Simple Summary
The juvenile hormones (JHs) play critical roles during insect development and reproduction. The numerous effects of JHs have generated multiple basic scientific questions, as well as prospects for the development of insecticidal endocrine disruptors. There is an increasing need for methods to identify and quantify endogenous JHs. The low titers and difficulties in working with these lipophilic compounds have often hindered the study of JH biology. In this article, we review the existing information on the detection and quantification of JH from insect samples, the development of approaches to manipulate JH titers, and the use of next-generation tools to modulate JH homeostasis.
Abstract
The juvenile hormones (JHs) are a group of sesquiterpenoids synthesized by the corpora allata. They play critical roles during insect development and reproduction. To study processes that are controlled by JH, researchers need methods to identify and quantify endogenous JHs and tools that can be used to increase or decrease JH titers in vitro and in vivo. The lipophilic nature of JHs, coupled with the low endogenous titers, make handling and quantification challenging. JH titers in insects can easily be increased by the topical application of JH analogs, such as methoprene. On the other hand, experimentally reducing JH titers has been more difficult. New approaches to modulate JH homeostasis have been established based on advances in RNA interference and CRISPR/Cas9-based genome editing. This review will summarize current advances in: (1) the detection and quantification of JHs from insect samples; (2) approaches to manipulating JH titers; and (3) next-generation tools to modulate JH homeostasis.
1. Introduction
Juvenile hormones (JHs), synthesized by the minuscule corpora allata glands (CA), are critical regulators of insect development and reproduction. Multiple processes are controlled by JHs, including the inhibition of metamorphosis, caste determination and differentiation, stimulation of flight and migration, regulation of reproduction, control of diapause, stress resistance and immunity, as well as aging and senescence [1,2,3]. The pleiotropic effects of JH have presented a range of intriguing basic scientific questions and opportunities in an applied context as insecticidal endocrine disruptors [4,5,6]. As with other sesquiterpenoid hormones, JHs are relatively non-polar, lipophilic, insoluble in aqueous solutions and fragile, making them difficult to physically manipulate in a laboratory setting. In vivo titers generally operate in the femtomole to picomole range. The combination of low titers and challenges in physical extraction and recovery have made JHs a notoriously difficult arena for basic and applied research [7]. In this article, we review the current knowledge on the detection and quantification of JHs from insect samples, the development of approaches to manipulate JH titers, and the use of next-generation tools to modulate JH homeostasis.
2. Detection and Quantification of JH from Insect Samples
Seven different epoxidated JH homologs and the non-epoxidated methyl farneosate (MF) have been described, with one or more of these sesquiterpenoids detected in more than 100 insect species [3]. All these JH homologs share physical properties that hinder their isolation and quantification [7]. Biological samples containing JHs have included whole-body extracts, hemolymph, and culture media from in vitro incubated CA preparations. From these samples, JH has been quantified using bioassays, radioimmunoassays, radiochemical assays, and physicochemical assays [7]. All JH homologs share structural features, which offer advantages and disadvantages when identifying and quantifying them. Bioassays, radioimmunoassays, and radiochemical assays measure total JH activity but often do not recognize individual homologs. Physicochemical assays are the most sensitive and informative methods. Gas chromatography (GC) coupled with mass spectrometry (MS) and high-performance liquid chromatography-MS (HPLC-MS) are the best-established protocols [8,9].
