Re-Visiting the Incidence of Environmental Factors on a Pre-Imaginal Population of the Red Gum Lerp Psyllid, Glycaspis brimblecombei Moore
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Data Collection
- (a)
- a total of 100 leaves were collected randomly from 5 plants (20 leaves/plant), regardless of the presence of specimens of the insect and trying to collect for each plant an average of 5 leaves per cardinal point (this method will be named “psyllid sampling” in the following pages);
- (b)
- a 30 min collection of leaves was carried out by two collectors (15 min per each) randomly on all Eucalyptus plants of the selected site, regardless of the number of leaves sampled (higher on less infested trees, of course, due to the longer time spent looking on the canopy for leaves bearing lerps) but oriented to pick up only leaves showing mature lerps of the insect (this method will be named as “infestation sampling” in the following pages). The number of leaves sampled in this method was not recorded.
2.2. Climatic Data Collection
2.3. Data Treatment
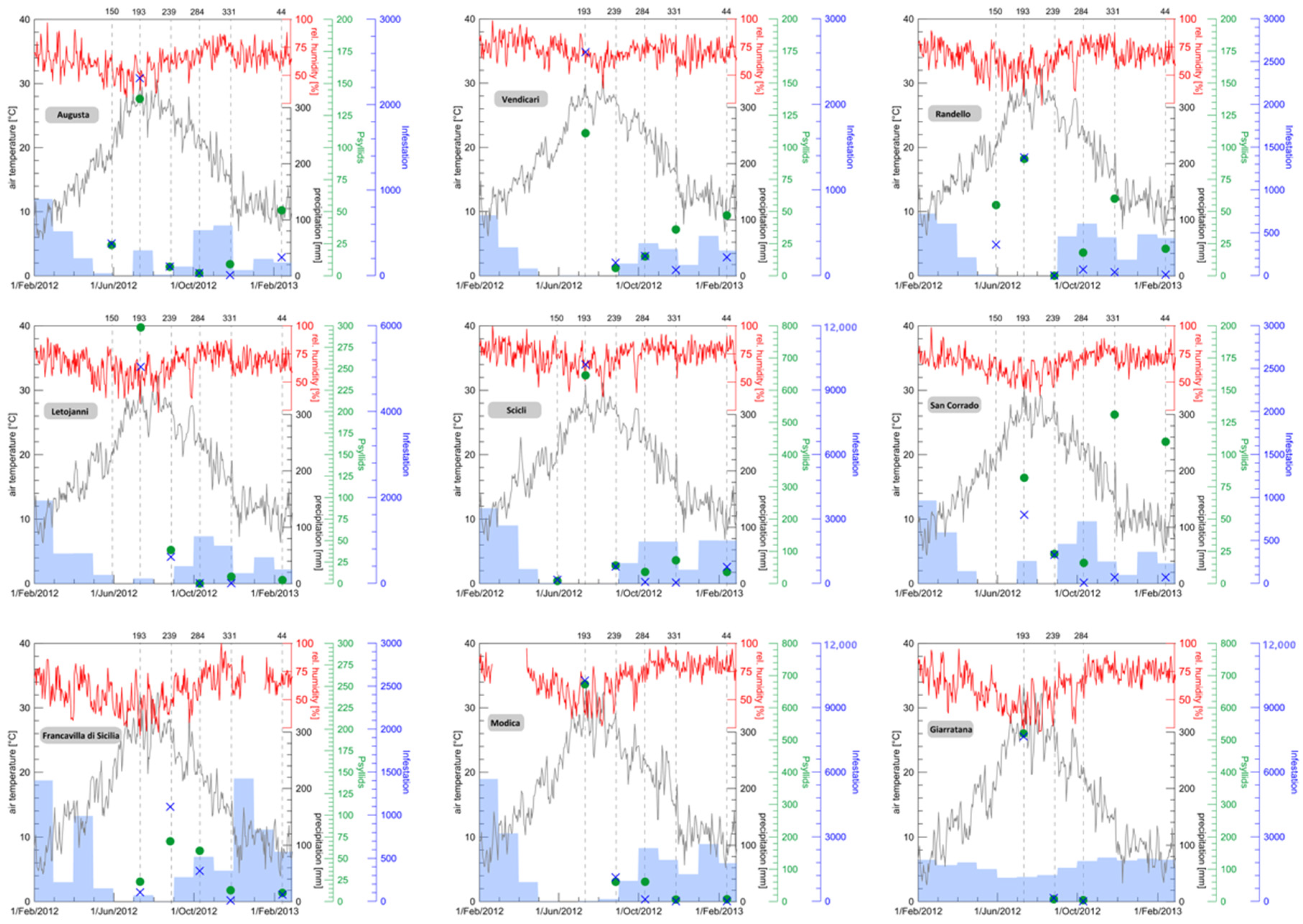
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cuello, E.M.; López, S.N.; Andorno, A.V.; Hernández, C.M.; Botto, E.N. Development of Glycaspis brimblecombei Moore (Hemiptera: Aphalaridae) on Eucalyptus camaldulensis Dehnh. and Eucalyptus dunnii Maiden. Agric. For. Entomol. 2018, 20, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Gill, R.J. New state record: Redgum lerp psyllid, Glycaspis brimblecombei. Calif. Plant Pest Dis. Rep. 1998, 17, 7–8. [Google Scholar]
- Brennan, E.B.; Gill, R.J.; Hrusa, G.F.; Weinbaum, S.A. First record of Glycaspis brimblecombei (Moore) (Homoptera: Psyllidae) in North America: Initial observations and predator associations of a potentially serious new pest of Eucalyptus in California. Pan Pac. Entomol. 1999, 75, 55–57. [Google Scholar]
- Bella, S.; Rapisarda, C. First record from Greece of the invasive red gum lerp psyllid Glycaspis brimblecombei Moore (Hemiptera: Psyllidae) and its associated parasitoid Psyllaephagus bliteus Riek (Hymenoptera: Encyrtidae). Redia 2013, 96, 33–35. [Google Scholar]
- Ben Attia, S.; Rapisarda, C. First record of the red gum lerp psyllid, Glycaspis brimblecombei Moore (Hemiptera Psyllidae), in Tunisia. Phytoparasitica 2014, 42, 535–539. [Google Scholar] [CrossRef]
