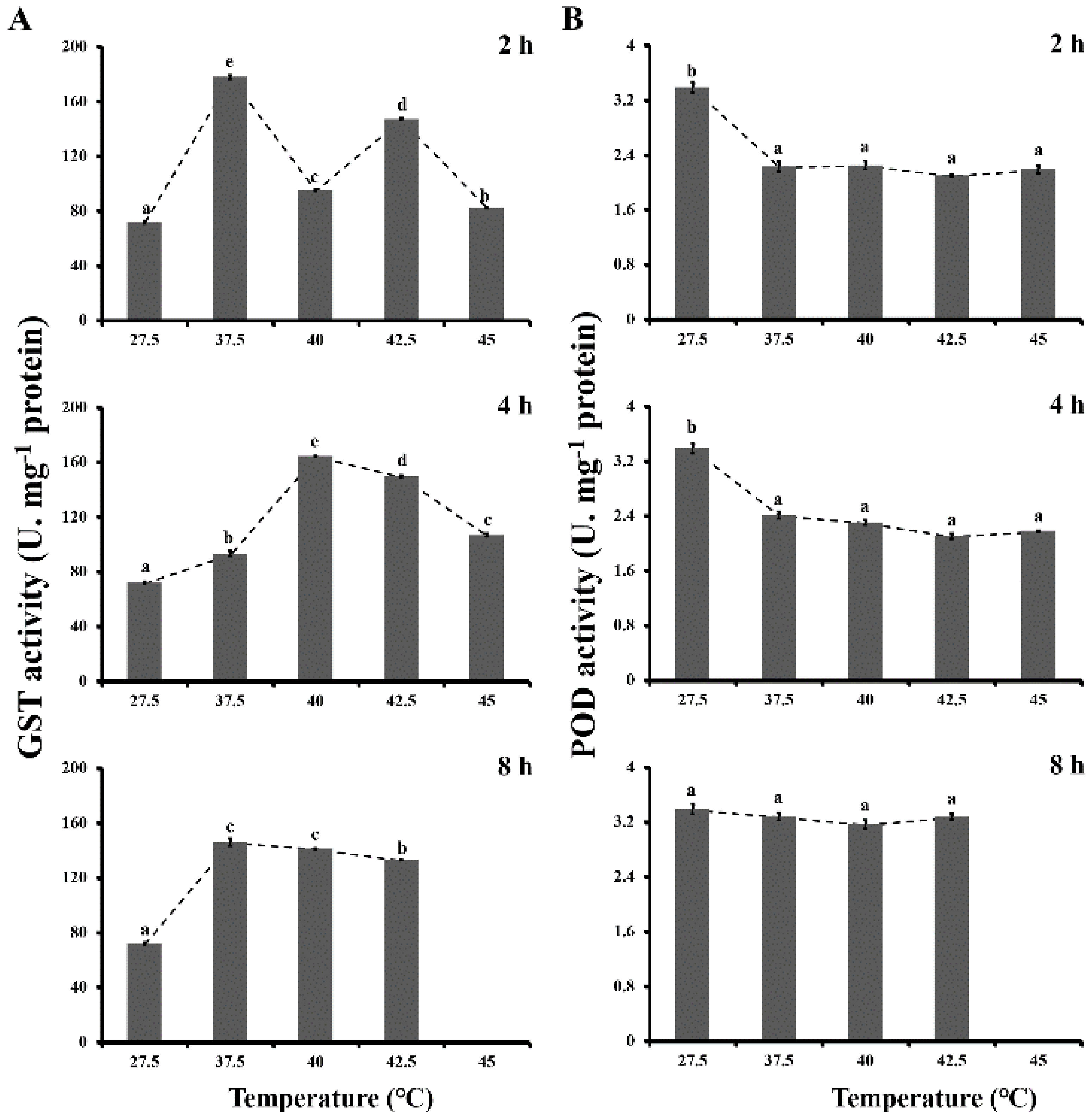
3.2. Antioxidant Enzyme Activities
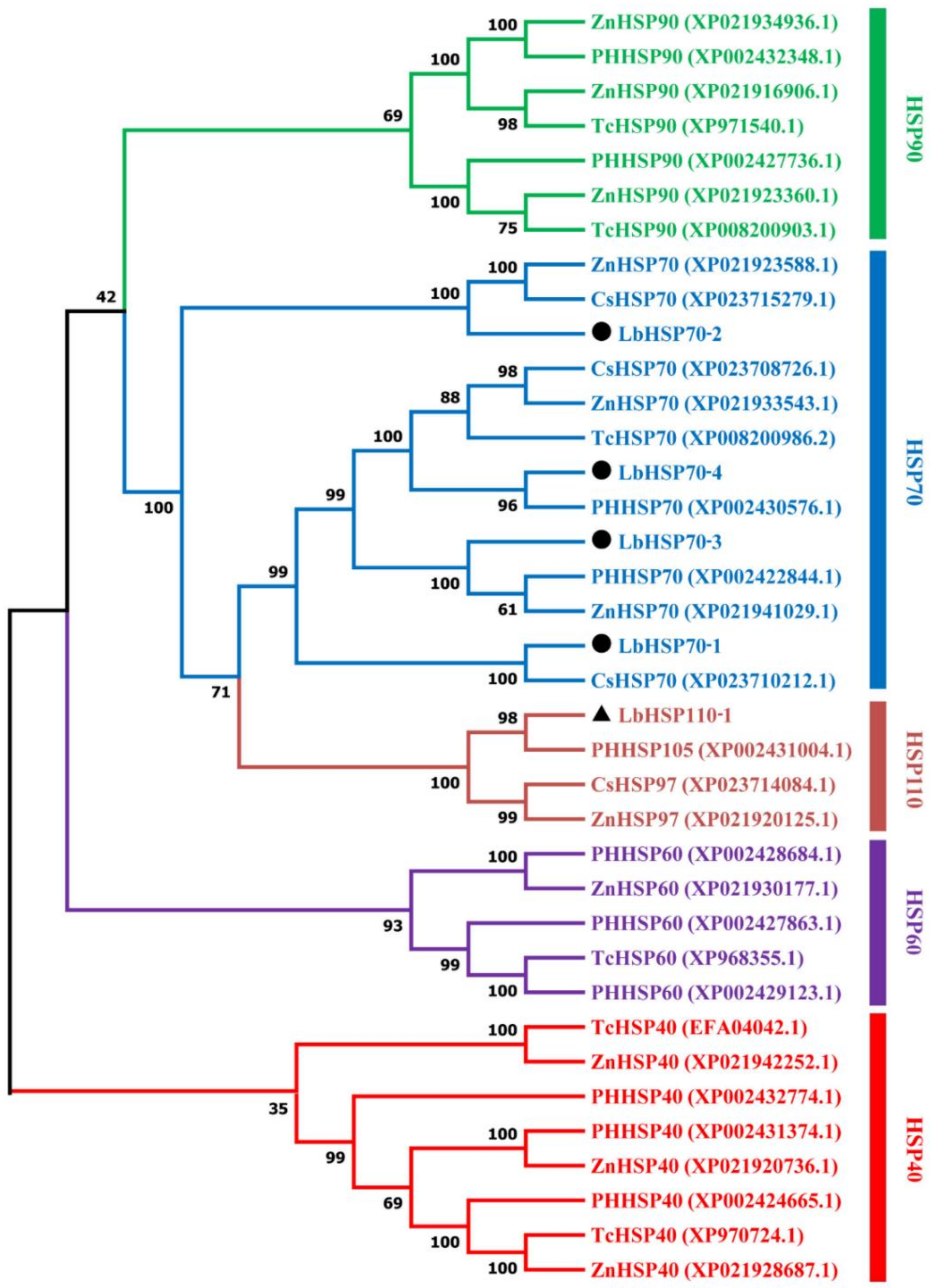
3.3. Sequence Analysis and Phylogenetic Tree
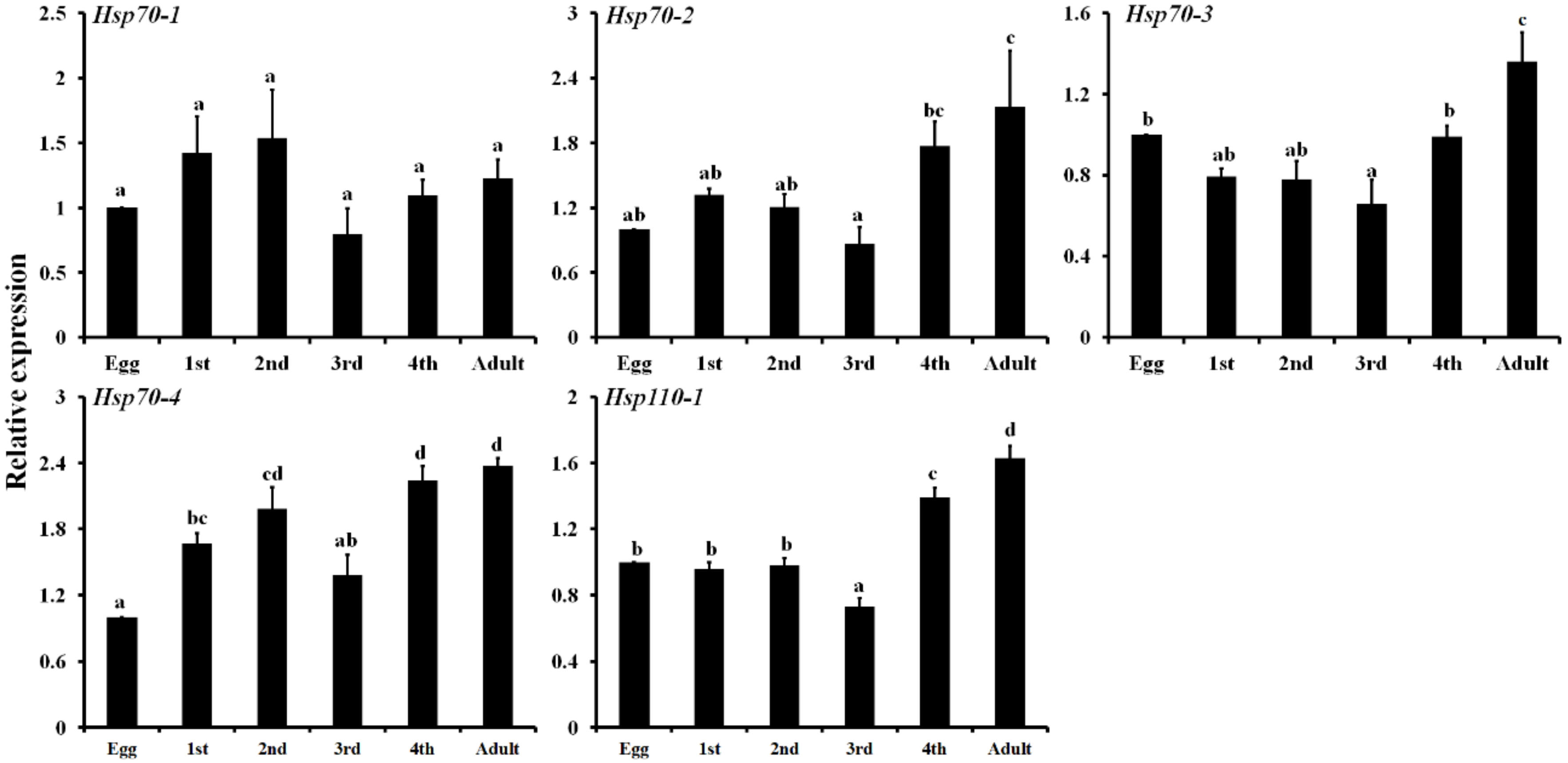
3.4. Expression Profiling of Five LbHsps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Wang, H.S.; Kang, L. Effect of cooling rates on the cold hardiness and cryoprotectant profiles of locust eggs. Cryobiology 2005, 51, 220–229. [Google Scholar] [CrossRef]
- Storey, K. Organic Solutes in Freezing Tolerance. Comp. Biochem. Physiol. A Physiol. 1997, 117, 319–326. [Google Scholar] [CrossRef]
- Franks, S.J.; Hoffmann, A.A. Genetics of climate change adaptation. Annu. Rev. Genet. 2012, 46, 185–208. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Liu, X.; Yu, W.B.; Tan, X.L.; Zhang, S.Z.; Tian, H.G.; Liu, T.X. The potential coordination of the heat shock proteins and antioxidant enzyme genes of Aphidius gifuensis in response to thermal stress. Front. Physiol. 2017, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.B.; Poupin, M.J.; Lardies, M.A. Plasticity of life-cycle, physiological thermal traits and Hsp70 gene expression in an insect along the ontogeny: Effect of temperature variability. J. Therm. Biol. 2011, 36, 355–362. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Huang, H.; Wang, J.J. Antioxidant responses of citrus red mite, Panonychus citri (McGregor) (Acari: Tetranychidae), exposed to thermal stress. J. Insect Physiol. 2010, 56, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Solangi, G.S.; Guo, J.; Wan, F.; Zhou, Z. Antioxidant responses of ragweed leaf beetle Ophraella communa (Coleoptera: Chrysomelidae) exposed to thermal stress. Front. Physiol. 2018, 9, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalouette, L.; Williams, C.M.; Hervant, F.; Sinclair, B.J.; Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Martemyanov, V.V.; Vorontsova, Y.L.; Rantala, M.J.; Gryzanova, E.V.; Glupov, V.V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.; Murhammer, D. Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef]
