Effect of Pheromones, Plant Volatiles and Spinosad on Mating, Male Attraction and Burrowing of Cadra cautella (Walk.) (Lepidoptera: Pyralidae)
Abstract
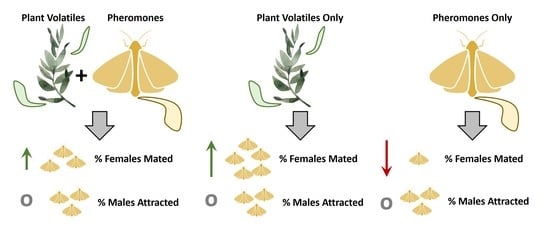
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Cultures
2.2. Experiment 1. Response of Cadra cautella to Sex Pheromone Components (Z, E)-9, 12-tetradecadienyl Acetate (ZETA) and (Z)-9-tetradecadien-1-yl Acetate (ZTA) in the Presence of Botanicals
2.2.1. Preparation of Adults
2.2.2. Cubicle Construction
2.2.3. Preparation of Pheromone Blend
2.2.4. Botanical Oils
2.2.5. Introduction of Botanical Oils and Pheromone Blend into the Cubicle
2.2.6. Introduction of Cadra cautella Moths to the Cubicle
2.2.7. Recording Environmental Conditions of Cubicles
2.2.8. Experimental Design and Data Analysis
2.3. Experiment 2. Burrowing of Cadra cautella Larvae in Different Flour Media Treated with Spinosad
2.3.1. Insects
2.3.2. Types of Flour and Insecticide Application
2.3.3. Introduction of Cadra cautella Larvae to Flour
2.3.4. Experimental Design and Data Analysis
3. Results
3.1. Experiment 1. Response of Cadra cautella to Sex Pheromone Components (Z, E)-9, 12-tetradecadienyl Acetate and (Z)-9-tetradecadien-1-yl Acetate in the Presence of Botanicals
3.2. Experiment 2. Burrowing of Cadra cautella Larvae in Different Types of Flours Treated with Spinosad
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248. 2017. Available online: https://www.un.org/development/desa/publications/world-population-prospects-the-2017-revision.html (accessed on 12 May 2020).
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijayaratne, L.K.W.; Rajapakse, R.H.S. Effects of spinosad on the heat tolerance and cold tolerance of Sitophilus oryzae L. (Coleoptera: Curculionidae) and Rhyzopertha dominica F. (Coleoptera: Bostrichidae). J. Stored Prod. Res. 2018, 77, 84–88. [Google Scholar] [CrossRef]
- Wijayaratne, L.K.W.; Fernando, M.D.; Palipane, K.B. Control of insect pests under ware- house conditions using smoke generated from partial combustion of rice (paddy) husk. J. Natl. Sci. Found. 2009, 37, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Dissanayaka, D.M.S.K.; Sammani, A.M.P.; Wijayaratne, L.K.W.; Samaranayaka, P.M.; Karunarathna, L.M.; Chandima, N.; Wijerathna, I.M.; Harshana, S.; Heshani, A.; Kalhari, D. Postharvest losses of agricultural commodities in Trincomalee, Sri Lanka. In Proceedings of the 12th International Working Conference on Stored Product Protection, Berlin, Germany, 11 October 2018; Adler, C.S., Opit, G., Furstenau, B., Muller-Blenkle, C., Kern, P., Arthur, F.H., Athanassiou, C.G., Bartosik, R., Campbell, J., Carvalho, M.O., et al., Eds.; Julius Kühn-Institut: Berlin, Germany, 2018; pp. 55–57. [Google Scholar]
- Kumari, J.M.P.; Wijayaratne, L.K.W.; Jayawardena, N.W.I.A.; Egodawatta, W.C.P. Quantitative and qualitative losses in paddy, maize and green gram stored under household conditions in Anuradhapura district of Sri Lanka. Sri Lankan J. Agri. Ecosys. 2020, 2, 99–106. [Google Scholar] [CrossRef]
- Sajeewani, P.A.H.; Karunarathne, E.V.U.P.; Wijerathne, K.B.T.T.; Mahalekam, M.P.S.; Rupasinghe, M.D.M.C.; Dissanayaka, D.M.S.K.; Wijayaratne, L.K.W.; Sammani, A.M.P. Abundance of insects in rice mills in Polonnaruwa, Sri Lanka. In Proceedings of the 12th International Working Conference on Stored Product Protection, Berlin, Germany, 11 October 2018; Adler, C.S., Opit, G., Furstenau, B., Muller-Blenkle, C., Kern, P., Arthur, F.H., Athanassiou, C.G., Bartosik, R., Campbell, J., Carvalho, M.O., et al., Eds.; Julius Kühn-Institut: Berlin, Germany, 2018; pp. 57–58. [Google Scholar]