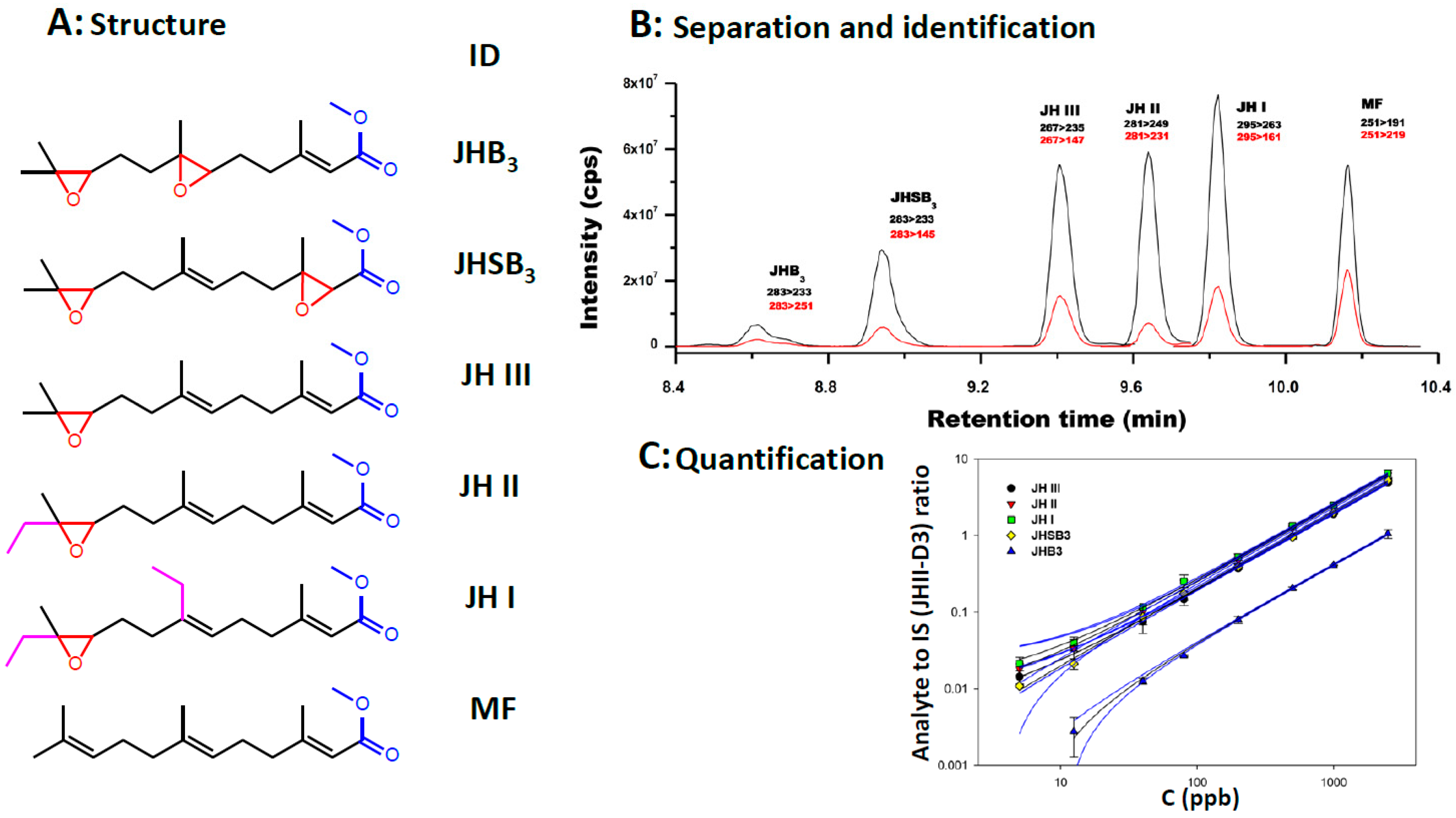
The shared structural features of the JH homologs facilitated the establishment of a robust and sensitive HPLC-MS/MS (tandem MS) method. It allows the simultaneous analysis of the five most common epoxidated JHs, including JH I, JH II, JH III, JH III bisepoxide (JHB3) and JH III skipped bisepoxide (JHSB3), as well as MF [10] (Figure 1). The protocol detects JHs in the low femtomole range (pg/mL) often allowing the analysis of JH in individual insects. When an individual species of JH homolog is fragmented, it produces a unique diagnostic set of ions that enables its identification and quantification. This approach has allowed the analysis of JHs in a phylogenetically and morphologically diverse range of individual insects. This method even led to the recognition that JHSB3 is the original homolog that Sir Vincent B. Wigglesworth described for the kissing-bug Rhodnius prolixus [11] and remained unidentified since 1934 [12]. Looking forward, new developments in HPLC-MS/MS and the advent of more affordable benchtop equipment should make it even easier to quantify JHs from insect samples.
Figure 1.
HLPC-MS/MS analysis of JH homologs. (A) Chemical structures of JH homologs: JHB3: juvenile hormone III bisepoxide. JHSB3: juvenile hormone III skipped bisepoxide. JH III: juvenile hormone III. JH II: juvenile hormone II. JH I: juvenile hormone I. MF: methyl farnesoate. Epoxide groups are in red. Methyl esters groups are in blue. Ethyl groups are in magenta. (B) HPLC separation of JH homologs: Typical LC-MS/MS peaks of JH homologs and MF. It shows the relationships between the retention times in minutes (x-axis) and the signal intensity (cps; counts per second) (y-axis). Injected mass was 125 pg (325 pg for MF and 32 pg for the internal standard –ISTD-). Retention times are in minutes. Black lines represent the signal intensity of the primary fragment used, and red lines represent the intensity of the secondary ion. (C) Standard curves for the quantification of JHs: relationships between the concentration of each of the five JH standards in parts per billions (ppb) (X-axis), and the signal intensities expressed as the ratio between the JH standard and the internal standard (IS, JH III-D3) (Y axis). JH III (black circle). JH II (red inverted triangle). JH I (green square), JHSB3 (yellow diamond) and JHB3 (blue triangle). The blue lines represent the 95% confidence bands, depicting the upper and lower confidence bounds for all points on a fitted line within the range of data.
3. Approaches to Modulate Endogenous JH Titers
JH titers are finely controlled, and alterations of JH homeostasis can interfere with a wide variety of biological functions including development, molting, and reproduction. Insect growth regulators (IGRs) are natural or synthetic compounds that interfere with JH homeostasis [13]. These compounds either decrease JH biosynthesis and transport, increase JH catabolism, or interfere with other elements of the JH signaling pathway [5,6]. In addition, JH analogs (JHA), such as methoprene, fenoxycarb, and pyriproxyfen, are functional mimics of the endogenous JHs that increase JH signaling, preventing metamorphosis [14] or interfering with normal reproduction [6,15] (Figure 2A). JHs act as an agonist by binding to the JH receptor [16,17]. This methoprene-tolerant (Met) protein is conserved in all insects that have been investigated and has a paralog in Drosophila melanogaster (Gce) [18]. Met is an intracellular receptor in the basic HLH (helix–loop–helix)—(bHLH–PAS) family of transcriptional regulators. All known JH homologs and JHAs operate by interacting with this unique Met protein [17,18].