- Jia, F.X.; Dou, W.; Hu, F.; Wang, J.J. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2011, 94, 956–963. [Google Scholar] [CrossRef]
- Dubrez, L.; Causse, S.; Bonan, N.B.; Dumetier, B.; Garrido, C. Heat shock proteins: Chaperoning DNA repair. Oncogene 2020, 39, 516–529. [Google Scholar] [CrossRef]
- Parsell, D.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef]
- Zhao, L.J.; Jones, W.A. Expression of heat shock protein genes in insect stress responses. Invert. Surviv. J. 2012, 9, 93–101. [Google Scholar] [CrossRef]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, Y.; Liu, Y.; Zhao, H. Genome wide identification and analysis of heat-shock proteins 70/110 to reveal their potential functions in Chinese soft-shelled turtle Pelodiscus sinensis. Ecol. Evol. 2019, 9, 6968–6985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattoo, R.U.; Sharma, S.K.; Priya, S.; Finka, A.; Goloubinoff, P. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J. Biol. Chem. 2013, 288, 21399–21411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.M.; Eves-van den Akker, S.; Hawle, P.V.; Atkinson, H.J.; Urwin, P.E. Duplication of Hsp-110 is implicated in differential success of globodera species under climate change. Mol. Biol. Evol. 2018, 35, 2401–2413. [Google Scholar] [CrossRef] [Green Version]
- Nayak, M.K.; Collins, P.J.; Throne, J.E.; Wang, J.J. Biology and management of psocids infesting stored products. Annu. Rev. Entomol. 2014, 59, 279–297. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Tsai, J.H.; Zhao, Z.M.; Li, L.S. Development and reproduction of the psocid Liposcelis bostrychophila (Psocoptera: Liposcelididae) as a function of temperature. Ann. Entomol. Soc. Am. 2000, 93, 261–270. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Zhu, K.Y.; Opit, G.P.; Throne, J.E. Differential heat shock tolerance and expression of heat-inducible proteins in two stored-product psocids. J. Econ. Entomol. 2008, 101, 1974–1982. [Google Scholar] [CrossRef]
