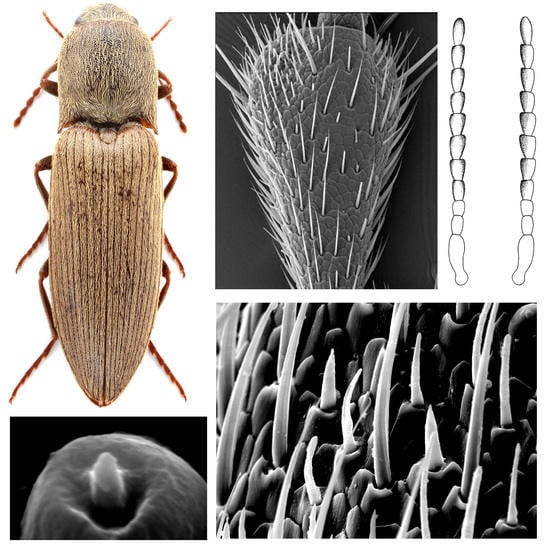
Comparative Antennal Morphology of Agriotes (Coleoptera: Elateridae), with Special Reference to the Typology and Possible Functions of Sensilla
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Gross Morphology of Antennae in Agriotes Species
3.2. Types of Sensilla in Agriotes Species
3.2.1. Sensilla chaetica (subtypes C1–C2)
3.2.2. Sensilla trichodea
3.2.3. Sensilla basiconica (subtypes B1–B9)
3.2.4. Dome-shaped Sensilla (subtypes D1–D2)
3.2.5. Sensilla campaniformia
3.2.6. Böhm sensilla
3.2.7. Glandular pores
4. Discussion
4.1. Sensilla chaetica
4.2. Sensilla trichodea
4.3. Sensilla basiconica
4.4. Dome-shaped sensilla
4.5. Sensilla campaniformia
4.6. Böhm sensilla
4.7. Glandular pores
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, D.S. Agricultural Insect Pests of Temperate Regions and Their Control; Cambridge University Press: Cambridge, UK, 1987; 272p. [Google Scholar]
- Parker, W.E.; Howard, J.J. The biology and management of wireworms (Agriotes spp.) on potato with particular reference to the U.K. Agric. For. Entomol. 2001, 3, 85–98. [Google Scholar] [CrossRef]
- Barsics, F.; Haubruge, E.; Verheggen, F.J. Wireworms’ management: An overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). Insects 2013, 4, 117–152. [Google Scholar] [CrossRef] [Green Version]
- Ritter, C.; Richter, E. Control methods and monitoring of Agriotes wireworms (Coleoptera: Elateridae). J. Plant Dis. Protect. 2013, 120, 4–15. [Google Scholar] [CrossRef]
- Keiser, A.; Häberli, M.; Stamp, P. Quality deficiencies on potato (Solanum tuberosum L.) tubers caused by Rhizoctonia solani, wireworms (Agriotes ssp.) and slugs (Deroceras reticulatum, Arion hortensis) in different farming systems. Field Crop Res. 2012, 128, 147–155. [Google Scholar] [CrossRef]
- Traugott, M.; Benefer, C.M.; Blackshaw, R.P.; van Herk, W.G.; Vernon, R.S. Biology, ecology, and control of elaterid beetles in agricultural land. Annu. Rev. Entomol. 2015, 60, 313–334. [Google Scholar] [CrossRef] [Green Version]
- Cate, P. Elateridae. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, pp. 89–209. [Google Scholar]
- Kabalak, M.; Sert, O.; Özgen, İ.; Platia, G. A new species of the Agriotes nuceus species group from Turkey. J. Insect Sci. 2013, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J. Elateridae Leach 1815. In American Beetles, Volume 2, Polyphaga: Scarabaeoidea through Curculionoidea; Arnett, R.H., Thomas, M.C., Skelley, P.E., Frank, J.H., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 160–173. [Google Scholar]
- Furlan, L.; Tóth, M.; Parker, W.E.; Ivezić, M.; Pancic, S.; Brmez, M.; Dobrinčić, R.; Barčić, J.I.; Mureşan, F.; Subchev, M.; et al. The efficacy of the new Agriotes sex pheromone traps in detecting wireworm population levels in different European countries. In Proceedings of the XXI IWGO Conference, Legnaro, Italy, 27 October–3 November 2001; Veneto Agricoltura: Legnaro, Italy, 2001; pp. 293–303. [Google Scholar]
- Tóth, M.; Furlan, L.; Xavier, A.; Vuts, J.; Toshova, T.; Subchev, M.; Szarukan, I.; Yatsynin, V. New sex attractant composition for the click beetle Agriotes proximus: Similarity to the pheromone of Agriotes lineatus. J. Chem. Ecol. 2008, 34, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, K.; Pitterl, P.; Furlan, L.; Cate, P.C.; Traugott, M. PCR-based species identification of Agriotes larvae. Bull. Entomol. Res. 2011, 101, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Vuts, J.; Tolasch, T.; Furlan, L.; Csonka, É.B.; Felföldi, T.; Márialigeti, K.; Toshova, T.B.; Subchev, M.; Xavier, A.; Tóth, M. Agriotes proximus and A. lineatus (Coleoptera: Elateridae): A comparative study on the pheromone composition and cytochrome c oxidase subunit I gene sequence. Chemoecology 2012, 22, 23–28. [Google Scholar] [CrossRef]