- Sajeewani, P.A.H.; Dissanayaka, D.M.S.K.; Wijayaratne, L.K.W. Progeny production by Stegobium paniceum (L.) (Coleoptera: Anobiidae) (drugstore beetle) in different spices. Rajarata Univ. J. 2020, 5, 13–17. [Google Scholar]
- Wijayaratne, L.K.W.; Dissanayaka, D.M.S.K.; Sammani, A.M.P. Variation in Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) progeny adult emergence in different animal feed stored under ventilated and non-ventilated conditions. J. Stored Prod. Res. 2019, 84, 101516. [Google Scholar] [CrossRef]
- Adhikarinayake, T.B.; Palipane, K.B.; Muller, J. Quality change and mass loss of paddy during airtight storage in a ferro-cement bin in Sri Lanka. J. Stored Prod. Res. 2006, 42, 377–390. [Google Scholar] [CrossRef]
- Hill, D.S. Types of damage. In Pests of Stored Products and Their Control; Belhaven Press: London, UK, 1990. [Google Scholar]
- Arbogast, R.T.; Chini, S.R. Erratum to abundance of Plodia interpunctella (Hubner) and Cadra cautella (Walker) infesting maize stored on South Carolina farms: Seasonal and non-seasonal variation. J. Stored Prod. Res. 2005, 41, 528–543. [Google Scholar] [CrossRef] [Green Version]
- Hagstrum, D.W.; Subramanyam, B. Fundamentals of Stored-Product Entomology; AACC International: St Paul, MN, USA, 2006. [Google Scholar]
- Ahmed, M. Disinfestation of stored grains, pulses, dried fruits and nuts, and other dried foods. In Food Irradiation: Principles and Applications; Molins, R.A., Ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Savoldelli, S.; Süss, L. Integrated control of Ephestia cautella (Walker) in a confectionary factory. In Proceedings of the 10th International Working Conference on Stored-Product Protection (IWCSPP), Berlin, Germany, 27 June–2 July 2010; Carvalho, M.O., Fields, P.G., Adler, C.S., Arthur, F.H., Athanassiou, C.G., Campbell, J.F., Fleurat-Lessard, F., Flinn, P.W., Hodges, R.J., Isikber, A.A., et al., Eds.; Julius-Kühn-Archiv: Berlin, Germany, 2010; pp. 991–992. [Google Scholar]
- Burks, C.S.; Johnson, J.A. Biology, behavior, and ecology of stored fruit and nut insects. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 21–32. [Google Scholar]
- Edde, P.A.; Eaton, M.; Kells, S.A.; Phillips, T.W. Biology, behavior, and ecology of pests in other durable commodities. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 45–62. [Google Scholar]
- Mason, L.J.; McDonough, M. Biology, behavior, and ecology of stored grain and legume insects. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 7–20. [Google Scholar]
- Arthur, F.H.; Starkus, L.A.; McKay, T. Degradation and residual efficacy of beta-cyfluthrin as a surface treatment for control of Tribolium castaneum Herbst: Effects of temperature and environment. J. Stored Prod. Res. 2019, 84, 101514. [Google Scholar] [CrossRef]
- Ridley, A.W.; Burrill, P.R.; Cook, C.C.; Daglish, G.J. Phosphine fumigation of silo bags. J. Stored Prod. Res. 2011, 47, 349–356. [Google Scholar] [CrossRef]
- Fields, P.G. Comparison of efficacy of methyl bromide and sulfuryl fluoride fumigations in Canadian pasta plants. In Proceedings of the Ninth International Controlled Atmosphere and Fumigation in Stored Products, Antalya, Turkey, 15–19 October 2012; Navarro, S., Banks, H.J., Jayas, D.S., Bell, C.H., Noyes, R.T., Ferizli, A.G., Emekci, M., Isikber, A., Alagusundaram, K., Eds.; ARBER Professional Congress Services: Antalya, Turkey, 2012; pp. 215–221. [Google Scholar]
- Hwaidi, M.I.; Collins, P.J.; Sissons, M. Does sorption of sulfuryl fluoride by wheat reduce its efficacy against adults and eggs of Rhyzopertha dominica? J. Stored Prod. Res. 2017, 74, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Beckett, S.J. Insect and mite control by manipulating temperature and moisture before and during chemical-free storage. J. Stored Prod. Res. 2011, 47, 284–292. [Google Scholar] [CrossRef]