- Beckett, S.J.; Morton, R. The mortality of three species of Psocoptera, Liposcelis bostrychophila Badonnel, Liposcelis decolor Pearman and Liposcelis paeta Pearman, at moderately elevated temperatures. J. Stored Prod. Res. 2003, 39, 103–115. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.Y.; He, M.; Yun, Y.L.; Peng, Y. Antioxidant responses of the pest natural enemy Hylyphantes graminicola (Araneae: Linyphiidae) exposed to short-term heat stress. J. Therm. Biol. 2020, 87, 6. [Google Scholar] [CrossRef]
- Dou, W.; Shen, G.M.; Niu, J.Z.; Ding, T.B.; Wei, D.D.; Wang, J.J. Mining genes involved in insecticide resistance of Liposcelis bostrychophila Badonnel by transcriptome and expression profile analysis. PLoS ONE 2013, 8, e79878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of Quantitative Real-Time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rael, L.T.; Thomas, G.W.; Craun, M.L.; Curtis, C.G.; Bar-Or, R.; Bar-Or, D. Lipid peroxidation and the thiobarbituric acid assay: Standardization of the assay when using saturated and unsaturated fatty acids. J. Biochem. Mol. Biol. 2004, 37, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Park, M.S.; Jo, P.G.; Choi, Y.K.; An, K.W.; Choi, C.Y. Characterization and mRNA expression of Mn-SOD and physiological responses to stresses in the Pacific oyster Crassostrea gigas. Mar. Biol. Res. 2009, 5, 451–461. [Google Scholar] [CrossRef]
- Ali, A.; Rashid, M.A.; Huang, Q.Y.; Wong, C.; Lei, C.L. Response of antioxidant enzymes in Mythimna separata (Lepidoptera: Noctuidae) exposed to thermal stress. Bull. Entomol. Res. 2017, 107, 382–390. [Google Scholar] [CrossRef]
- Cai, P.M.; Wang, Y.; Yi, C.D.; Zhang, Q.W.; Xia, H.M.; Li, J.; Zhang, H.H.; Yang, J.Q.; Ji, Q.G.; Chen, J.H. Effects of temperature on the activity of antioxidant enzymes in larvae of Bactrocera dorsalis (Diptera: Tephritidae) parasitized by Diachasmimorpha longicaudata (Hymenoptera: Braconidae): Optimizing the mass rearing of this braconid by varying the temperature. Eur. J. Entomol. 