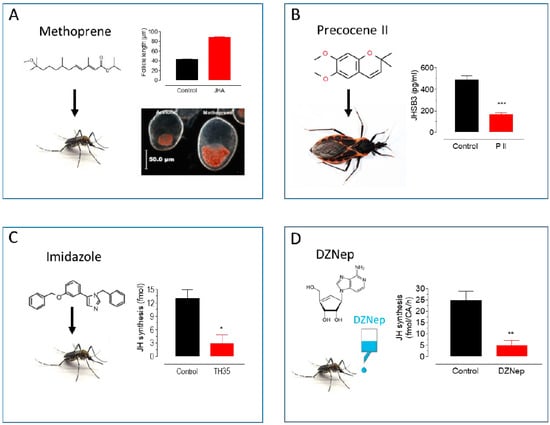
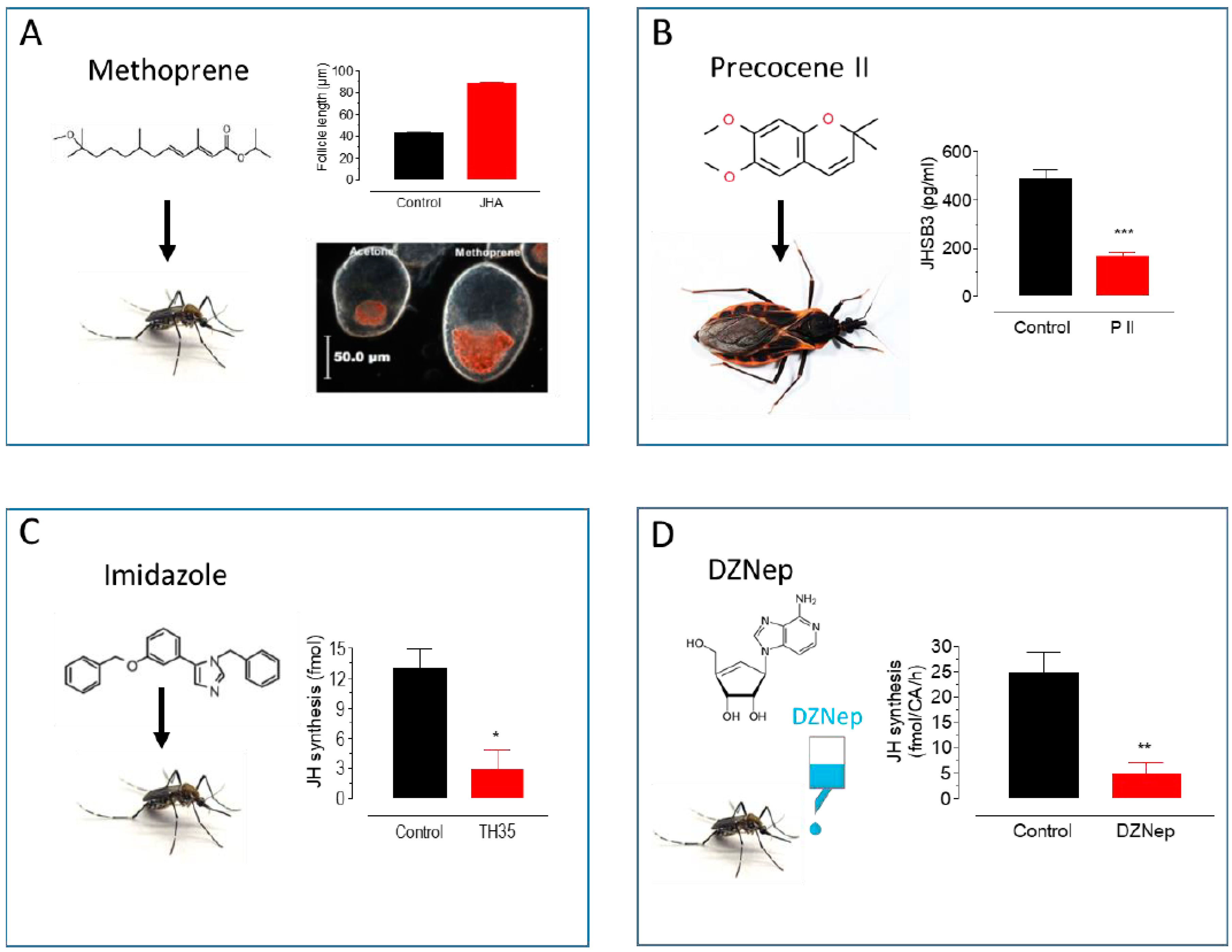
Figure 2.
Approaches to modulate endogenous JH titers. (A) Methoprene topical application: Methoprene stimulates follicle development and lipid accumulation in Ae. aegypti females [20]. (B) Precocene II topical application: P II decreases JHSB3 titers in Dipetalogaster maxima [35]. (C) Imidazole treatment: Imidazoles inhibit JH synthesis in Ae. aegypti females [30]. (D) DZNep in vivo treatment: DZNep added to the sugar inhibits JH synthesis in Ae. aegypti females [34]. * p < 0.05, ** p < 0.01, *** p < 0.001.
In addition to their utility as insecticides, synthetic JH mimics are essential research tools to study the role of JH signaling in vivo and in vitro systems [6]. For example, JH signaling can be easily increased with the topical application of methoprene [19,20]. The JH analogs are more stable than natural JHs, and are highly active in a broader spectrum of insects. This feature is useful when trying to assess whether JH signaling is important in controlling the physiological process under study. On the other hand, the relative molecular stability of JH analogs becomes a problem when studying processes that require only temporary increases in JH titer; for instance, treating mosquito or Drosophila larvae with JHA results in lethality, preventing pupae from reaching adulthood [21,22].
