Oogenesis of Hematophagous Midge Forcipomyia taiwana (Diptera: Ceratopogonidae) and Nuage Localization of Vasa in Germline Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Female Midge Collection, Culture, and Ovary Dissection
2.2. Electron Microscopy
2.3. Whole-Mount Immunostaining
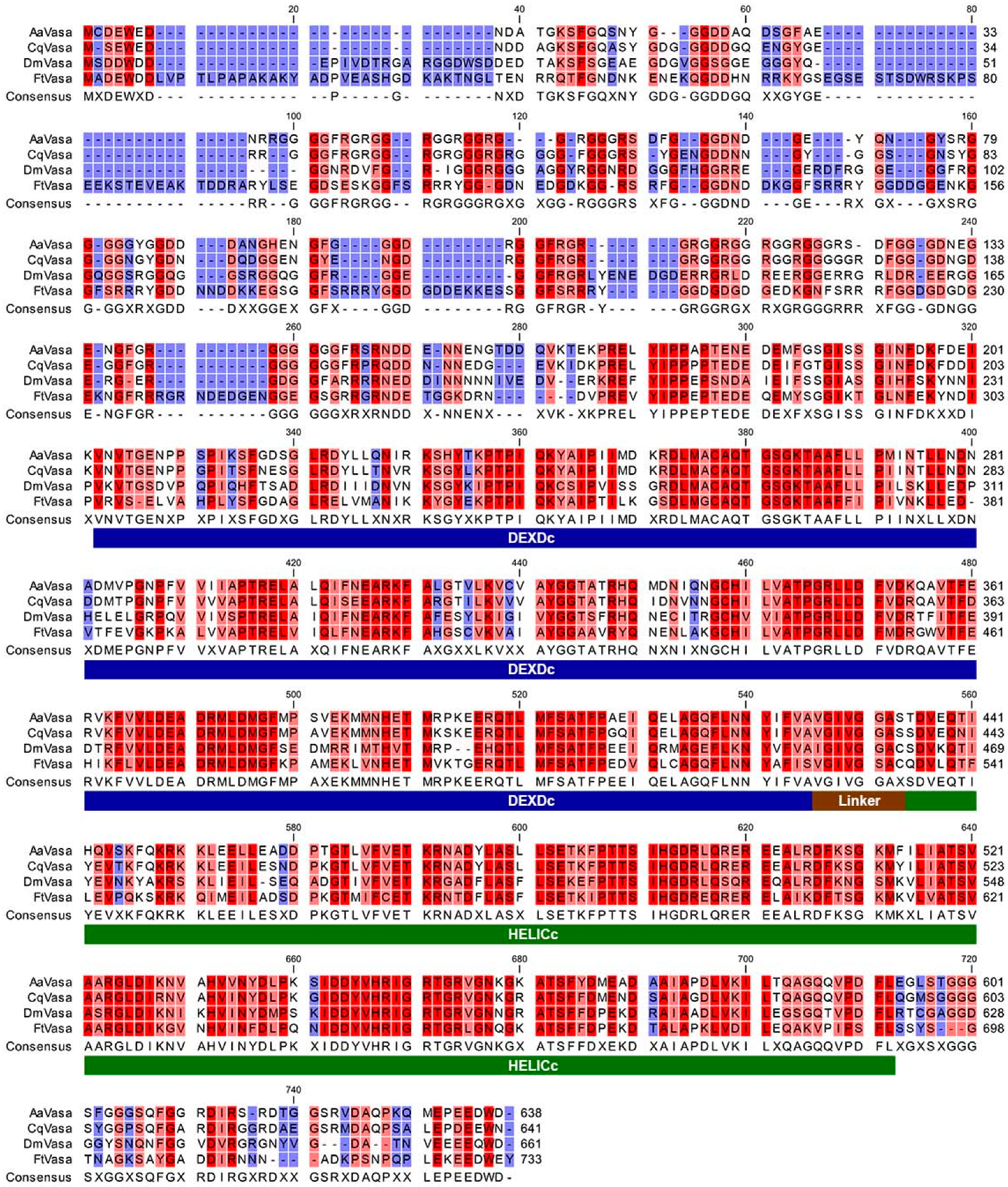
2.4. Cloning of FtVasa, Transgenes, and Drosophila Stocks
3. Results
3.1. Synchronous Development of F. taiwana Follicles
3.2. Ovarian Structure of F. taiwana
3.3. Polytrophic-Meroistic Ovariole of F. taiwana
3.4. F. taiwana Vasa Could be Localized to the Perinuclear Nuage in Drosophila Nurse Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiraki, T. Investigation on general injurious insects. Taiwan Sotokufu Noji Shikenjo Tolubetsu Hokodu 1913, 8, 286–297. [Google Scholar]
- Chuang, Y.Y.; Lin, C.S.; Wang, C.H.; Yeh, C.C. Distribution and seasonal occurrence of Forcipomyia taiwana (Diptera: Ceratopogonidae) in the nantou area in taiwan. J. Med. Entomol. 2000, 37, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Lien, J.C.; Lin, Y.N.; Hsu, S.J. The diurnal biting pattern of a bloodsucking midge Forcipomyia (Lasiohelea) taiwana (Shiraki) (Diptera, Ceratopogonidae). Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 1981, 14, 54–56. [Google Scholar] [PubMed]
- Shu, H.Y.; Chen, C.Y.; Hsu, Y.T.; Lin, G.H. A pilot study for the establishment of a bloodsucking model for Forcipomyia taiwana (biting midge). Int. J. Clin. Med. Microbiol. 2017, 2, 7. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hwang, G.Y.; Chen, P.C.; Tu, W.C.; Lee, M.F. Molecular cloning and immunologic characterization of for t 2: A major allergen from the biting midge Forcipomyia taiwana. Allergy 2011, 66, 703–705. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lee, M.F.; Lan, J.L.; Chen, C.S.; Wang, H.L.; Hwang, G.Y.; Wu, C.H. Hypersensitivity to Forcipomyia taiwana (biting midge): Clinical analysis and identification of major for t 1, for t 2 and for t 3 allergens. Allergy 2005, 60, 1518–1523. [Google Scholar] [CrossRef]
- Boorman, J. Biting midges (Ceratopogonidae). In Medical Insects and Arachnids; Lane, R.P., Crosskey, R.W., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 288–309. [Google Scholar]
- Wirth, W.W.; Ratanaworabhan, C.; Blanton, F.S. Synopsis of the genera of Ceratopogonidae (Diptera). Ann. Parasitol. Hum. Comp. 1974, 49, 595–613. [Google Scholar] [CrossRef]
- Campbell, M.M.; Kettle, D.S. Oogenesis in Culicoides brevitarsis kieffer (Diptera: Ceratopogonidae) and the development of a plastron-like layer on the egg. Aust. J. Zool. 1975, 23, 203–218. [Google Scholar] [CrossRef]
- Filimonova, S.A.; Brodskaya, N.K. Ultrastructural investigation of the oogenesis in bloodsucking biting midges of the genus Culicoides latr.(Diptera, Ceratopogonidae). Entomol. Rev. 1998, 78, 808–821. [Google Scholar]
