First Insight into Microbiome Profiles of Myrmecophilous Beetles and Their Host, Red Wood Ant Formica polyctena (Hymenoptera: Formicidae)—A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. 16S rRNA Gene Amplification and Sequencing
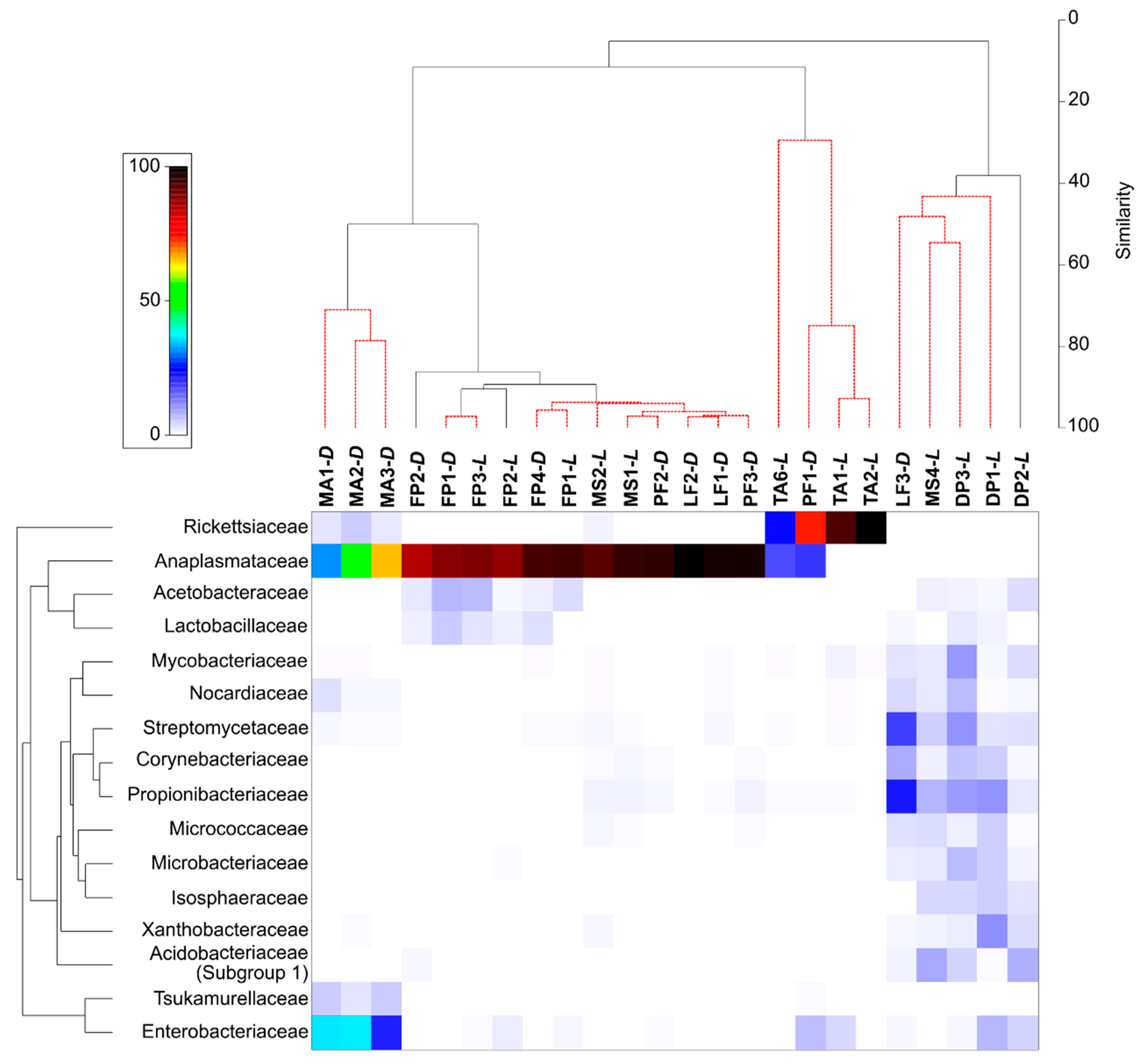
3. Results
3.1. General Description of 16S rRNA Gene Sequencing Results
3.2. Bacterial Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kronauer, D.J.C.; Pierce, N.E. Myrmecophiles. Curr. Biol. 2011, 21, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, T.; Bouillon, S.; Dekoninck, W.; Wenseleers, T. Trophic interactions in an ant nest microcosm: A combined experimental and stable isotope (δ13C/δ15N) approach. Oikos 2016, 1182–1192. [Google Scholar] [CrossRef]
- Parker, J. Myrmecophily in beetles (Coleoptera): Evolutionary patterns and biological mechanisms. Myrmecol. News 2016, 22, 65–108. [Google Scholar]
- Mynhardt, G. Declassifying myrmecophily in the coleoptera to promote the study of ant-beetle symbioses. Psyche 2013. [Google Scholar] [CrossRef]
- Staniec, B.; Pietrykowska-Tudruj, E.; Zagaja, M. Adaptive External Larval Ultrastructure of Lomechusa Gravenhorst, 1806 (Coleoptera: Staphylinidae: Aleocharinae), an Obligate Myrmecophilous Genus. Ann. Zool. 2017, 67, 609–626. [Google Scholar] [CrossRef]
- O’Keefe, S.T. Ant-Like Stone Beetles, Ants, and Their Associations (Coleoptera: Scydmaenidae; Hymenoptera: Formicidae; Isoptera). J. N. Y. Entomol. Soc. 2000, 108, 273–303. [Google Scholar] [CrossRef]
- Päivinen, J.; Ahlroth, P.; Kaitala, V. Ant-associated beetles of Fennoscandia and Denmark. Entomol. Fenn. 2002, 13, 20–40. [Google Scholar] [CrossRef]
- Kaminski, L.A.; Carvalho-Filho, F.S. Life History of Aricoris propitia (Lepidoptera: Riodinidae)—A Myrmecophilous Butterfly Obligately Associated with Fire Ants. Psyche 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Parmentier, T.; Dekoninck, W.; Wenseleers, T. A highly diverse microcosm in a hostile world: A review on the associates of red wood ants (Formica rufa group). Insectes Soc. 2014, 61, 229–237. [Google Scholar] [CrossRef]
- Robertson, J.A.; Moore, W. Phylogeny of Paussus L. (Carabidae: Paussinae): Unravelling morphological convergence associated with myrmecophilous life histories. Syst. Entomol. 2017, 42, 134–170. [Google Scholar] [CrossRef]
- Ivens, A.B.F.; Gadau, A.; Kiers, E.T.; Kronauer, D.J.C. Can social partnerships influence the microbiome? Insights from ant farmers and their trophobiont mutualists. Mol. Ecol. 2018, 27, 1898–1914. [Google Scholar] [CrossRef] [PubMed]
- Liberti, J.; Sapountzis, P.; Hansen, L.H.; Sørensen, S.J.; Adams, R.M.M.; Boomsma, J.J. Bacterial symbiont sharing in Megalomyrmex social parasites and their fungus-growing ant hosts. Mol. Ecol. 2015, 24, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Véle, A.; Holuša, J.; Frouz, J. Ecological requirements of some ant species of the genus Formica (Hymenoptera, Formicidae) in spruce forests. J. For. Sci. 2009, 55, 32–40. [Google Scholar] [CrossRef]
- Bernasconi, C.; Cherix, D.; Seifert, B.; Pamilo, P. Molecular taxonomy of the Formica rufa group (red wood ants) (Hymenoptera: Formicidae): A new cryptic species in the Swiss Alps? Myrmecol. News 2010, 14, 37–47. [Google Scholar]
- Gosswald, K. The wood ant. Volume 2. The wood ant in the forest ecosystem, its uses and protection. In Die Waldameise. Band 2. Die Waldameise Okosystem Wald, ihr Nutzen und ihre Hege; AULA: Wiesbaden, Germany, 1989. [Google Scholar]
- Bernasconi, C.; Pamilo, P.; Cherix, D. Genetic differentiation of disjunct populations of the ants Formica aquilonia and Formica lugubris in Europe. Insectes Soc. 1992, 39, 15–29. [Google Scholar] [CrossRef]