2019, 116, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.H.; Liu, H.; Wang, J.J.; Wang, Z.Y. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of the predatory mite, Neoseiulus cucumeris (Acari: Phytoseiidae). Exp. Appl. Acarol. 2014, 64, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Du, Y.; Lu, M.; Qiang, C. Antioxidant responses of Chilo suppressalis (Lepidoptera: Pyralidae) larvae exposed to thermal stress. J. Therm. Biol. 2011, 36, 292–297. [Google Scholar] [CrossRef]
- Felton, G.W.; Duffey, S.S. Protective action of midgut catalase in lepidopteran larvae against oxidative plant defenses. J. Chem. Ecol. 1991, 17, 1715–1732. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G.; Menon, D. Glutathione transferases, regulators of cellular metabolism and physiology. BBA-Gen. Subj. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef]
- Jena, K.; Kumar Kar, P.; Kausar, Z.; Babu, C.S. Effects of temperature on modulation of oxidative stress and antioxidant defenses in testes of tropical tasar silkworm Antheraea mylitta. J. Therm. Biol. 2013, 38, 199–204. [Google Scholar] [CrossRef]
- Lu, K.; Chen, X.; Liu, W.; Zhang, Z.; Wang, Y.; You, K.; Li, Y.; Zhang, R.; Zhou, Q. Characterization of heat shock protein 70 transcript from Nilaparvata lugens (Stål): Its response to temperature and insecticide stresses. Pestic. Biochem. Phys. 2017, 142, 102–110. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, Y.; Bai, L.; Zhang, Y.; Xiao, Y.; Xiao, L.; Tan, Y.; Shen, Y. Characterizing heat shock protein 90 gene of Apolygus lucorum (Meyer-Dur) and its expression in response to different temperature and pesticide stresses. Cell Stress Chaperones 2014, 19, 725–739. [Google Scholar] [CrossRef] [Green Version]
- Mahroof, R.; Yan Zhu, K.; Neven, L.; Subramanyam, B.; Bai, J. Expression patterns of three heat shock protein 70 genes among developmental stages of the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 247–256. [Google Scholar] [CrossRef]