- Briegel, H.; Gut, T.; Lea, A.O. Sequential deposition of yolk components during oogenesis in an insect, Aedes aegypti (Diptera: Culicidae). J. Insect Physiol. 2003, 49, 249–260. [Google Scholar] [CrossRef]
- Fiil, A. Oogenesis in the malaria mosquito Anopheles gambiae. Cell Tissue Res. 1976, 167, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.A.; Shalaby, A.M.; Abdel-Ghaffar, H.; Abdel-Sabour, S. Histological studies of developmental stages during oogenesis in Culex pipiens molestus (Forskal). Ii. The adult stage. Folia Morphol. 1986, 34, 153–168. [Google Scholar]
- Bastock, R.; St Johnston, D. Drosophila oogenesis. Curr. Biol. 2008, 18, R1082–R1087. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, E.; Cooley, L. Looking at oogenesis. Methods Cell Biol. 1994, 44, 545–561. [Google Scholar] [PubMed]
- Yokota, S. Nuage proteins: Their localization in subcellular structures of spermatogenic cells as revealed by immunoelectron microscopy. Histochem. Cell Biol. 2012, 138, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Arkov, A.L. Next generation organelles: Structure and role of germ granules in the germline. Mol. Reprod. Dev. 2013, 80, 610–623. [Google Scholar] [CrossRef]
- Mahowald, A.P. Polar granules of Drosophila. Ii. Ultrastructural changes during early embryogenesis. J. Exp. Zool. 1968, 167, 237–261. [Google Scholar] [CrossRef]
- Mahowald, A.P.; Strassheim, J.M. Intercellular migration of centrioles in the germarium of Drosophila melanogaster. An electron microscopic study. J. Cell Biol. 1970, 45, 306–320. [Google Scholar] [CrossRef]
- Mahowald, A.P. Polar granules of Drosophila. 3. The continuity of polar granules during the life cycle of Drosophila. J. Exp. Zool. 1971, 176, 329–343. [Google Scholar] [CrossRef]
- Findley, S.D.; Tamanaha, M.; Clegg, N.J.; Ruohola-Baker, H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and Rde1/Ago1 homolog, Aubergine, in nuage. Development 2003, 130, 859–871. [Google Scholar] [CrossRef]
- Liang, L.; Diehl-Jones, W.; Lasko, P. Localization of Vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development 1994, 120, 1201–1211. [Google Scholar] [PubMed]
- Snee, M.J.; Macdonald, P.M. Live imaging of nuage and polar granules: Evidence against a precursor-product relationship and a novel role for Oskar in stabilization of polar granule components. J. Cell Sci. 2004, 117, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.; Koonin, E.V. An expanding family of helicases within the ‘DEAD/H’ superfamily. Nucleic Acids Res. 1993, 21, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Sengoku, T.; Nureki, O.; Nakamura, A.; Kobayashi, S.; Yokoyama, S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 2006, 125, 287–300. [Google Scholar] [CrossRef]
- Durdevic, Z.; Ephrussi, A. Germ cell lineage homeostasis in Drosophila requires the Vasa RNA helicase. Genetics 2019, 213, 911–922. [Google Scholar] [CrossRef]