- Goropashnaya, A.V.; Fedorov, V.B.; Pamilo, P. Recent speciation in the Formica rufa group ants (Hymenoptera, Formicidae): Inference from mitochondrial DNA phylogeny. Mol. Phylogenet. Evol. 2004, 32, 198–206. [Google Scholar] [CrossRef]
- Stockan, J.A.; Robinson, E.J.H. Wood Ant Ecology and Conservation; Cambridge University Press: Cambridge, UK, 2016; ISBN 9781107261402. [Google Scholar]
- Staniec, B.; Zagaja, M. Rove-beetles (Coleoptera, Staphylinidae) of ant nests of the vicinities of Leżajsk. Ann. UMCS Biol. 2008, 63, 111–127. [Google Scholar] [CrossRef][Green Version]
- Härkönen, S.K.; Sorvari, J. Species richness of associates of ants in the nests of red wood ant Formica polyctena (Hymenoptera, Formicidae). Insect Conserv. Divers. 2014, 7, 485–495. [Google Scholar] [CrossRef]
- Zagaja, M.; Staniec, B.; Pietrykowska-Tudruj, E.; Trytek, M. Biology and defensive secretion of myrmecophilous Thiasophila spp. (Coleoptera: Staphylinidae: Aleocharinae) associated with the Formica rufa species group. J. Nat. Hist. 2017, 51, 2759–2777. [Google Scholar] [CrossRef]
- Parmentier, T.; De Laender, F.; Wenseleers, T.; Bonte, D. Prudent behavior rather than chemical deception enables a parasite to exploit its ant host. Behav. Ecol. 2018, 29, 1225–1233. [Google Scholar] [CrossRef]
- Mazur, S. Część XIX Chrząszcze—Coleoptera, zeszyt 11-12, Sphaeritidae i gniliki—Histeridae; Klucze do.; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1973. [Google Scholar]
- Shavrin, A.V.; Anichtchenko, A.V.; Barsevskis, A. Contribution to the knowledge of myrmecophilous beetles (Coleoptera) of Latvia. Linzer Biol. Beiträge 2015, 47, 1829–1842. [Google Scholar]
- Assing, V.; Schülke, M. Freude–Harde–Lohse–Klausnitzer—Die Käfer Mitteleuropas. Band 4. Staphylinidae I. Zweite neubearbeitete Auflage; Spektrum Akademischer Verlag, I-XII: Heidelberg, Germany, 2011. [Google Scholar]
- Besuchet, C. Ptiliidae. Die Kafer Mitteleuropus, 3. Krefeld; Freude, H., Harde, K.W., Lohse, G.A., Eds.; Goecke und Evers: Krefeld, Germany, 1971; pp. 311–334. [Google Scholar]
- Freude, H.; Harde, K.; Lohse, W. Die Käfer Mitteleuropas. Bd. 5. Staphylinidae II (Hypocyphtinae und Aleocharinae), Pselaphidae; Goecke und Evers Verlag: Krefeld, Germany, 1974. [Google Scholar]
- Stebnicka, Z. Chrząszcze—Coleoptera, Czarnuchowate—Tenebrionidae, Boridae; Klucze do Oznaczania Owadów Polski, cz. XIX: Wroclaw, Poland, 1991. [Google Scholar]
- Ślipiński, S. Chrząszcze—Coleoptera, Monotomidae; Klucze do Oznaczania Owadów Polski, cz. XIX: Wroclaw, Poland, 1981. [Google Scholar]
- Witzgall, K. Histeridae. In Die Käfer Mitteleuropas. Bd. 3; Freude, H., Harde, K.W., Lohse, G.A., Eds.; Goecke & Evers Verlag: Krefeld, Germany, 1971; pp. 156–189. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Pena, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Naufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Accids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.; DeSantis, T.; Andersen, G.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- McDonald, D.; Clemente, J.C.; Kuczynski, J.; Rideout, J.R.; Stombaugh, J.; Wendel, D.; Wilke, A.; Huse, S.; Hufnagle, J.; Meyer, F.; et al. The Biological Observation Matrix (BIOM) format or: How I learned to stop worrying and love the ome-ome. Gigascience 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A tool for visualizing high-throughput microbial community data. Gigascience 2013, 26, 2–16. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Gorley, R.N. Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. Ecol. 2008, 366, 56–69. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R. PRIMER version 7: User manual/tutorial. Available online: http://updates.primer-e.com/primer7/manuals/Getting_started_with_PRIMER_7.pdf (accessed on 1 December 2019).