- Benefer, C.M.; van Herk, W.G.; Ellis, J.S.; Blackshaw, R.P.; Vernon, R.S.; Knight, M.E. The molecular identification and genetic diversity of economically important wireworm species (Coleoptera: Elateridae) in Canada. J. Pest Sci. 2013, 86, 19–27. [Google Scholar] [CrossRef]
- Tóth, M.; Furlan, L.; Yatsynin, V.G.; Ujvary, I.; Szarukan, I.; Imrei, Z.; Tolasch, T.; Francke, W.; Jossi, W. Identification of pheromones and optimization of bait composition for click beetle pests (Coleoptera: Elateridae) in Central and Western Europe. Pest Manag. Sci. 2003, 59, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.S.; Tóth, M. Evaluation of pheromones and a new trap for monitoring Agriotes lineatus and Agriotes obscurus in the Fraser valley of British Columbia. J. Chem. Ecol. 2007, 32, 345–351. [Google Scholar] [CrossRef]
- Tóth, M. Pheromones and attractants of click beetles: An overview. J. Pest Sci. 2013, 86, 3–17. [Google Scholar] [CrossRef]
- Blackshaw, R.P.; van Herk, W.G.; Vernon, R.S. Determination of Agriotes obscurus (Coleoptera: Elateridae) sex pheromone attraction range using target male behavioural responses. Agric. For. Entomol. 2018, 20, 228–233. [Google Scholar] [CrossRef]
- Tóth, M.; Furlan, L.; Szarukán, I.; Nagy, A.; Vuts, J.; Toshova, T.; Velchev, D.; Lohonyai, Z.; Imrei, Z. The addition of a pheromone to a floral lure increases catches of females of the click beetle Agriotes ustulatus (Schaller) (Coleoptera: Elateridae). J. Chem. Ecol. 2019, 45, 667–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg-Karlson, A.-K.; Agren, L.; Dobson, H.; Bergström, G. Identification and electroantennographic activity of sex-specific geranyl esters in an abdominal gland of female Agriotes obscurus (L.) and A. lineatus (L.) (Coleoptera, Elateridae). Experientia 1988, 44, 531–534. [Google Scholar] [CrossRef]
- Merivee, E.; Erm, A. Studies on sex pheromone gland morphology and pheromone components in female elaterid beetles Agriotes obscurus L. and Agriotes lineatus L. (Coleoptera, Elateridae). Eesti. NSV Tead. Akad. Toim. Biol. 1993, 42, 108–117. [Google Scholar]
- Yatsynin, V.G.; Rubanova, E.V.; Okhrimenko, N.V. Identification of female-produced sex pheromones and their geographical differences in pheromone gland extract composition from click beetles (Col., Elateridae). J. Appl. Entomol. 1996, 120, 463–466. [Google Scholar] [CrossRef]
- Tóth, M.; Furlan, L.; Yatsynin, V.G.; Ujvary, I.; Szarukan, I.; Imrei, Z.; Subchev, M.; Tolasch, T.; Francke, W. Identification of sex pheromone composition of click beetle Agriotes brevis Candèze. J. Chem. Ecol. 2002, 28, 1641–1652. [Google Scholar] [CrossRef]
- Tolasch, T.; von Fragstein, M.; Steidle, J.L. Sex pheromone of Agriotes acuminatus (Stephens, 1830) (Coleoptera: Elateridae). J. Chem. Ecol. 2010, 36, 314–318. [Google Scholar] [CrossRef]
- Vuts, J.; Furlan, L.; Tóth, M. Female responses to synthetic pheromone and plant compounds in Agriotes brevis Candèze (Coleoptera: Elateridae). J. Insect Behav. 2018, 31, 106–117. [Google Scholar] [CrossRef]
- Merivee, E. Antennal sensilla of the female and male elaterid beetle Agriotes obscurus L. (Coleoptera: Elateridae). Proc. Estonian Acad. Sci. Biol. 1992, 41, 189–215. [Google Scholar]