- Wang, S.C.; Hsu, H.J.; Lin, G.W.; Wang, T.F.; Chang, C.C.; Lin, M.D. Germ plasm localisation of the HELICc of Vasa in Drosophila: Analysis of domain sufficiency and amino acids critical for localisation. Sci. Rep. 2015, 5, 14703. [Google Scholar] [CrossRef]
- Lim, A.K.; Kai, T. Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2007, 104, 6714–6719. [Google Scholar] [CrossRef]
- Xiol, J.; Spinelli, P.; Laussmann, M.A.; Homolka, D.; Yang, Z.; Cora, E.; Coute, Y.; Conn, S.; Kadlec, J.; Sachidanandam, R.; et al. RNA clamping by Vasa assembles a piRNA amplifier complex on transposon transcripts. Cell 2014, 157, 1698–1711. [Google Scholar] [CrossRef]
- Webster, A.; Li, S.; Hur, J.K.; Wachsmuth, M.; Bois, J.S.; Perkins, E.M.; Patel, D.J.; Aravin, A.A. Aub and Ago3 are recruited to nuage through two mechanisms to form a ping-pong complex assembled by Krimper. Mol. Cell 2015, 59, 564–575. [Google Scholar] [CrossRef]
- Dehghani, M.; Lasko, P. C-terminal residues specific to Vasa among DEAD-box helicases are required for its functions in piRNA biogenesis and embryonic patterning. Dev. Genes Evol. 2016, 226, 401–412. [Google Scholar] [CrossRef]
- Yu, Y. Catalogue and Keys of Chinese Ceratopogonidae (Insecta, Diptera); Military Medical Science Press: Beijing, China, 2005. [Google Scholar]
- Luo, Y.P. Establishing and maintaining colonies of Forcipomyia taiwana in the laboratory. J. Vector Ecol. 2018, 43, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, A.J.; Adams, J.H.; Davidson, C.M.; Micklem, D.R.; Haseloff, J.; St Johnston, D.; Brand, A.H. Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev. 1998, 12, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Ringrose, L. Transgenesis in Drosophila melanogaster. Methods Mol. Biol. 2009, 561, 3–19. [Google Scholar] [PubMed]
- Hay, B.; Jan, L.Y.; Jan, Y.N. A protein component of Drosophila polar granules is encoded by Vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 1988, 55, 577–587. [Google Scholar] [CrossRef]
- Glukhova, V.M. Blood-Sucking Midges of the Genera Culicoides and Forcipomyia (Ceratopogonidae); Nauka, Leningradskoe Otdelenie: Leningrad, Russia, 1989. [Google Scholar]
- Linley, J.R. The ovarian cycle in Culicoides barbosai Wirth & Blanton and C. furens (Poey) (Diptera, Ceratopogonidae). Bull. Entomol. Res. 1966, 57, 1–17. [Google Scholar]
- Kupriianova, E.S.; Aksenova, A.S. gonotrophic harmony in Culex pipians l. Mosquitoes. Med. Parazitol. 1977, 46, 10–15. [Google Scholar]
- Detinova, T.S. Mechanism of gonotrophic harmony in the common malarial mosquito. Zool. Zh. 1953, 32, 1178–1188. [Google Scholar]
- Watts, R.B.; Smith, S.M. Oogenesis in Toxorhynchites rutilus (Diptera: Culicidae). Can. J. Zool. 1978, 56, 136–139. [Google Scholar] [CrossRef]
- Bertram, D.S.; World Health Organization. The Ovary and Ovarioles of Mosquitos (An Illustrated Note, with Glossary)/by Bertram, D.S.; World Health Organization: Geneva, Switzerland, 1959. [Google Scholar]