In contrast, deliberately reducing JH titers has proven more difficult. However, some efficient anti-juvenile hormones compounds have been described. Precocenes are strong inhibitors of CA activity (anti-allatotropins) in several species of insects [23,24]. Precocenes have anti-JH effects by causing necrosis of the CA (pro-allatocidins) [25] (Figure 2B). An alternative approach is to modulate hormonal catabolism. JH degradation by JH esterase (JHE) plays an important role in lowering JH titers [1]. JHE over-expression in early larval instars has been shown to cause precocious metamorphosis through the elimination of JH [26]. In addition, recombinant JHE (rJHE), injected in vivo, has been employed as an anti-JH agent in several insects [27,28,29]. Alternatively, imidazole-containing compounds are potent anti-JH compounds [30]. They effectively inhibited JH biosynthesis by the CA of the cockroach Diploptera punctata. Imidazoles were also effective inhibitors of purified cockroach epoxidase (CYP15A1) in the conversion of MF to JH III [31]. Imidazoles are also strong inhibitors of JH synthesis in Diptera as well [32] (Figure 2C).
The modulation of JH biosynthesis can also modify JH titers. A critical enzyme in JH synthesis is juvenile hormone acid methyltransferase (JHAMT) [33]. An inhibitor of JHAMT is 3-deazaneplanocin A (DZNep), which inhibits JH synthesis in vitro by the CA of female adult Aedes aegypti in a dose-response fashion [34]. In vivo experiments, with the addition of DZNep to the sugar ingested by mosquitoes, also resulted in a dose-response decrease in JH synthesis and JH hemolymph titers (Figure 2D), as well as a decrease in the expression of early trypsin, a JH-dependent gene. These results suggest that DZNep can lower JH synthesis and titer in experiments evaluating JH-controlled processes in insects.
4. Next-Generation Tools to Modulate JH Homeostasis
Changes in JH homeostasis can be generated by modulating the expression of many different critical genes involved in diverse aspects of JH biology (Figure 3). Reducing gene expression can be accomplished with RNA interference (RNAi), while enhancing gene expression can be done by driving ectopic genes with tissue-specific promoters in a transgenic context. Gene editing has become a highly efficient method to alter insect genomes. It can be used to either express genes with known JH-modulatory effects or to knock out genes playing critical roles in modulating JH titers or in JH signaling pathways.
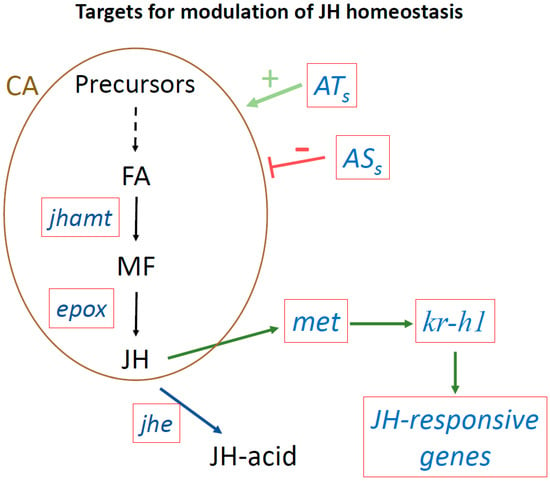
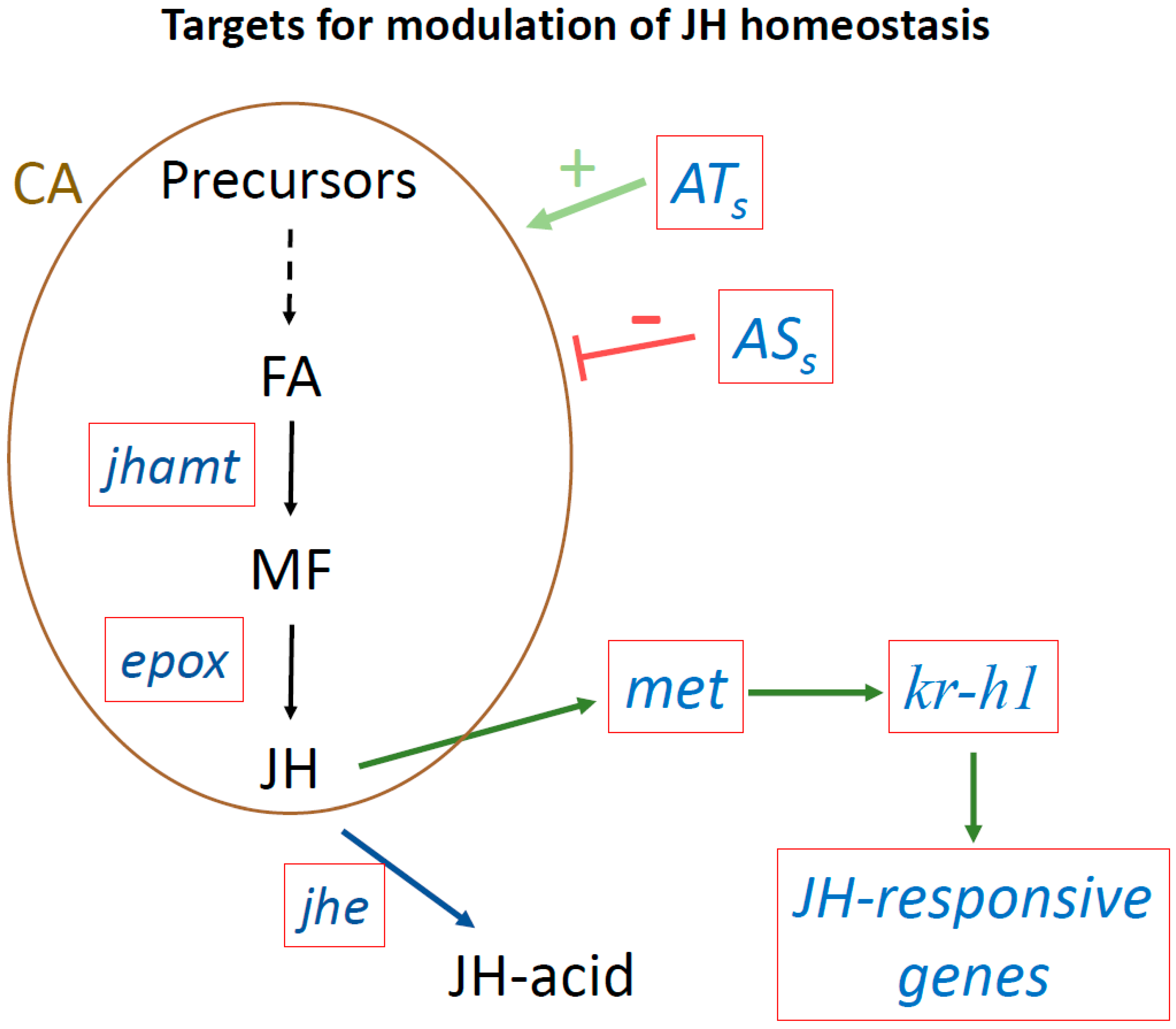
Figure 3.
Targets to modulate JH homeostasis using next generation tools. Targeting genes playing critical roles modulating JH titers or JH signaling. Red boxes show target genes. Juvenile acid methyl transferase (jhamt) transforms farnesoic acid (FA) into methyl farneosate (MF). Epoxidase (epox) converts MF into JH. JH esterase (jhe) converts JH into JH-acid. The met gene encodes the JH receptor. Krüppel-homolog 1 (Kr-h1) is as an early inducible gene in the JH signaling pathway, that activates the transcription of many JH-inducible genes. ATs, allatotropins that stimulate JH biosynthesis; ASs, allatostatins that inhibit JH synthesis; CA, corpora allata.