- Merivee, E.; Rahi, M.; Luik, A. Distribution of olfactory and some other antennal sensilla in the male click beetle Agriotes obscurus L. (Coleoptera: Elateridae). Int. J. Insect Morphol. Embryol. 1997, 26, 75–83. [Google Scholar] [CrossRef]
- Merivee, E.; Rahi, M.; Bresciani, J.; Ravn, H.P.; Luik, A. Antennal sensilla of the click beetle, Limonius aeruginosus (Olivier) (Coleoptera: Elateridae). Int. J. Insect Morphol. Embryol. 1998, 27, 311–318. [Google Scholar] [CrossRef]
- Merivee, E.; Rahi, M.; Luik, A. Antennal sensilla of the click beetle, Melanotus villosus (Geoffroy) (Coleoptera: Elateridae). Int. J. Insect Morphol. Embryol. 1999, 28, 41–51. [Google Scholar] [CrossRef]
- Peng, G.; Shu, J.; Zhang, A.; Wang, J.; Xu, T.; Wang, H. Antennal sensilla of the click beetle, Melanotus cribricollis (Coleoptera: Elateridae). Sci. Silvae Sin. 2012, 48, 106–112. [Google Scholar]
- Ren, L.-L.; Wu, Y.; Shi, J.; Zhang, L.; Luo, Q. Antennal morphology and sensilla ultrastructure of Tetrigus lewisi Candèze (Coleoptera: Elateridae). Micron 2014, 60, 29–38. [Google Scholar] [CrossRef]
- Zauli, A.; Maurizi, E.; Carpaneto, G.M.; Chiari, S.; Merivee, E.; Svensson, G.P.; Di Giulio, A. Scanning electron microscopy analysis of the antennal sensilla in the rare saproxylic beetle Elater ferrugineus (Coleoptera, Elateridae). Ital. J. Zool. 2016, 83, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Faucheux, M.J.; Kundrata, R. Comparative antennal morphology of male Drilini with special reference to the sensilla (Coleoptera: Elateridae: Agrypninae). Zool. Anz. 2017, 266, 105–119. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Ann. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Internat. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Faucheux, M.J. Biodiversité et Unité des Organes Sensoriels des Insectes Lépidoptères; Société des Sciences Naturelles de l’Ouest de la France: Nantes, France, 1999; pp. 1–296. [Google Scholar]
- Chen, J.M.; Qiao, H.L.; Xhen, J.; Xu, C.Q.; Lian, Z.M.; Guo, K. Observation of antennal sensilla in Xylotrechus grayii (Coleoptera: Cerambycidae) with scanning electron microscopy. Microsc. Res. Tech. 2014, 77, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, M.J. Persistence of larval characteristics on the antennae of the neotenic female of Drilus mauritanicus Lucas, 1849 (Coleoptera, Elateridae, Agrypninae, Drilini). Bull. Inst. Sci. Rabat Sect. Sci. Vie 2014, 36, 65–76. [Google Scholar]
- Faucheux, M.J.; Beaulieu, G. Antennal sensilla in the male imago of Drilus mauritanicus Lucas 1849. Comparison with Malacogaster passerinii Bassi 1833 (Coleoptera: Elateridae: Agrypninae: Drilini). Bull. Soc. Sci. Nat. Ouest Fr. (N.S.) 2016, 38, 149–163. [Google Scholar]
- Altner, H. Insect sensillum specificity and structure: An approach to a new typology. In Olfaction and Taste; Le Magnen, J., MacLeod, P., Eds.; Information Retrieval: London, UK, 1977; Volume 6, pp. 295–303. [Google Scholar]
- Zacharuk, R.Y. Antennae and sensilla. In Comparative Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: London, UK, 1985; Volume 6, pp. 1–69. [Google Scholar]
- Faucheux, M.J. Sensilles antennaires du mâle du Ver luisant, Lampyris noctiluca (Linnaeus, 1767) (Coleoptera: Elateroidea: Lampyridae). Bull. Soc. Sci. Nat. Ouest Fr. (N.S.) 2015, 37, 145–152. [Google Scholar]
- Dyer, L.J.; Seabrook, W.D. Sensilla on the antennal flagellum of the sawyer beetles Monochamus notatus (Drury) and Monochamus scutellatus (Say) (Coleoptera: Cerambycidae). J. Morphol. 1975, 146, 513–532. [Google Scholar] [CrossRef]