- Duron, O.; Hurst, G.D. Arthropods and inherited bacteria: From counting the symbionts to understanding how symbionts count. BMC Biol. 2013, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Santo Domingo, J.W.; Kaufman, M.G.; Klug, M.J.; Holben, W.E.; Harris, D.; Tiedje, J.M. Influence of diet on the structure and function of the bacterial hindgut community of crickets. Mol. Ecol. 1998, 7, 761–767. [Google Scholar] [CrossRef]
- Schmitt-Wagner, D.; Friedrich, M.W.; Wagner, B.; Brune, A. Axial Dynamics, Stability, and Interspecies Similarity of Bacterial Community Structure in the Highly Compartmentalized Gut of Soil-Feeding Termites (Cubitermes spp.). Appl. Environ. Microbiol. 2003, 69, 6018–6024. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, Y.; Deevong, P.; Inoue, T.; Moriya, S.; Trakulnaleamsai, S.; Ohkuma, M.; Vongkaluang, C.; Noparatnaraporn, N.; Kudo, T. Intra-and interspecific comparisons of bacterial diversity community structure support co-evolution of gut microbiota and termite host. Appl. Environ. Microbiol. 2005, 71, 6590–6599. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.I.; Tebbe, C.C. Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ. Microbiol. 2006, 8, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.; Yuval, B.; Jurkevitch, E. Community Structure of the Mediterranean Fruit Fly Microbiota: Seasonal and Spatial Sources of Variation. Isr. J. Ecol. Evol. 2008, 54, 181–191. [Google Scholar] [CrossRef]
- Ramalho, M.O.; Moreau, C.S.; Bueno, O.C. The Potential Role of Environment in Structuring the Microbiota of Camponotus across Parts of the Body. Adv. Entomol. 2019, 7, 47–70. [Google Scholar] [CrossRef][Green Version]
- Anderson, K.E.; Russell, J.A.; Moreau, C.S.; Kautz, S.; Sullam, K.E.; Hu, Y.; Basinger, U.; Mott, B.M.; Buck, N.; Wheeler, D.E. Highly similar microbial communities are shared among related and trophically similar ant species. Mol. Ecol. 2012, 21, 2282–2296. [Google Scholar] [CrossRef]
- Ramalho, M.O.; Bueno, O.C.; Moreau, C.S. Microbial composition of spiny ants (Hymenoptera: Formicidae: Polyrhachis) across their geographic range. BMC Evol. Biol. 2017, 17, 96. [Google Scholar] [CrossRef]
- Sanders, J.G.; Powell, S.; Kronauer, D.J.C.; Vasconcelos, H.L.; Frederickson, M.E.; Pierce, N.E. Stability and phylogenetic correlation in gut microbiota: Lessons from ants and apes. Mol. Ecol. 2014, 23, 1268–1283. [Google Scholar] [CrossRef]
- Tholen, A.; Schink, B.; Brune, A. The gut microflora of Reticulitermes flavipes, its relation to oxygen, and evidence for oxygen-dependent acetogenesis by the most abundant Enterococcus sp. FEMS Microbiol. Ecol. 2006, 24, 137–149. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Goldman-Huertas, B.; Moreau, C.S.; Baldo, L.; Stahlhut, J.K.; Werren, J.H.; Pierce, N.E. Specialization and geographic isolation among Wolbachia symbionts from ants and lycaenid butterflies. Evolution 2009, 63, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Funaro, C.F.; Kronauer, D.J.C.; Moreau, C.S.; Goldman-Huertas, B.; Pierce, N.E.; Russell, J.A. Army ants harbor a host-specific clade of Entomoplasmatales bacteria. Appl. Environ. Microbiol. 2011, 77, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Martinson, V.G.; Danforth, B.N.; Minckley, R.L.; Rueppell, O.; Tingek, S.; Moran, N.A. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol. Ecol. 2011, 20, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Martinson, V.G.; Moran, N.A.; Robinson, G.E. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA 2012, 109, 11002–11007. [Google Scholar] [CrossRef]
- Köhler, T.; Dietrich, C.; Scheffrahn, R.H.; Brune, A. High-Resolution Analysis of Gut Environment and Bacterial Microbiota Reveals Functional Compartmentation of the Gut in Wood-Feeding Higher Termites (Nasutitermes spp.). Appl. Environ. Microbiol. 2012, 78, 4691–4701. [Google Scholar] [CrossRef]