- Laudonia, S.; Garonna, A.P. The red gum lerp psyllid, Glycaspis brimblecombei, a new exotic pest of Eucalyptus camaldulensis in Italy. Bull. Insectol. 2010, 63, 233–236. [Google Scholar]
- Garonna, A.P.; Sasso, R.; Laudonia, S. Glycaspis brimblecombei (Hem.: Psyllidae), la psilla dal follicolo bianco ceroso, altra specie aliena dell’Eucalipto rosso in Italia. Forest@ 2011, 18, 71–77. [Google Scholar] [CrossRef]
- Lo Verde, G.; Bella, S.; Caleca, V.; Rapisarda, C.; Sidoti, A. Presenza in Sicilia di Glycaspis brimblecombei Moore (Hemiptera Psyllidae) su Eucalyptus camaldulensis Dehnh. Nat. Sicil. 2011, 4, 425–434. [Google Scholar]
- Peris-Felipo, F.J.; Mancusi, G.; Turrisi, G.F.; Jiménez-Peydró, R. New corological and biological data of the Red Gum Lerp Psyllid, Glycaspis brimblecombei Moore, 1964 in Italy (Hemiptera, Psyllidae). Biodivers. J. 2011, 2, 13–17. [Google Scholar]
- Suma, P.; Nucifora, S.; Caleca, V.; Lo Verde, G.; Tortorici, F.; Rapisarda, C.; Bella, S. A review of introduced alien insect pests and their associated parasitoids on Eucalyptus trees in Sicily. Redia 2018, 101, 81–88. [Google Scholar] [CrossRef]
- Moore, K.M. Observations on some Australian forest insects. 23. A revision of the genus Glycaspis (Homoptera: Psyllidae) with descriptions of seventy-three new species. Aust. Zool. 1970, 15, 248–342. [Google Scholar]
- Moore, K.M. The Glycaspis spp. (Hemiptera: Psyllidae)—Eucalyptus camaldulensis associations. J. Aust. Entomol. Soc. 1970, 7, 3–7. [Google Scholar]
- Brennan, E.B.; Hrusa, G.F.; Weinbaum, S.A.; Levison, W., Jr. Resistance of Eucalyptus species to Glycaspis brimblecombei (Homoptera: Psyllidae) in the San Francisco Bay area. Pan Pac. Entomol. 2001, 77, 249–253. [Google Scholar]
- Hollis, D. Australian Psylloidea: Jumping Plant Lice and Lerps Insects. Australia Biological Resources Study; CSIRO Publishing: Canberra, Australia, 2004. [Google Scholar]
- Tuller, J.; Oliveira, K.N.; Silva, J.O.; Lopes de Faria, M.; do-Espírito Santo, M.M.; Serrão, J.E.; Zanuncio, J.C. Glycaspis brimblecombei (Hemiptera: Psyllidae) attack patterns on different Eucalyptus genotypes. PeerJ 2017, 5, e3864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halbert, S.E.; Gill, R.J.; Nisson, J.N. Two Eucalyptus Psyllids New to Florida (Homoptera: Psyllidae); Entomology Circular No. 407; Florida Department of Agriculture and Consumer Services: Gainesville, FL, USA, 2001.