In insects, RNAi generally causes loss-of-function phenotypes by the degradation of specific transcripts, though it may also exert translational inhibition. It is an efficient and simple reverse functional genomics approach widely used to study the actions of insect genes [36]. This gene knock-down technique allows insect’s loss-of-function (LOF) analyses without creating null mutants. Novel RNAi delivery systems, including ingestion, injection, cuticular penetration, delivery by microbes, and the use of nanoparticles, are facilitating the use of RNAi in insect’s research and control [37]. The RNAi effect acts systemically or with tissue-specificity if driven by transgenes [38,39]. RNAi has been a valuable technique in different areas of JH research. RNAi knock-down of Met in the beetle Tribolium castaneum produced precocious metamorphosis phenotypes consistent with disrupted JH signaling, thus helping to establish the identity of the JH receptor [40]. Later, the study of Drosophila Met-Gce double mutants provided conclusive genetic evidence that definitively confirmed the JH receptor role of Met-Gce [18]. A yeast-derived Gal4-UAS system, achieving temporal and tissue specific control, was used to validate that ecdysis-triggering hormone (ETH) functions as an allatotropin, inducing JH synthesis by the CA of Drosophila [38,39]. RNAi constructs targeting the ETH receptor gene (ethr) were introduced into the genome using the CA-specific driver jhamt-GAL4, resulting in a marked reduction (>70%) of JH levels [38]. Meanwhile, cell-specific Gal4 driver-mediated expression of pro-apoptotic genes in Inka cells (the cells that produce ecdysis triggering hormone) also resulted in a significant decrease in JH signaling [38,39].
Krüppel-homolog 1 (Kr-h1) is an early inducible gene in the JH signaling pathway downstream of the Met receptor in insects [41]. RNAi has been successfully used to validate the roles of the Met receptor and Kr-h1 in JH signaling in R. prolixus. Premature depletion of Kr-h1 [42] or Met [43] triggered precocious adult development. Modulation of Kr-h1 expression by RNAi has often been used to investigate JH/Met-mediated control of gene activity [44]. Moreover, the study of the expression of JH-regulated genes is a reliable and straightforward approach to validate the putative role of JH in modulating physiological or behavioral processes. In mosquitoes, the early trypsin mRNA, a well-known JH-dependent gene [45], has been widely used as a model for JH signaling studies [46], as well as a marker for JH titers. A study has described an early-trypsin-GAL4 > UAS-enhanced green fluorescent protein (EGFP) system to monitor JH signaling [47]. The system revealed that in early-trypsin GAL4 > UAS-EGFP female mosquitoes, the intensity of the midgut-specific EGFP signal was significantly and strongly correlated to the early trypsin gene transcript levels, as well the JH titer; probing the usefulness of using the expression of this gene as a marker for JH-signaling activation [47].