- Arthur, F.H.; Hartzer, K.L.; Throne, J.E.; Flinn, P. Susceptibility of Tribolium castaneum (Coleoptera: Tenebrionidae) and Trogoderma inclusum (Coleoptera: Dermestidae) to cold temperatures. J. Stored Prod. Res. 2015, 64, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Obeng-Ofori, D. The use of botanicals by resource poor farmers in Africa and Asia for the protection of stored agricultural products. Stewart Postharvest Rev. 2007, 6, 1–8. [Google Scholar] [CrossRef]
- Arthur, F.H. Grain protectants: Current status and prospects for the future. J. Stored Prod. Res. 1996, 32, 293–302. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored product insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Opit, G.P.; Phillips, T.W.; Aikins, M.J.; Hasan, M.M. Phosphine resistance in Tribolium castaneum and Rhyzopertha dominica from stored wheat in Oklahoma. J. Econ. Entomol. 2012, 105, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Wijayaratne, L.K.W.; Arthur, F.H.; Whyard, S. Methoprene and control of stored-product insects. J. Stored Prod. Res. 2018, 76, 161–169. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Larson, N.L.; Brabec, D.; Zhang, A. Methyl benzoate as a putative alternative, environmentally-friendly fumigant for the control of stored product insects. J. Econ. Entomol. 2019, 112, 2458–2468. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Bruce, A.; Wilkins, R.V.; Albin, C.E.; Arthur, F.H. Sanitation improves stored product insect pest management. Insects 2019, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Subramanyam, B.; Roesli, R. Inert dusts. In Alternatives to Pesticides in Stored-Product IPM; Subramanyam, B., Hagstrum, D.W., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 321–380. [Google Scholar]
- Morrison, W.R., III; Cullum, J.P.; Leskey, T.C. Evaluation of trap designs and deployment strategies for capturing Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2015, 108, 1683–1692. [Google Scholar] [CrossRef] [Green Version]
- Morrison, W.R., III; Lee, D.H.; Short, B.D.; Khrimian, A.; Leskey, T.C. Establishing the behavioral basis for an attract-and-kill strategy to manage the invasive Halyomorpha halys in apple orchards. J. Pest. Sci. 2016, 89, 81–86. [Google Scholar] [CrossRef]
- Ahmad, T.R. Effects of pheromone trap design and placement on capture of almond moth, Cadra cautella (Lepidoptera: Pyralidae). J. Econ. Entomol. 1987, 80, 897–900. [Google Scholar] [CrossRef]
- Ryne, C.; Ekeberg, M.; Jonzen, N.; Oehlschlager, C.; Lofstedt, C.; Anderbrant, O. Reduction in an almond moth Ephestia cautella (Lepidoptera: Pyralidae) population by means of mating disruption. Pest Manag. Sci. 2006, 62, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Plarre, R. More than a pest management tool—45 years of practical experience with insect pheromones in stored-product and material protection. J. Plant Dis. Protect. 2013, 120, 145–152. [Google Scholar] [CrossRef]
- Allison, J.; Cardé, R. Bidirectional selection for novel pheromone blend ratios in the almond moth, Cadra cautella. J. Chem. Ecol. 2007, 33, 2293–2307. [Google Scholar] [CrossRef] [PubMed]
- Read, J.S.; Haines, C.P. The functions of the female sex pheromones of Ephestia cautella (Walker) (Lepidoptera, Phycitidae). J. Stored Prod. Res. 1976, 12, 49–53. [Google Scholar] [CrossRef]