- Luo, S.; Ahola, V.; Shu, C.; Xu, C.; Wang, R. Heat shock protein 70 gene family in the Glanville fritillary butterfly and their response to thermal stress. Gene 2015, 556, 132–141. [Google Scholar] [CrossRef]
- Cheng, W.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef]
- Lu, M.X.; Li, H.B.; Zheng, Y.T.; Shi, L.; Du, Y.Z. Identification, genomic organization and expression profiles of four heat shock protein genes in the western flower thrips, Frankliniella occidentalis. J. Therm. Biol. 2016, 57, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Raviol, H.; Sadlish, H.; Rodriguez, F.; Mayer, M.P.; Bukau, B. Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. EMBO J. 2006, 25, 2510–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuermann, J.P.; Jiang, J.; Cuellar, J.; Llorca, O.; Wang, L.; Gimenez, L.E.; Jin, S.; Taylor, A.B.; Demeler, B.; Morano, K.A. Structure of the Hsp110: Hsc70 nucleotide exchange machine. Mol. Cell 2008, 31, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, T.; Barakat, A.Z.; Mohamed, B.A.; Paprotta, I.; Meinhardt, A.; Engel, W.; Adham, I.M. Heat-shock protein HSPA4 is required for progression of spermatogenesis. Reproduction 2011, 142, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Experiments | Primer Names | Primer Sequences (5′-3′) | Product Length | Efficiency (%) | R2 |
|---|---|---|---|---|---|
| Full-length confirmation | LbHsp70-1F | ATCGTCAGGATCATTTAGTGGCT | 2137 bp | - | - |
| LbHsp70-1R | CGTTTTCGCACAATAAATTCACG | ||||
| LbHsp70-2F | CGCGTTGTTAAAATATGGCTGCTC | 1541 bp | - | - | |
| LbHsp70-2R | TTACCCACCTCGTCAGGAAATGGA | ||||
| LbHsp70-3F | ATAGTTCCTGAGAAATTAACCGAA | 2012 bp | - | - | |
| LbHsp70-3R | TCCAGAAGAGGCGTTTAATCAACT | ||||
| LbHsp70-4F | TTAAAATATGATCCGTTATAGGAT | 1996 bp | - | - | |
| LbHsp70-4R | TCACCCTAATTTCAGTCTTTACAA | ||||
| LbHsp110-1F | GTGTGGGTGTTTTAATTTTGTTAT | 2532 bp | - | - | |
| LbHsp110-1R | AACGTTATTTATGATTTATTCTGT | ||||
| qPCR | q LbHsp70-1F | GGGAGAAGATGCCGATCCAG | 113 bp | 107.5 | 0.991 |
| q LbHsp70-1R | AACCTTGGTTTTCGCTTGCC | ||||
| q LbHsp70-2F | GAAGCTGCTGTTGGTGGAAA | 148 bp | 105.8 | 0.981 | |