- Büning, J. The Insect Ovary; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Gutzeit, H.O. Oosome formation during in vitro oogenesis in Bradysia tritici (syn. Sciara ocellaris). Wilhelm Roux’ Archiv. Dev. Biol. 1985, 194, 404–410. [Google Scholar] [CrossRef]
- Brauns, F. Die entstehung der nahrzelle und die bedeutung derselben für das wachsende ei bei Forficula auricularia L. Sitz. Abh. Nat. Gessellschaft Rostock 1912, 4, 99–141. [Google Scholar]
- Hawkins, N.C.; Thorpe, J.; Schupbach, T. Encore, a gene required for the regulation of germ line mitosis and oocyte differentiation during Drosophila oogenesis. Development 1996, 122, 281–290. [Google Scholar] [PubMed]
- Ohlmeyer, J.T.; Schupbach, T. Encore facilitates SCF-Ubiquitin-proteasome-dependent proteolysis during Drosophila oogenesis. Development 2003, 130, 6339–6349. [Google Scholar] [CrossRef] [PubMed]
- Van Buskirk, C.; Hawkins, N.C.; Schupbach, T. Encore is a member of a novel family of proteins and affects multiple processes in Drosophila oogenesis. Development 2000, 127, 4753–4762. [Google Scholar]
- Lilly, M.A.; de Cuevas, M.; Spradling, A.C. Cyclin a associates with the fusome during germline cyst formation in the Drosophila ovary. Dev. Biol. 2000, 218, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.Z.; Hong, A.; Lilly, M.A.; Lehmann, R. Twin, a Ccr4 homolog, regulates Cyclin poly(a) tail length to permit Drosophila oogenesis. Development 2005, 132, 1165–1174. [Google Scholar] [CrossRef]
- Jeske, M.; Muller, C.W.; Ephrussi, A. The LOTUS domain is a conserved DEAD-box RNA helicase regulator essential for the recruitment of Vasa to the germ plasm and nuage. Genes Dev. 2017, 31, 939–952. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-C.; Ching, Y.-H.; Krishnaraj, P.; Chen, G.-Y.; Radhakrishnan, A.S.; Lee, H.-M.; Tu, W.-C.; Lin, M.-D. Oogenesis of Hematophagous Midge Forcipomyia taiwana (Diptera: Ceratopogonidae) and Nuage Localization of Vasa in Germline Cells. Insects 2020, 11, 106. https://doi.org/10.3390/insects11020106
Wang S-C, Ching Y-H, Krishnaraj P, Chen G-Y, Radhakrishnan AS, Lee H-M, Tu W-C, Lin M-D. Oogenesis of Hematophagous Midge Forcipomyia taiwana (Diptera: Ceratopogonidae) and Nuage Localization of Vasa in Germline Cells. Insects. 2020; 11(2):106. https://doi.org/10.3390/insects11020106
Chicago/Turabian StyleWang, Szu-Chieh, Yung-Hao Ching, Preethi Krishnaraj, Guan-Yu Chen, Anna Shiny Radhakrishnan, Hsien-Min Lee, Wu-Chun Tu, and Ming-Der Lin. 2020. "Oogenesis of Hematophagous Midge Forcipomyia taiwana (Diptera: Ceratopogonidae) and Nuage Localization of Vasa in Germline Cells" Insects 11, no. 2: 106. https://doi.org/10.3390/insects11020106
APA StyleWang, S.-C., Ching, Y.-H., Krishnaraj, P., Chen, G.-Y., Radhakrishnan, A. S., Lee, H.-M., Tu, W.-C., & Lin, M.-D. (2020). Oogenesis of Hematophagous Midge Forcipomyia taiwana (Diptera: Ceratopogonidae) and Nuage Localization of Vasa in Germline Cells. Insects, 11(2), 106. https://doi.org/10.3390/insects11020106