- Prevett, P.F.; Benton, F.P.; Hall, D.R.; Hodges, R.J.; dos Serodio, S.R. Suppression of mating in Ephestia cautella (Walker) (Lepidoptera: Phycitidae) using microencapsulated formulations of synthetic sex pheromone. J. Stored Prod. Res. 1989, 25, 147–154. [Google Scholar] [CrossRef]
- Pease, G.; Storm, C.G. Efficacy of pheromone-based control system, Exosex™ SPTab, against moth pests in European food processing facilities. In Proceedings of the 10th International Working Conference on Stored-Product Protection (IWCSPP), Berlin, Germany, 27 June–2 July 2010; Carvalho, M.O., Fields, P.G., Adler, C.S., Arthur, F.H., Athanassiou, C.G., Campbell, J.F., Fleurat-Lessard, F., Flinn, P.W., Hodges, R.J., Isikber, A.A., et al., Eds.; Julius-Kühn-Archiv: Berlin, Germany, 2010; pp. 183–189. [Google Scholar]
- Sammani, A.M.P.; Dissanayaka, D.M.S.K.; Wijayaratne, L.K.W.; Morrison, W.R., III. Effects of spinosad and spinetoram on larval mortality, adult emergence, progeny production and mating in Cadra cautella (Walk.) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2020, 88, 101665. [Google Scholar] [CrossRef]
- Rajapakse, R.H.S. Effect of five botanicals as protectants of green gram against the pulse beetle Callosobruchus maculatus. In Proceedings of the Second International Symposium on Bruchids and Legumes, Okayama, Japan, 1 July 1990; Fujii, K., Gatehouse, A.M.R., Johnson, C.D., Mitchel, R., Yoshida, T., Eds.; Kluwer Academic Publishers: Okayama, Japan, 1990; pp. 85–90. [Google Scholar]
- Rajapakse, R.H.S.; Van Emden, H.F. Potential of four vegetable oils and ten botanical powders for reducing infestation of cowpeas by Callosobruchus maculatus, C. Chinensis and C. rhodesianus. J. Stored Prod. Res. 1997, 33, 59–68. [Google Scholar] [CrossRef]
- Rajapakse, R.H.S. Pesticidal potential of tropical plants-insecticidal activity of some selected natural products against Callosobruchus maculatus and C. chinensis. Proc. Entomo. Congress, Trivandrum India. 2000, 11, 69–71. [Google Scholar]
- Belmain, S.R.; Neal, G.E.; Ray, D.E.; Golob, P. Insecticidal and vertebrate toxicity associated with ethno botanicals used as post-harvest protectants in Ghana. Food Chem. Toxicol. 2001, 39, 287–291. [Google Scholar] [CrossRef]
- Said, P.; Pashte, V. Botanicals: The protectants of stored grains pests. Trends Biosci. 2015, 8, 3750–3755. [Google Scholar]
- Lal, M.; Ram, B.; Tiwari, P. Botanicals to cope stored grain insect pests: A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- Dissanayaka, D.M.S.K.; Sammani, A.M.P.; Wijayaratne, L.K.W. Food oils as kairomones for trapping Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) adults. J. Stored Prod. Res. 2018, 79, 83–88. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, A. Control of insect pests in crop plants and stored food grains using plant saponins: A review. LWT Food Sci. Technol. 2018, 87, 93–101. [Google Scholar] [CrossRef]
- Allotey, J.; Goswami, L. Damage caused and control of the moths Plodia interpunctella (Hubn.) and Ephestia cautella (Wlk.) on maize and groundnuts using local plant materials. Insect Sci. Appl. 1994, 15, 323–329. [Google Scholar] [CrossRef]
- Rajendran, S. Insect infestation and control in stored grain sorghum and millets. Int. J. Food Sci. Tech. 2003, 40, 451–457. [Google Scholar]
- Klerkx, L.; van Mierlo, B.; Leeuwis, C. Evolution of systems approaches to agricultural innovation: Concepts, analysis and interventions. In Farming Systems Research into the 21st Century; Darnhofer, I., Gibbon, D., Dedieu, B., Eds.; The New Dynamic, Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Rentokil. Common Species of Pests in Food© 2020 Rentokil Initial plc. 2020. Available online: https://www.rentokil.tt/pests-in-food/species (accessed on 25 April 2020).