In many insect systems, RNAi does not always mediate efficient gene knock-down, and sometimes mRNA levels are reduced by less than 75% [48]. Residual gene expression can mask phenotypes in RNAi experiments, which complete loss-of function alleles induced by mutagenesis might uncover. In this context, CRISPR/Cas9-based editing approaches offer a more clearly defined tool for manipulating gene expression in insects [49]. Highly efficient and specific knockout of genes can be achieved by injecting embryos with an in vitro-synthesized single guide RNA (sgRNA) and Cas9 mRNA or protein [50,51].
In Drosophila, precise temporal-spatial activation of the expression of transgenes that modulate JH titers or signaling can be achieved using binary CRISPR/Cas9 systems. These systems utilize a driver line to promote high-level, tissue- or developmental stage-specific expression of the “JH-modulatory” gene in a responder line. Spatial and temporal control of gene manipulation in Drosophila has been achieved via drug-activated Cas9 nucleases [52]. Drug-inducible CRISPR/Cas9 systems permit the study of genes at later stages where early lethality is a problem; combined with tissue-specific expression of Cas9 or sgRNAs, it results in spatiotemporal control [52]. Genome editing with “first-generation methods” such as TALENs has been successfully employed to knock out genes playing critical roles modulating JH titers or JH signaling. For example, this method was used to produce a knockout mutant of the rate-limiting JH acid methyl transferase (jhamt) and the two JH receptor genes (Met1 and Met2) in the silkworm Bombyx mori [53]. Experiments with these Bombyx null mutants revealed that the larval status can be maintained by a JH independent mechanism in very early larval instars [53]. CRISPR/Cas9- generated null mutants of juvenile hormone esterase showed extended larval growth in Bombyx [54]. Similarly, the knockout of jhamt resulted in a significant reduction in fecundity in Drosophila [55]. Using CRISPR/Cas9 gene editing, a study with Ae. aegypti mosquitoes showed that silencing the JH receptor (Met) has effects similar to those attributable to a drop in JH titer [56]. The functions of the juvenile hormone binding protein (mJHBP) have been studied in an mJHBP-deficient null mutant Ae. aegypti mosquito; the studies revealed that JH functions in mosquito immunity and hemocyte development in a manner that is perhaps independent of canonical JH signaling [57].
5. Conclusions
The study of different aspects of JH biology has been traditionally hampered by the difficulty of working with this lipophilic hormone, elusive in low physiological concentrations. In this review, we summarized some of the traditional and novel tools to study the roles of JH in controlling insect biology. Mass-spectrometry methods represent the gold standard for the detection and quantification of JH from insect samples, providing fast, simple, accurate, ultra-trace quantitation of all major JH homologs. The ability to modulate endogenous JH titers, accomplished using JH analogs or JH-synthesis modifying drugs, now can be more efficiently attained using next-generation strategies. These, with their potential for precise temporal- and tissue-specific control, can modulate the expression of many different critical genes involved in diverse aspects of JH biology.
Author Contributions
Both authors contributed equally to the designing and writing of the review. Both authors have read and agree to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health U.S.A., grants R01AI04554 and R21AI153689.
Acknowledgments
We would like to express our thanks to Mark E. Clifton and Marten J. Edwards for comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Goodman, W.G.; Cusson, M. The Juvenile Hormones. In Insect Endocrinology; Gilbert, L.I., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 310–365. [Google Scholar]
- Zhu, J.; Noriega, F.G. The role of juvenile hormone in mosquito development and reproduction. In Advances in Insect Physiology; Progress in Mosquito Research; Raikhel, A., Ed.; Elsevier: Oxford, UK, 2016; Volume 51, pp. 93–113. [Google Scholar]
- Rivera-Pérez, C.; Clifton, M.E.; Noriega, F.G.; Jindra, M. Juvenile hormone regulation and action. In Advances in Invertebrate (Neuro) Endocrinology; Saleuddin, S., Lange, A.B., Orchard, I., Eds.; Apple Academic Press, Inc.: Oakville, ON, Canada, 2020; Volume 2, pp. 1–76. [Google Scholar]
- Slama, K.; Romanuk, M.; Sorm, F. Insect Hormones and Bioanalogues; Springer: New York, NY, USA, 1974. [Google Scholar]
- Cusson, M.; Sen, S.E.; Shinoda, T. Juvenile hormone biosynthetic enzymes as targets for insecticide discovery. In Advanced Technologies for Managing Insect Pests; Ishayya, I., Palli, S.R., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 31–55. [Google Scholar]
- Jindra, M.; Bittova, L. The juvenile hormone receptor as a target of juvenoid “insect growth regulators”. Arch. Insect Biochem. Physiol. 2020, 103, e21615. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Perez, C.; Nouzova, M.; Noriega, F.G. New approaches to study juvenile hormone biosynthesis in insects. Short Views Insect Biochem. Molec. Biol. 2014, 7, 185–216. [Google Scholar]