- Poulsen, M.; Sapountzis, P. Behind every great ant, there is a great gut. Mol. Ecol. 2012, 21, 2054–2057. [Google Scholar] [CrossRef]
- Su, L.; Yang, L.; Huang, S.; Su, X.; Li, Y.; Wang, F.; Wang, E.; Kang, N.; Xu, J.; Song, A. Comparative Gut Microbiomes of Four Species Representing the Higher and the Lower Termites. J. Insect Sci. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Hornung, B.V.H.; Zwittink, R.D.; Kuijper, E.J. Issues and current standards of controls in microbiome research. FEMS Microbiol. Ecol. 2019, 95, 1–7. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T.; Sanchez, L.G.; Fierer, N. A cross-taxon analysis of insect-associated bacterial diversity. PLoS ONE 2013, 8, e61218. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Choi, M.Y.; Kim, J.W.; Lee, S.A.; Ahn, J.H.; Song, J.; Kim, S.H.; Weon, H.Y. Effects of diet type, developmental stage, and gut compartment in the gut bacterial communities of two Cerambycidae species (Coleoptera). J. Microbiol. 2017, 55, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.S.; Ramalho, M.O.; Martins, C.; Martins, V.G.; Bueno, O.C. Microbial Communities in Different Tissues of Atta sexdens rubropilosa Leaf-cutting Ants. Curr. Microbiol. 2017, 74, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Kellner, K.; Ishak, H.D.; Linksvayer, T.A.; Mueller, U.G. Bacterial community composition and diversity in an ancestral ant fungus symbiosis. FEMS Microbiol. Ecol. 2015, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lanan, M.C.; Rodrigues, P.A.P.; Agellon, A.; Jansma, P.; Wheeler, D.E. A bacterial filter protects and structures the gut microbiome of an insect. ISME J. 2016, 10, 1866–1876. [Google Scholar] [CrossRef]
- Meirelles, L.A.; McFrederick, Q.S.; Rodrigues, A.; Mantovani, J.D.; De Melo Rodovalho, C.; Ferreira, H.; Bacci, M.; Mueller, U.G. Bacterial microbiomes from vertically transmitted fungal inocula of the leaf-cutting ant Atta texana. Environ. Microbiol. Rep. 2016, 8, 630–640. [Google Scholar] [CrossRef]
- Scott, J.J.; Budsberg, K.J.; Suen, G.; Wixon, D.L.; Balser, T.C.; Currie, C.R. Microbial community structure of leaf-cutter ant fungus gardens and refuse dumps. PLoS ONE 2010, 5, e9922s. [Google Scholar] [CrossRef]
- Sapountzis, P.; Zhukova, M.; Hansen, L.H.; Sørensen, S.J.; Schiøtt, M.; Boomsma, J.J. Acromyrmex leaf-cutting ants have simple gut microbiota with nitrogen-fixing potential. Appl. Environ. Microbiol. 2015, 81, 5527–5537. [Google Scholar] [CrossRef]
- Di Salvo, M.; Calcagnile, M.; Talà, A.; Tredici, S.M.; Maffei, M.E.; Schönrogge, K.; Barbero, F.; Alifano, P. The Microbiome of the Maculinea-Myrmica Host-Parasite Interaction. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.R.L.; Salzman, S.; Sanders, J.; Kaltenpoth, M.; Pierce, N.E. Microbial communities of lycaenid butterflies do not correlate with larval diet. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Szenteczki, M.A.; Pitteloud, C.; Casacci, L.P.; Kešnerová, L.; Whitaker, M.R.L.; Engel, P.; Vila, R.; Alvarez, N. Bacterial communities within Phengaris (Maculinea) alcon caterpillars are shifted following transition from solitary living to social parasitism of Myrmica ant colonies. Ecol. Evol. 2019, 9, 4452–4464. [Google Scholar] [CrossRef] [PubMed]
- Seipke, R.F.; Org Barke, J.; Heavens, D.; Yu, D.W.; Hutchings, M.I. Analysis of the bacterial communities associated with two ant–plant symbioses. Microbiol. Open 2013, 2, 276–283. [Google Scholar] [CrossRef]
- Chua, K.O.; Song, S.L.; Yong, H.S.; See-Too, W.S.; Yin, W.F.; Chan, K.G. Microbial Community Composition Reveals Spatial Variation and Distinctive Core Microbiome of the Weaver Ant Oecophylla smaragdina in Malaysia. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Zakalyukina, Y.V.; Biryukov, M.V.; Golichenkov, M.V.; Netrusov, A.I. Phenotypic and phylogenetic characterization of actinomycetes isolated from Lasius niger and Formica cunicularia ants. Mosc. Univ. Biol. Sci. Bull. 2017, 72, 13–19. [Google Scholar] [CrossRef]