| q LbHsp70-2R | CTGCTGCACATGGTTCATCA | ||||
| q LbHsp70-3F | TTGGGTGGAGAGGACTTCGA | 163 bp | 105.1 | 0.995 | |
| q LbHsp70-3R | TGCTGGCTTGAGTTGACGAT | ||||
| q LbHsp70-4F | GGGAAAGAACCGAGTCGAGG | 255 bp | 103.4 | 0.993 | |
| q LbHsp70-4R | GACTTGGATGGTGACGGTGT | ||||
| q LbHsp110-1F | GGGCAGGAGGAATCGAAACA | 102 bp | 109.7 | 0.995 | |
| q LbHsp110-1R | CGGCAGCTACGCCCATTATA | ||||
| β-actin-F | CACGGTATCGTCACCAACTG | 207 bp | 98.4 | 0.998 | |
| β-actin-R | AGACAATACGGCTTGGATGG |
| Temperature (°C) | MDA Concentration (nmol mg−1 Protein) | ||
|---|---|---|---|
| 2 h | 4 h | 8 h | |
| 27.5 | 0.795 ± 0.087 a 1 | 0.795 ± 0.087 a | 0.795 ± 0.087 a |
| 37.5 | 1.237 ± 0.147 a | 2.116 ± 0.295 b | 1.711 ± 0.085 a |
| 40 | 1.022 ± 0.107 a | 2.263 ± 0.376 b | 1.170 ± 0.498 a |
| 42.5 | 7.763 ± 0.332 b | 17.50 ± 0.263 c | 5.720 ± 1.088 b |
| 45 | 0.846 ± 0.159 a | 2.943 ± 0.289 b | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Z.Q.; Tu, Y.Q.; Guo, P.Y.; He, W.; Jing, T.X.; Wang, J.J.; Wei, D.D. Antioxidant Enzymes and Heat Shock Protein Genes from Liposcelis bostrychophila Are Involved in Stress Defense upon Heat Shock. Insects 2020, 11, 839. https://doi.org/10.3390/insects11120839
Miao ZQ, Tu YQ, Guo PY, He W, Jing TX, Wang JJ, Wei DD. Antioxidant Enzymes and Heat Shock Protein Genes from Liposcelis bostrychophila Are Involved in Stress Defense upon Heat Shock. Insects. 2020; 11(12):839. https://doi.org/10.3390/insects11120839
Chicago/Turabian StyleMiao, Ze Qing, Yan Qing Tu, Peng Yu Guo, Wang He, Tian Xing Jing, Jin Jun Wang, and Dan Dan Wei. 2020. "Antioxidant Enzymes and Heat Shock Protein Genes from Liposcelis bostrychophila Are Involved in Stress Defense upon Heat Shock" Insects 11, no. 12: 839. https://doi.org/10.3390/insects11120839
APA StyleMiao, Z. Q., Tu, Y. Q., Guo, P. Y., He, W., Jing, T. X., Wang, J. J., & Wei, D. D. (2020). Antioxidant Enzymes and Heat Shock Protein Genes from Liposcelis bostrychophila Are Involved in Stress Defense upon Heat Shock. Insects, 11(12), 839. https://doi.org/10.3390/insects11120839