- Ramirez, C.E.; Nouzova, M.; Benigni, P.; Quirke, J.M.E.; Noriega, F.G.; Fernandez-Lima, F. Fast, ultra-trace detection of juvenile hormone III from mosquitoes using mass spectrometry. Talanta 2016, 159, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bergot, B.J.; Ratcliff, M.; Schooley, D.A. Method for quantitative determination of the four known juvenile hormones in insect tissue using gas chromatography-mass spectroscopy. J. Chromatogr. 1981, 204, 231–244. [Google Scholar] [CrossRef]
- Ramirez, C.E.; Nouzova, M.; Michalkova, V.; Fernandez-Lima, F.; Noriega, F.G. Common structural features facilitate the simultaneous identification and quantification of the five most common juvenile hormones by liquid chromatography-tandem mass spectrometry. Insect Biochem. Molec. Biol. 2020, 116, 103287. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, V.B. The physiology of ecdysis in Rhodnius prolixus (Hemiptera). II. Factors controlling moulting and ‘metamorphosis’. Q. J. Microsc. Sci. 1934, 77, 191–222. [Google Scholar]
- Villalobos-Sambucaro, M.J.; Nouzova, M.; Ramirez, C.E.; Alzugaray, M.E.; Fernandez-Lima, F.; Ronderos, J.R.; Noriega, F.G. The juvenile hormone described in Rhodnius prolixus by Wigglesworth is juvenile hormone III skipped bisepoxide. Sci. Rep. 2020, 10, 3091. [Google Scholar] [CrossRef]
- Dhadialla, T.S.; Retnakaran, A.; Smagghe, G. Insect growth and development disrupting insecticides. In Comprehensive Insect Molecular Science; Gilbert, L.I., Iatrou, K., Gill, S., Eds.; Elsevier/Pergamon: New York, NY, USA, 2005; Volume 6, pp. 55–116. [Google Scholar]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Staal, G.B. Anti juvenile hormone agents. Annu. Rev. Entomol. 1986, 31, 391–429. [Google Scholar] [CrossRef]
- Charles, J.-P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor, methoprene-tolerant. Proc. Natl. Acad. Sci. USA 2011, 108, 21128–21133. [Google Scholar] [CrossRef]
- Bittova, L.; Jedlicka, P.; Dracinsky, M.; Kirubakaran, P.; Vondrasek, J.; Hanus, R.; Jindra, M. Exquisite ligand stereoselectivity of a Drosophila juvenile hormone receptor contrasts with its broad agonist repertoire. J. Biol. Chem. 2019, 294, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Uhlirova, M.; Charles, J.-P.; Smykal, V.; Hill, R.J. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet 2015, 11, e1005394. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.E.; Noriega, F.G. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. J. Insect Physiol. 2011, 57, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.E.; Noriega, F.G. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. J. Insect Physiol. 2012, 58, 1007–1019. [Google Scholar] [CrossRef]
- Paul, A.; Harrington, L.C.; Scott, J.G. Evaluation of novel insecticides for control of the dengue vector Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 55–60. [Google Scholar] [CrossRef]
- Jones, G.; Jones, D.; Li, X.; Tang, L.; Ye, L.; Teal, P.; Riddiford, L.; Sandifer, C.; Borovsky, D.; Martin, J.-R. Activities of natural methyl farnesoids on pupariation and metamorphosis of Drosophila melanogaster. J. Insect Physiol. 2010, 56, 1456–1464. [Google Scholar] [CrossRef]
- Bowers, W.S.; Ohta, T.; Cleere, J.S.; Marsella, P.A. Discovery of insect anti-juvenile hormone in plants. Science 1976, 193, 542–547. [Google Scholar] [CrossRef]
- Bowers, W.S.; Martinez-Pardo, R. Antiallatotropins: Inhibition of corpus allatum development. Science 1977, 197, 1369–1371. [Google Scholar] [CrossRef]
- Pratt, G.E.; Jennings, R.C.; Hamnett, A.F.; Brooks, G.T. Lethal metabolism of precocene-1 to a reactive epoxide by locust corpora allata. Nature 1980, 284, 320–323. [Google Scholar] [CrossRef]
- Tan, A.; Tanaka, H.; Tamura, T.; Shiotsuki, T. Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc. Natl. Acad. Sci. USA 2005, 102, 11751–11756. [Google Scholar] [CrossRef]
- Philpott, M.L.; Hammock, B.D. Juvenile hormone esterase is a biochemical anti-juvenile hormone agent. Insect Biochem. 1990, 20, 451–459. [Google Scholar] [CrossRef]
- Bonning, B.C.; Loher, W.; Hammock, B.D. Recombinant juvenile hormone esterase as a biochemical anti-juvenile hormone agent: Effects on ovarian development in Acheta domesticus. Arch. Insect Biochem. Physiol. 1997, 34, 359–368. [Google Scholar] [CrossRef]
- Edgar, K.; Noriega, F.G.; Bonning, B.C.; Wells, M.A. Recombinant juvenile hormone esterase, an effective tool to modify juvenile hormone-dependent expression of the early trypsin gene in mosquitoes. Insect Molec. Biol. 2000, 9, 27–31. [Google Scholar] [CrossRef]
- Kuwano, E.; Takeya, R.; Eto, M. Terpenoid imidazoles: New anti-juvenile hormones. Agric. Biol. Chem. 1983, 47, 921–923. [Google Scholar]
- Helvig, C.; Koener, J.F.; Unnithan, G.C.; Feyereisen, R. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc. Natl. Acad. Sci. USA 2004, 101, 4024–4029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kuwano, E.; Noriega, F.G. 1,5-disubstituted imidazoles inhibit juvenile hormone biosynthesis by the corpora allata of the mosquito Aedes aegypti. J. Insect Physiol. 2003, 49, 1005–1011. [Google Scholar] [CrossRef]