- Currie, C.R.; Poulsen, M.; Mendenhall, J.; Boomsma, J.J.; Billen, J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 2006, 311, 81–83. [Google Scholar] [CrossRef]
- Sen, R.; Ishak, H.D.; Estrada, D.; Dowd, S.E.; Hong, E.; Mueller, U.G. Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc. Natl. Acad. Sci. USA 2009, 106, 17805–17810. [Google Scholar] [CrossRef]
- Mueller, U.G.; Scott, J.J.; Ishak, H.D.; Cooper, M.; Rodrigues, A. Monoculture of leafcutter ant gardens. PLoS ONE 2010, 5, 1–7. [Google Scholar] [CrossRef]
- Zucchi, T.D.; Guidolin, A.S.; Consoli, F.L. Isolation and characterization of actinobacteria ectosymbionts from Acromyrmex subterraneus brunneus (Hymenoptera, Formicidae). Microbiol. Res. 2011, 166, 68–76. [Google Scholar] [CrossRef]
- Currie, C.R.; Scott, J.A.; Summerbell, R.C.; Malloch, D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 1999, 398, 701–704. [Google Scholar] [CrossRef]
- Haeder, S.; Wirth, R.; Herz, H.; Spiteller, D. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4742–4746. [Google Scholar] [CrossRef] [PubMed]
- Barke, J.; Seipke, R.F.; Grüschow, S.; Heavens, D.; Drou, N.; Bibb, M.J.; Goss, R.J.; Yu, D.W.; Hutchings, M.I. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol. 2010, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Schoenian, I.; Spiteller, M.; Ghaste, M.; Wirth, R.; Herz, H.; Spiteller, D. Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants. Proc. Natl. Acad. Sci. USA 2011, 108, 1955–1960. [Google Scholar] [CrossRef]
- Mattoso, T.C.; Moreira, D.D.O.; Samuels, R.I. Symbiotic bacteria on the cuticle of the leaf-cutting ant Acromyrmex subterraneus subterraneus protect workers from attack by entomopathogenic fungi. Biol. Lett. 2012, 8, 461–464. [Google Scholar] [CrossRef]
- Matarrita-Carranza, B.; Moreira-Soto, R.D.; Murillo-Cruz, C.; Mora, M.; Currie, C.R.; Pinto-Tomas, A.A. Evidence for widespread associations between neotropical hymenopteran insects and Actinobacteria. Front. Microbiol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Esposti, M.D.; Romero, E.M. The functional microbiome of arthropods. PLoS ONE 2017, 12, e0176573. [Google Scholar] [CrossRef]
- Russell, J.A.; Moreau, C.S.; Goldman-Huertas, B.; Fujiwara, M.; Lohman, D.J.; Pierce, N.E. Bacterial gut symbionts are tightly linked with the evolution of herbivory in ants. Proc. Natl. Acad. Sci. USA 2009, 106, 21236–21241. [Google Scholar] [CrossRef]
- Brown, B.P.; Wernegreen, J.J. Deep divergence and rapid evolutionary rates in gut-associated Acetobacteraceae of ants. BMC Microbiol. 2016, 16, 1–17. [Google Scholar] [CrossRef]
- Hu, Y.; Holway, D.A.; Łukasik, P.; Chau, L.; Kay, A.D.; LeBrun, E.G.; Miller, K.A.; Sanders, J.G.; Suarez, A.V.; Russell, J.A. By their own devices: Invasive Argentine ants have shifted diet without clear aid from symbiotic microbes. Mol. Ecol. 2017, 26, 1608–1630. [Google Scholar] [CrossRef]
- Crotti, E.; Rizzi, A.; Chouaia, B.; Ricci, I.; Favia, G.; Alma, A.; Sacchi, L.; Bourtzis, K.; Mandrioli, M.; Cherif, A.; et al. Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 2010, 76, 6963–6970. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J.; Inkerman, P.A. Acetic acid bacterial biota of the pink sugar cane mealybug, Saccharococcus sacchari, and its environs. Appl. Environ. Microbiol. 1990, 56, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.W.; Nam, Y.D.; Chang, H.W.; Kim, K.H.; Kim, M.S.; Ryu, J.H.; Kim, S.H.; Lee, W.J.; Bae, J.W. Phylogenetic characterization of two novel commensal bacteria involved with innate immune homeostasis in Drosophila melanogaster. Appl. Environ. Microbiol. 2008, 74, 6171–6177. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lewis, Z.; Lizé, A. Insect behaviour and the microbiome. Curr. Opin. Insect Sci. 2015, 9, 86–90. [Google Scholar] [CrossRef]