- Bella, S. New alien insect pests to Portugal on urban ornamental plants and additional data on recently introduced species. Ann. Soc. Entomol. Fr. 2013, 49, 374–382. [Google Scholar] [CrossRef]
- Morgan, F.D. Psylloidea of South Australia; Government of South Australian Government Printer: Adelaide, Australia, 1984.
- Mannu, R.; Buffa, F.; Pinna, C.; Deiana, V.; Satta, A.; Floris, I. Preliminary results on spatio-temporal variability of Glycaspis brimblecombei (Hemiptera Psyllidae) populations from a three-year monitoring program in Sardinia (Italy). Redia 2018, 101, 107–114. [Google Scholar] [CrossRef]
- Dahlsten, D.L.; Daane, K.M.; Paine, T.D.; Sime, K.R.; Lawson, A.B.; Rowney, D.L.; Roltsch, W.J.; Andrews, J.W., Jr.; Kabashima, J.N.; Shaw, D.A.; et al. Imported parasitoid helps control red gum lerp psyllid. Calif. Agric. 2005, 59, 229–234. [Google Scholar] [CrossRef]
- Queiroz, D.L.; Majer, J.; Burckhardt, D.; Zanetti, R.; Fernandez, J.I.R.; Queiroz, E.C.; Garrastazu, M.; Fernandes, B.V.; Anjos, N. Predicting the geographical distribution of Glycaspis brimblecombei (Hemiptera: Psylloidea) in Brazil. Aust. J. Entomol. 2013, 52, 20–30. [Google Scholar] [CrossRef]
- Floris, I.; Pusceddu, M.; Mannu, R.; Buffa, F.; Quaranta, M.; Satta, A. Impact of sap-sucking insect pests (Blastopsylla occidentalis Taylor and Glycaspis brimblecombei Moore, Hemiptera: Psyllidae) on unifloral eucalyptus honey. Ann. Silvic. Res. 2020, 44, 66–70. [Google Scholar]
- Daane, K.M.; Sime, K.R.; Paine, T.D. Climate and the effectiveness of Psyllaephagus bliteus as a parasitoid of the red gum lerp psyllid. Biocontrol Sci.Technol. 2012, 22, 1305–1320. [Google Scholar] [CrossRef]
- Margiotta, M.; Bella, S.; Buffa, F.; Caleca, V.; Floris, I.; Giorno, V.; Lo Verde, G.; Rapisarda, C.; Sasso, R.; Suma, P.; et al. Modeling Environmental Influences in the Psyllaephagus bliteus (Hymenoptera: Encyrtidae—Glycaspis brimblecombei (Hemiptera: Aphalaridae) Parasitoid–Host System. J. Econ. Entomol. 2017, 110, 491–501. [Google Scholar] [PubMed]
- Laudonia, S.; Margiotta, M.; Sasso, R. Seasonal occurrence and adaptation of the exotic Glycaspis brimblecombei Moore (Hemiptera: Aphalaridae) in Italy. J. Nat. Hist. 2014, 48, 675–689. [Google Scholar] [CrossRef] [Green Version]
- Caleca, V.; Bella, S.; La Pergola, A.; Lombardo, A.; Lo Verde, G.; Maltese, M.; Nucifora, S.; Rizzo, R.; Suma, P.; Tortorici, F.; et al. Environmental factors and influence of parasitism of Psyllaephagus bliteus Riek (Hymenoptera Encyrtidae) on populations of Glycaspis brimblecombei Moore (Hemiptera Aphalaridae) in Mediterranean climatic areas. Redia 2018, 101, 89–100. [Google Scholar]