- Subramanyam, B.; Hartzer, M.; Boina, D.R. Performance of pre-commercial release formulations of spinosad against five stored-product insect species on four stored commodities. J. Pest Sci. 2012, 85, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Athanassiou, C.G.; Kavallieratos, N.G.; Yiatilis, A.E.; Vayias, B.J.; Mavrotas, C.S.; Tomanovic, Z. Influence of temperature and humidity on the efficacy of spinosad against four stored-grain beetle species. J. Insect Sci. 2008, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hertlein, M.B.; Thompson, G.D.; Subramanyam, B.; Athanassiou, C.G. Spinosad: A new natural product for stored grain protection. J. Stored Prod. Res. 2011, 47, 131–146. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.K.; Sammani, A.M.P.; Wijayaratne, L.K.W. Orientation of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) adults at various distances to different concentrations of aggregation pheromone 4,8-dimethyldecanal. J. Stored Prod. Res. 2020, 87, 101631. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.K.; Sammani, A.M.P.; Wijayaratne, L.K.W. Response of different population sizes to traps and effect of spinosad on the trap catch and progeny adult emergence in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2020, 86, 101576. [Google Scholar] [CrossRef]
- Zhu, J.W.; Ryne, C.; Unelius, C.R.; Valeur, P.G.; Lofstedt, C. Reidentification of the female sex pheromone of the Indian meal moth, Plodia interpunctella: Evidence for a four-component pheromone blend. Entomol. Exp. Appl. 1999, 92, 137–146. [Google Scholar] [CrossRef]
- Wijayaratne, L.K.W.; Burks, C.S. Persistence of mating suppression of the Indian Meal Moth Plodia Interpunctella in the presence and absence of commercial mating disruption dispensers. Insects 2020, 11, 701. [Google Scholar] [CrossRef]
- Ryne, C.; Svensson, G.P.; Lofstedt, C. Mating disruption of Plodia interpunctella in small-scale plots: Effects of pheromone blend, emission rates and population density. J. Chem. Ecol. 2001, 27, 2109–2124. [Google Scholar] [CrossRef]
- Drummond, B.A. Multiple mating and sperm competition in the Lepidoptera. In Sperm Competition; Smith, R.L., Ed.; Academic Press: London, UK, 1984; pp. 291–370. [Google Scholar]
- Mafra–Neto, A.; Baker, T.C. Timed, metered sprays of pheromone disrupt mating of Cadra cautella (Lepidoptera: Pyralidae). J. Agric. Entomol. 1996, 13, 149–168. [Google Scholar]
- Vayias, B.J.; Athanassiou, C.G.; Milonas, D.N.; Mavrotas, C. Persistence and efficacy of spinosad on wheat, maize and barley grains against four major stored product pests. J. Crop Prot. 2010, 29, 496–505. [Google Scholar] [CrossRef]
- SAS Institute. The SAS system for windows, Release 9.1; Statistical Analysis System Institute: Cary, NC, USA, 2002–2008. [Google Scholar]
- Sakuma, M. Virtual reality experiments on a digital servosphere: Guiding male silkworm moths to a virtual odour source. Comput. Electron. Agric. 2002, 35, 243–254. [Google Scholar] [CrossRef]
- Willis, M.A.; Avonder, J.L.; Zheng, E. The role of vision in odor-plume tracking by walking and flying insects. J. Exp. Biol. 2011, 214, 4121–4132. [Google Scholar] [CrossRef] [Green Version]
- Nansen, C.; Phillips, T.W. Ovipositional responses of the Indianmeal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) to oils. Ann. Entomol. Soc. Am. 2003, 96, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Hendrikse, A.; Vos-Bunnemeyer, E. Role of host-plant stimuli in sexual behaviour of small ermine moths (Yponomeuta). Ecol. Entomol. 1987, 12, 363–371. [Google Scholar] [CrossRef]
- Landolt, P.J.; Reed, H.C.; Heath, R.R. Attraction of female papaya fruit fly (Diptera: Tephritidae) to male pheromone and host fruit. Environ. Entomol. 1992, 21, 1154–1159. [Google Scholar] [CrossRef]
- Nakamuta, K.; Leal, W.S.; Nakashima, T.; Tokoro, M.; Ono, M.; Nakanishi, M. Increase of trap catches by a combination of male sex pheromones and floral attractant in longhorn beetle, Anaglyptus subfasciatus. J. Chem. Ecol. 1997, 23, 1635–1640. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. J. Agric. Food Chem. 2000, 48, 6025–6029. [Google Scholar] [CrossRef]