- Frost, C.L.; Fernández-Marín, H.; Smith, J.E.; Hughes, W.O.H. Multiple gains and losses of Wolbachia symbionts across a tribe of fungus-growing ants. Mol. Ecol. 2010, 19, 4077–4085. [Google Scholar] [CrossRef]
- Frost, C.L.; Pollock, S.W.; Smith, J.E.; Hughes, W.O.H. Wolbachia in the flesh: Symbiont intensities in germ-line and somatic tissues challenge the conventional view of Wolbachia transmission routes. PLoS ONE 2014, 9, e95122. [Google Scholar] [CrossRef][Green Version]
- Van Borm, S.; Wenseleers, T.; Billen, J.; Boomsma, J.J. Cloning and sequencing of wsp encoding gene fragments reveals a diversity of co-infecting Wolbachia strains in Acromyrmex leafcutter ants. Mol. Phylogenet. Evol. 2003, 26, 102–109. [Google Scholar] [CrossRef]
- Ramalho, M.O.; Vieira, A.S.; Pereira, M.C.; Moreau, C.S.; Bueno, O.C. Transovarian Transmission of Blochmannia and Wolbachia Endosymbionts in the Neotropical Weaver Ant Camponotus textor (Hymenoptera, Formicidae). Curr. Microbiol. 2018, 75, 866–873. [Google Scholar] [CrossRef]
- Ramalho, M.O.; Martins, C.; Silva, L.M.R.; Martins, V.G.; Bueno, O.C. Intracellular Symbiotic Bacteria of Camponotus textor, Forel (Hymenoptera, Formicidae). Curr. Microbiol. 2017, 74, 589–597. [Google Scholar] [CrossRef]
- Reeves, D.D.; Price, S.L.; Ramalho, M.O.; Moreau, C.S. The Diversity and Distribution of Wolbachia, Rhizobiales, and Ophiocordyceps within the Widespread Neotropical Turtle Ant, Cephalotes atratus (Hymenoptera: Formicidae). Neotrop. Entomol. 2020. [Google Scholar] [CrossRef]
- Kelly, M.; Price, S.L.; De Oliveira Ramalho, M.; Moreau, C.S. Diversity of Wolbachia Associated with the Giant Turtle Ant, Cephalotes atratus. Curr. Microbiol. 2019, 76, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.O.; Bueno, O.C.; Moreau, C.S. Species-specific signatures of the microbiome from Camponotus and Colobopsis ants across developmental stages. PLoS ONE 2017, 12, e0187461. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Liautard, C.; Reuter, M.; Brown, W.D.; Sundström, L.; Chapuisat, M. Sex ratio and Wolbachia infection in the ant Formica exsecta. Heredity 2001, 87, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Viljakainen, L.; Reuter, M.; Pamilo, P. Wolbachia transmission dynamics in Formica wood ants. BMC Evol. Biol. 2008, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Ishak, H.D.; Plowes, R.; Sen, R.; Kellner, K.; Meyer, E.; Estrada, D.A.; Dowd, S.E.; Mueller, U.G. Bacterial Diversity in Solenopsis invicta and Solenopsis geminata Ant Colonies Characterized by 16S amplicon 454 Pyrosequencing. Microb. Ecol. 2011, 61, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Souza, R.F.; Bueno, O.C. Presence and distribution of the endosymbiont Wolbachia among Solenopsis spp. (Hymenoptera: Formicidae) from Brazil and its evolutionary history. J. Invertebr. Pathol. 2012, 109, 287–296. [Google Scholar] [CrossRef]
- Souza, R.F.; Martins, C.; Pereira, R.M.; Bueno, O.C. Analysis of the Hypervariable Regions (HVRs) of the wsp Gene of Wolbachia from Solenopsis invicta Ants in Southeastern Brazil. Adv. Entomol. 2014, 2, 135–143. [Google Scholar] [CrossRef][Green Version]
- Russell, J.A.; Funaro, C.F.; Giraldo, Y.M.; Goldman-Huertas, B.; Suh, D.; Kronauer, D.J.C.; Moreau, C.S.; Pierce, N.E. A veritable menagerie of heritable bacteria from ants, butterflies, and beyond: Broad molecular surveys and a systematic review. PLoS ONE 2012, 7, e51027. [Google Scholar] [CrossRef]