- Beyer, M.; El Jarroudi, M.; Junk, J.; Pogoda, F.; Dubos, T.; Görgen, K.; Hoffmann, L. Spring air temperature accounts for the bimodal temporal distribution of Septoria tritici epidemics in the winter wheat stands of Luxembourg. Crop Prot. 2012, 42, 250–255. [Google Scholar] [CrossRef]
- Junk, J.; Jonas, M.; Eickermann, M. Assessing meteorological key factors influencing crop invasion by pollen beetle (Meligethes aeneus F.)—Past observations and future perspectives. Meteorol. Z. 2015, 25, 357–364. [Google Scholar] [CrossRef]
- Yates, K.L.; Bouchet, P.J.; Caley, M.J.; Mengersen, K.; Randin, C.F.; Parnell, S.; Fielding, A.H.; Bamford, A.J.; Ban, S.; Barbosa, A.M.; et al. Outstanding Challenges in the Transferability of Ecological Models. Trends Ecol. Evol. 2018, 33, 790–802. [Google Scholar] [CrossRef] [Green Version]
- Boavida, G.; Garcia, A.; Branco, M.I. How effective is Psyllaephagus bliteus (Hymenoptera: Encyrtidae) in controlling Glycaspis brimblecombei (Hemiptera: Psylloidea)? Biol. Control 2016, 99, 1–7. [Google Scholar] [CrossRef]
- Oliveira, K.N.; Jesus, F.M.; Silva, J.O.; Espírito-Santo, M.M.; Faria, M.L. An experimental test of rainfall as a control agent of Glycaspis brimblecombei Moore (Hemiptera, Psyllidae) on seedlings of Eucalyptus camaldulensis Dehn (Myrtaceae). Rev. Bras. Entomol. 2012, 56, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, I.; Denno, R.F. Interspecific interactions in phytophagous insects revisited: A quantitative assessment of competition theory. Ecol. Lett. 2007, 10, 977–994. [Google Scholar] [CrossRef]
- Suma, P.; Nucifora, S.; Bella, S. New distribution record of the invasive bronze bug Thaumastocoris peregrinus Carpintero and Dellapé (Heteroptera, Thaumastocoridae) in Italy. EPPO Bull. 2014, 44, 179–182. [Google Scholar] [CrossRef]
- Martínez, G.; González, A.; Dicke, M. Effect of the eucalypt lerp psyllid on adult feeding, oviposition-site selection, and offspring performance of the bronze bug. Entomol. Exp. Appl. 2018, 166, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Zavala, J.; Gog, L.; Giacometti, R. Anthropogenic increase in carbon dioxide modifies plant–insect interactions. Ann. Appl. Biol. 2017, 170, 68–77. [Google Scholar] [CrossRef]



| No. | Locality | Province | Altitude (m a.s.l.) | Latitude | Longitude |