- Hayes, J.L.; Strom, B.L.; Roton, L.M.; Ingram, L.L. Repellent properties of the host compound4-allylanisole to the southern pine beetle. J. Chem. Ecol. 1994, 20, 1595–1615. [Google Scholar] [CrossRef]
- Dickens, J.C.; Billings, R.F.; Payne, T.L. Green leaf volatiles interrupt aggregation pheromone response in bark beetles infesting southern pines. Experientia 1992, 48, 523–524. [Google Scholar] [CrossRef]
- Poland, T.M.; Borden, J.H.; Stock, A.J.; Chong, L.J. Green leaf volatiles disrupt responses by the spruce beetle, Dendroctonus rufipennis, and the western pine beetle Dendroctonus brevicomis (Coleoptera: Scolytidae) to attractant-baited traps II. J. Entomol. Soc. B.C. 1998, 95, 17–24. [Google Scholar]
- Cha, D.H.; Adams, T.; Rogg, H. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila szuukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Bell, C.H. Insect and mite penetration and contamination of packaged foods. In Food and Beverage Stability and Shelf Life; Woodhead Publishing: Sawston, UK, 2011; pp. 106–131. [Google Scholar]
- Mullen, M.A.; Vardeman, J.M.; Bagwell, J. Fumigation. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 157–178. [Google Scholar]
- Cline, L.D. Clinging and climbing ability of larvae of eleven species of stored-product insects on nine flexible packaging materials and glass. J. Econ. Entomol. 1978, 71, 689–691. [Google Scholar] [CrossRef]
- Cline, L.D. Penetration of seven common flexible packaging materials by larvae and adults of eleven species of stored-product insects. J. Econ. Entomol. 1978, 71, 726–729. [Google Scholar] [CrossRef]
- Bowditch, T.G. Penetration of polyvinyl chloride and polypropylene packaging films by Ephestia cautella (Lepidoptera: Pyralidae) and Plodia interpunctella (Lepidoptera: Pyralidae) larvae, and Tribolium confusum (Coleoptera: Tenebrionidae) adults. J. Econ. Entomol. 1997, 90, 1028–1031. [Google Scholar] [CrossRef]
- Shinoda, K.; Tanaka, S.; Yoshida, T.; Nakasuji, F. Penetration of polyethylene film for packaging by larvae of Indian meal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) and their dispersal. Jpn. J. Appl. Entomol. Z. 1990, 2, 128–132. [Google Scholar]
- Scheff, D.S.; Sehgal, B.; Subramanyam, B. Evaluating Penetration Ability of Plodia interpunctella (Hubner) (Lepidoptera: Pyralidae) Larvae into Multilayer Polypropylene Packages. Insects 2018, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, H. Experimental invasion of a food container by first-instar larvae of the Indian meal moth, Plodia internpuctella Hübner, through pinholes. Med. Entomol. Zool. 1998, 49, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Scheff, D.S.; Subramanyam, B.; Arthur, F. Effect of methoprene treated polymer packaging on fecundity, egg hatchability, and egg-to-adult emergence of Tribolium castaneum and Trogoderma variabile. J. Stored Prod. Res. 2016, 69, 227–234. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammani, A.M.P.; Dissanayaka, D.M.S.K.; Wijayaratne, L.K.W.; Bamunuarachchige, T.C.; Morrison, W.R., III. Effect of Pheromones, Plant Volatiles and Spinosad on Mating, Male Attraction and Burrowing of Cadra cautella (Walk.) (Lepidoptera: Pyralidae). Insects 2020, 11, 845. https://doi.org/10.3390/insects11120845
Sammani AMP, Dissanayaka DMSK, Wijayaratne LKW, Bamunuarachchige TC, Morrison WR III. Effect of Pheromones, Plant Volatiles and Spinosad on Mating, Male Attraction and Burrowing of Cadra cautella (Walk.) (Lepidoptera: Pyralidae). Insects. 2020; 11(12):845. https://doi.org/10.3390/insects11120845
Chicago/Turabian StyleSammani, Abeysinghe M. P., Dissanayaka M. S. K. Dissanayaka, Leanage K. W. Wijayaratne, Thushara C. Bamunuarachchige, and William R. Morrison, III. 2020. "Effect of Pheromones, Plant Volatiles and Spinosad on Mating, Male Attraction and Burrowing of Cadra cautella (Walk.) (Lepidoptera: Pyralidae)" Insects 11, no. 12: 845. https://doi.org/10.3390/insects11120845
APA StyleSammani, A. M. P., Dissanayaka, D. M. S. K., Wijayaratne, L. K. W., Bamunuarachchige, T. C., & Morrison, W. R., III. (2020). Effect of Pheromones, Plant Volatiles and Spinosad on Mating, Male Attraction and Burrowing of Cadra cautella (Walk.) (Lepidoptera: Pyralidae). Insects, 11(12), 845. https://doi.org/10.3390/insects11120845