- Pontieri, L.; Schmidt, A.M.; Singh, R.; Pedersen, J.S.; Linksvayer, T.A. Artificial selection on ant female caste ratio uncovers a link between female-biased sex ratios and infection by Wolbachia endosymbionts. J. Evol. Biol. 2017, 30, 225–234. [Google Scholar] [CrossRef]
- Wenseleers, T.; Ito, F.; Van Borm, S.; Huybrechts, R.; Volckaert, F.; Billen, J. Widespread Occurrence of the Microorganism Wolbachia in Ants. Proc. Biol. Sci. 1998, 265, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Wolbachia Pipientis: Microbial Manipulator of Arthropod Reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef] [PubMed]
- Charlat, S.; Hurst, G.D.D.; Merçot, H. Evolutionary consequences of Wolbachia infections. Trends Genet. 2003, 19, 217–223. [Google Scholar] [CrossRef]
- Correa, C.C.; Ballard, J.W.O. Wolbachia Associations with Insects: Winning or Losing Against a Master Manipulator. Front. Ecol. Evol. 2016, 3, 1–18. [Google Scholar] [CrossRef]
- Pietri, J.E.; Debruhl, H.; Sullivan, W. The rich somatic life of Wolbachia. Microbiol. Open 2016, 5, 923–936. [Google Scholar] [CrossRef]
- Hedges, L.M.; Brownlie, J.C.; O’neill, S.L.; Johnson, K.N. Wolbachia and Virus Protection in Insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Goodacre, S.L.; Martin, O.Y. Modification of Insect and Arachnid Behaviours by Vertically Transmitted Endosymbionts: Infections as Drivers of Behavioural Change and Evolutionary Novelty. Insects 2012, 3, 246–261. [Google Scholar] [CrossRef]
- Andersen, S.B.; Boye, M.; Nash, D.R.; Boomsma, J.J. Dynamic Wolbachia prevalence in Acromyrmex leaf-cutting ants: Potential for a nutritional symbiosis. J. Evol. Biol. 2012, 25, 1340–1350. [Google Scholar] [CrossRef]
- Russell, J.A.; Sanders, J.G.; Moreau, C.S. Hotspots for symbiosis: Function, evolution, and specificity of ant-microbe associations from trunk to tips of the ant phylogeny (Hymenoptera: Formicidae). Myrmecol. News 2017, 24, 43–69. [Google Scholar]
- Russell, J.A. The ants (Hymenoptera: Formicidae) are unique and enigmatic hosts of prevalent Wolbachia (Alphaproteobacteria) symbionts. Myrmecol. News 2012, 16, 7–23. [Google Scholar]
- Reuter, M.; Pedersen, J.S.; Keller, L. Loss of Wolbachia infection during colonisation in the invasive Argentine ant Linepithema humile. Heredity 2005, 94, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Tolley, S.J.A.; Nonacs, P.; Sapountzis, P. Wolbachia Horizontal Transmission Events in Ants: What Do We Know and What Can We Learn? Front. Microbiol. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dedeine, F.; Ahrens, M.; Calcaterra, L.; Shoemaker, D.D. Social parasitism in fire ants (Solenopsis spp.): A potential mechanism for interspecies transfer of Wolbachia. Mol. Ecol. 2005, 14, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Cordaux, R.; Michel-Salzat, A.; Bouchon, D. Wolbachia infection in crustaceans: Novel hosts and potential routes for horizontal transmission. J. Evol. Biol. 2001, 14, 237–243. [Google Scholar] [CrossRef]
- Dyson, E.A.; Kamath, M.K.; Hurst, G.D.D. Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): Evidence for horizontal transmission of a butterfly male killer. Heredity 2002, 88, 166–171. [Google Scholar] [CrossRef]
- Li, S.J.; Ahmed, M.Z.; Lv, N.; Shi, P.Q.; Wang, X.M.; Huang, J.L.; Qiu, B.L. Plantmediated horizontal transmission of Wolbachia between whiteflies. ISME J. 2017, 11, 1019–1028. [Google Scholar] [CrossRef]
- Kajtoch, Ł.; Kotásková, N. Current state of knowledge on Wolbachia infection among Coleoptera: A systematic review. Peer J. 2018, 6, e4471. [Google Scholar] [CrossRef]
- Hughes, G.L.; Dodson, B.L.; Johnson, R.M.; Murdock, C.C.; Tsujimoto, H.; Suzuki, Y.; Patt, A.A.; Cui, L.; Nossa, C.W.; Barry, R.M.; et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad. Sci. USA 2014, 111, 12498–12503. [Google Scholar] [CrossRef]