|---|---|---|---|---|---|
| 1 | Augusta | Siracusa | 3 | 37°14′36.35″ N | 15°12′16.20″ E |
| 2 | Vendicari (Noto) | Siracusa | 5 | 36°48′15.70″ N | 15°05′34.10″ E |
| 3 | Randello (Ragusa) | Ragusa | 15 | 36°50′25.00″ N | 14°27′38.80″ E |
| 4 | Letojanni | Messina | 30 | 37°52′55.25″ N | 15°18′09.55″ E |
| 5 | Scicli | Ragusa | 200 | 36°48′45.00″ N | 14°42′57.00″ E |
| 6 | San Corrado (Noto) | Siracusa | 320 | 36°55′55.00″ N | 15°03′32.90″ E |
| 7 | Francavilla di Sicilia | Messina | 425 | 37°54′36.65″ N | 15°07′42.60″ E |
| 8 | Modica | Ragusa | 460 | 36°52′38.70″ N | 14°45′12.70″ E |
| 9 | Giarratana | Ragusa | 520 | 37°02′53.50″ N | 14°47′58.00″ E |
| Parameters | Precipitation | Humidity | Accumulated Temperature | Accumulated Precipitation | Psyllids | Infestation |
|---|---|---|---|---|---|---|
| Temperature | −0.49 (0.00) | −0.52 (0.00) | 0.24 (0.08) | −0.24 (0.08) | 0.60 0.00 | 0.75 0.00 |
| Precipitation | 0.55 (0.00) | 0.37 (0.01) | 0.46 (0.00) | −0.10 (0.45) | −0.29 (0.04) | |
| Humidity | 0.41 (0.00) | 0.15 (0.27) | −0.19 (0.17) | −0.40 (0.00) | ||
| Accumulated Temperature | 0.40 (0.00) | 0.42 (0.00) | 0.29 (0.03) | |||
| Accumulated Precipitation | 0.08 (0.56) | −0.07 (0.64) | ||||
| Psyllids | 0.91 (0.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junk, J.; Eickermann, M.; Milenovic, M.; Suma, P.; Rapisarda, C. Re-Visiting the Incidence of Environmental Factors on a Pre-Imaginal Population of the Red Gum Lerp Psyllid, Glycaspis brimblecombei Moore. Insects 2020, 11, 860. https://doi.org/10.3390/insects11120860
Junk J, Eickermann M, Milenovic M, Suma P, Rapisarda C. Re-Visiting the Incidence of Environmental Factors on a Pre-Imaginal Population of the Red Gum Lerp Psyllid, Glycaspis brimblecombei Moore. Insects. 2020; 11(12):860. https://doi.org/10.3390/insects11120860
Chicago/Turabian StyleJunk, Jürgen, Michael Eickermann, Milan Milenovic, Pompeo Suma, and Carmelo Rapisarda. 2020. "Re-Visiting the Incidence of Environmental Factors on a Pre-Imaginal Population of the Red Gum Lerp Psyllid, Glycaspis brimblecombei Moore" Insects 11, no. 12: 860. https://doi.org/10.3390/insects11120860
APA StyleJunk, J., Eickermann, M., Milenovic, M., Suma, P., & Rapisarda, C. (2020). Re-Visiting the Incidence of Environmental Factors on a Pre-Imaginal Population of the Red Gum Lerp Psyllid, Glycaspis brimblecombei Moore. Insects, 11(12), 860. https://doi.org/10.3390/insects11120860