- Shinoda, T.; Itoyama, K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc. Natl. Acad. Sci. USA 2003, 100, 11986–11991. [Google Scholar] [CrossRef]
- Nouzova, M.; Michalkova, V.; Ramirez, C.E.; Fernandez-Lima, F.; Noriega, F.G. Inhibition of juvenile hormone synthesis in mosquitoes by the methylation inhibitor 3-deazaneplanocin A (DZNep). Insect Biochem. Molec. Biol. 2019, 113, 103183. [Google Scholar] [CrossRef]
- Ramos, F.O.; Leyria, J.; Nouzova, M.; Fruttero, L.L.; Noriega, F.G.; Canavoso, L.E. Juvenile hormone mediates lipid storage in the oocytes of Dipetalogaster maxima. Insect Biochem. Mol. Biol. 2020, 103499. [Google Scholar] [CrossRef]
- Belles, X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu. Rev. Entomol. 2010, 55, 111–128. [Google Scholar] [CrossRef]
- Whitten, M.M.A. Novel RNAi delivery systems in the control of medical and veterinary pests. Curr. Opin. Insect Sci. 2019, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Meiselman, M.; Lee, S.S.; Tran, R.T.; Dai, H.; Ding, Y.; Rivera-Perez, C.; Wijesekera, T.P.; Dauwalder, B.; Noriega, F.G.; Adams, M.E. An endocrine network essential for reproductive success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E3849–E3858. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Ding, Y.; Karapetians, N.; Rivera-Perez, C.; Noriega, F.G.; Adams, M.E. Hormonal signaling cascade during an early adult critical period required for courtship memory retention in Drosophila. Curr. Biol. 2017, 227, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Konopova, B.; Jindra, M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2007, 104, 10488–10493. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, C.; Namiki, T.; Shinoda, T. Kruppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 2009, 325, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Konopova, B.; Smykal, V.; Jindra, M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE 2011, 6, e28728. [Google Scholar] [CrossRef]
- Villalobos Sambucaro, M.J.; Riccillo, F.L.; Calderón-Fernández, G.M.; Sterkel, M.; Diambra, L.A.; Ronderos, J.R. Genomic and functional characterization of a methoprene-tolerant gene in the kissing-bug Rhodnius prolixus. Gen. Comp. Endocrinol. 2015, 216, 1–8. [Google Scholar] [CrossRef]
- Saha, T.T.; Roy, S.; Pei, G.; Dou, W.; Zou, Z.; Raikhel, A.S. Synergistic action of the transcription factors Kruppel homolog 1 and Hairy in juvenile hormone/Methoprene-tolerant-mediated gene-repression in the mosquito Aedes aegypti. PLoS Genet. 2019, 15, e1008443. [Google Scholar] [CrossRef]
- Noriega, F.G.; Shaa, D.; Wells, M.A. Juvenile Hormone controls early trypsin gene expression in the midgut of Aedes aegypti. Insect Molec. Biol. 1997, 6, 63–66. [Google Scholar] [CrossRef]
- Li, M.; Mead, E.A.; Zhu, J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. USA 2011, 108, 638–643. [Google Scholar] [CrossRef]
- Zhao, B.; Hou, Y.; Wang, J.; Kokoza, V.A.; Saha, T.T.; Wang, X.-L.; Lin, L.; Zou, Z.; Raikhel, A.S. Determination of juvenile hormone titers by means of LC-MS/MS/MS and a juvenile hormone-responsive Gal4/UAS system in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2016, 77, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Perkins, L.A.; Holderbaum, L.; Tao, R.; Hu, Y.; Sopko, R.; McCall, K.; Yang-Zhou, D.; Flockhart, I.; Binari, R.; Shim, H.-S.; et al. The transgenic RNAi project at Harvard medical school: Resources and validation. Genetics 2015, 201, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Guo, Z.; Liu, Y.; Zhang, Y. Progress and prospects of CRISPR/Cas systems in insects and other arthropods. Front. Physiol. 2017, 8, 608. [Google Scholar] [CrossRef]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Huynh, N.; Wang, S.; King-Jones, K. Spatial and temporal control of gene manipulation in Drosophila via drug-activated Cas9 nucleases. Insect Biochem. Mol. Biol. 2020, 120, 103336. [Google Scholar] [CrossRef]
- Daimon, T.; Uchibori, M.; Nakao, H.; Sezutsu, H.; Shinoda, T. Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc. Natl Acad. Sci. USA 2015, 112, E4226–E4235. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Shiotsuki, T.; Wang, Z.; Xu, X.; Huang, Y.; Tan, A. Depletion of juvenile hormone esterase extends larval growth in Bombyx mori. Insect Biochem. Mol. Biol. 2017, 81, 72–79. [Google Scholar] [CrossRef]
- Wen, D.; Rivera-Perez, C.; Abdou, M.; Jia, Q.; He, Q.; Zyaan, O.; Bendena, W.B.; Tobe, S.S.; Noriega, F.G.; Palli, S.R.; et al. Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLoS Genet. 2015, 11, e1005038. [Google Scholar] [CrossRef]
- Guan-Heng Zhu, G.-H.; Jiao, Y.; Chereddy, S.C.R.R.; Noh, M.Y.; Palli, S.R. Knockout of juvenile hormone receptor, Methoprene tolerant, induces black larval phenotype in the yellow fever mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. USA 2019, 116, 21501–21507. [Google Scholar]
- Kim, I.H.; Castillo, J.C.; Aryan, A.; Martin-Martin, I.; Nouzova, M.; Noriega, F.G.; Barletta, A.B.F.; Calvo, E.; Adelman, Z.N.; Ribeiro, J.M.; et al. A mosquito juvenile hormone binding protein (mJHBP) regulates the activation of innate immune defenses and hemocyte development. PLoS Pathog. 2020, 16, e1008288. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).