- Giorgini, M.; Bernardo, U.; Monti, M.M.; Nappo, A.G.; Gebiola, M. Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl. Environ. Microbiol. 2010, 76, 2589–2599. [Google Scholar] [CrossRef]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Hendry, T.A.; Hunter, M.S.; Baltrus, D.A. The Facultative Symbiont Rickettsia Protects an Invasive Whitefly against Entomopathogenic Pseudomonas syringae Strains. Appl. Environ. Microbiol. 2014, 80, 7161–7168. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, A.; Pamilo, P. Multiple endosymbionts in populations of the ant Formica cinerea. BMC Evol. Biol. 2010, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Tinsley, M.C.; Temperley, M.; Jiggins, F.M. Are we underestimating the diversity and incidence of insect bacterial symbionts? A case study in ladybird beetles. Biol. Lett. 2007, 3, 678–681. [Google Scholar] [CrossRef]
- White, J.A.; Richards, N.K.; Laugraud, A.; Saeed, A.; Curry, M.M.; McNeill, M.R. Endosymbiotic Candidates for Parasitoid Defense in Exotic and Native New Zealand Weevils. Microb. Ecol. 2015, 70, 274–286. [Google Scholar] [CrossRef]
- Perotti, A.M.; Young, D.K.; Braig, H.R. The ghost sex-life of the paedogenetic beetle Micromalthus debilis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The Gut Bacteria of Insects: Nonpathogenic Interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Hu, Y.; Łukasik, P.; Moreau, C.S.; Russell, J.A. Correlates of gut community composition across an ant species (Cephalotes varians) elucidate causes and consequences of symbiotic variability. Mol. Ecol. 2014, 23, 1284–1300. [Google Scholar] [CrossRef]
- Rubin, B.E.R.; Kautz, S.; Wray, B.D.; Moreau, C.S. Dietary specialization in mutualistic acacia-ants affects relative abundance but not identity of host-associated bacteria. Mol. Ecol. 2019, 28, 900–916. [Google Scholar] [CrossRef]



| Host Species | D. pygmaeus | F. polyctena | L. formicetorum | M. angusticollis | M. subterraneus | P. formicetorum | T. angulata |
|---|---|---|---|---|---|---|---|
| D. pygmaeus | 51.066 | ||||||
| F. polyctena | 22.568 | 48.954 | |||||
| L. formicetorum | 51.785 | 17.534 | 65.864 | ||||
| M. angusticollis | 16.151 | 11.77 | 13.216 | 77.465 | |||
| M. subterraneus | 53.743 | 18.352 | 62.895 | 11.372 | 58.866 | ||
| P. formicetorum | 35.998 | 16.628 | 40.068 | 35.943 | 43.313 | 33.288 | |
| T. angulata | 28.933 | 13.482 | 30.611 | 27.323 | 28.206 | 26.899 | 18.352 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarczyk-Ziemba, A.; Zagaja, M.; Wagner, G.K.; Pietrykowska-Tudruj, E.; Staniec, B. First Insight into Microbiome Profiles of Myrmecophilous Beetles and Their Host, Red Wood Ant Formica polyctena (Hymenoptera: Formicidae)—A Case Study. Insects 2020, 11, 134. https://doi.org/10.3390/insects11020134
Kaczmarczyk-Ziemba A, Zagaja M, Wagner GK, Pietrykowska-Tudruj E, Staniec B. First Insight into Microbiome Profiles of Myrmecophilous Beetles and Their Host, Red Wood Ant Formica polyctena (Hymenoptera: Formicidae)—A Case Study. Insects. 2020; 11(2):134. https://doi.org/10.3390/insects11020134
Chicago/Turabian StyleKaczmarczyk-Ziemba, Agnieszka, Mirosław Zagaja, Grzegorz K. Wagner, Ewa Pietrykowska-Tudruj, and Bernard Staniec. 2020. "First Insight into Microbiome Profiles of Myrmecophilous Beetles and Their Host, Red Wood Ant Formica polyctena (Hymenoptera: Formicidae)—A Case Study" Insects 11, no. 2: 134. https://doi.org/10.3390/insects11020134
APA StyleKaczmarczyk-Ziemba, A., Zagaja, M., Wagner, G. K., Pietrykowska-Tudruj, E., & Staniec, B. (2020). First Insight into Microbiome Profiles of Myrmecophilous Beetles and Their Host, Red Wood Ant Formica polyctena (Hymenoptera: Formicidae)—A Case Study. Insects, 11(2), 134. https://doi.org/10.3390/insects11020134