- Dai, H.-G.; Honda, H. Sensilla on the antennal flagellum of the yellow spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1990, 25, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Faucheux, M.J. Le Grand Capricorne, Cerambyx cerdo Linné. In Les Insectes du Bois: Capricornes, Vrillettes, Termites et Autres Xylophages; Faucheux, M.J., Lebrun, D., Sadorge, A., Eds.; Société des Sciences Naturelles de l’Ouest de la France: Nantes, France, 2001; pp. 115–134. [Google Scholar]
- Faucheux, M.J. Antennal sensilla of the yellow beetle Phoracantha recurva Newman, 1840: Distribution and comparison with Phoracantha semipunctata (Fabricius, 1775) (Coleoptera: Cerambycidae). Bull. Inst. Sci. Rabat, Sect. Sci. Vie 2011, 33, 19–29. [Google Scholar]
- Hatfield, L.D.; Frazier, J.L.; Coons, L.B. Antennal sensilla of the pecan weevil, Curculio caryae (Horn) (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 1976, 5, 279–287. [Google Scholar] [CrossRef]
- Faucheux, M.J. Distribution and abundance of antennal sensilla from two populations of the pine engraver beetle, Ips pini (Say) (Coleoptera: Scolytidae). Ann. Sci. Nat. Zool. 1994, 15, 15–31. [Google Scholar]
- Merivee, E.; Ploomi, A.; Rahi, M.; Luik, A.; Sammelselg, V. Antennal sensilla of the ground beetle Bembidion lampros Hbst. (Coleoptera, Carabidae). Acta Zool. 2000, 81, 339–350. [Google Scholar] [CrossRef]
- Merivee, E.; Ploomi, A.; Luik, A.; Rahi, M.; Sammelselg, V. Antennal sensilla of the ground beetle Platynus dorsalis (Pontoppidan, 1763) (Coleoptera, Carabidae). Microsc. Res. Tech. 2001, 55, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D. Electrophysiological investigation of the olfactory specificity of sexual attracting substances in different species of moths. J. Insect Physiol. 1962, 8, 15–30. [Google Scholar] [CrossRef]
- Tóth, M.; Szöcs, G.; Löfstedt, C.; Hansson, B.S.; Subchev, M. Sex pheromone components of Mamestra suasa: Chemical analysis, electrophysiological activity, wind tunnel activity and field tests in two European countries. Entomol. Exp. Appl. 1986, 42, 291–299. [Google Scholar] [CrossRef]
- Greenfield, M.D. Sexual selection. In Pheromone Communication in Moths. Evolution, Behavior, and Application; Allison, J.D., Cardé, R.T., Eds.; University of California Press: Oakland, CA, USA, 2016; pp. 79–88. [Google Scholar]
- Cook, B.J.; Shelton, W.D.; Staten, R.T. Antennal responses of the pink bollworm to gossypiella. Southwest. Entomol. 1978, 3, 141–146. [Google Scholar]
- Van der Pers, J.N.C. Responses from olfactory receptors in females of three species of small ermine moths (Lepidoptera: Yponomeutidae) to plant odours. Entomol. Exp. Appl. 1978, 24, 394–398. [Google Scholar]
- Lopes, O.; Barata, E.N.; Mustaparta, H.; Araújo, J. Fine structure of antennal sensilla basiconica and their detection of plant volatiles in the eucalyptus woodborer, Phoracantha semipunctata Fabricius (Coleoptera: Cerambycidae). Arthropod Struct. Dev. 2002, 31, 1–13. [Google Scholar] [CrossRef]
- Sun, L.; Xiao, H.; Gu, S.H.; Zhou, J.J.; Guo, Y.Y.; Liu, Z.W.; Zhang, Y.J. The antenna-specific odorant binding protein AlinOBP13 of the alphalpha plant bug Adelphocoris lineolatus is expressed specifically in basiconic sensilla and has high binding affinity to terpenoids. Insect Mol. Biol. 2014, 23, 417–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, L.; Zhang, L.; Luo, Y. Ultrastructure of antennal and posterior abdominal sensilla in Chlorophorus caragana females. Micron 2015, 75, 45–57. [Google Scholar] [CrossRef]
- Hallberg, E. Sensory organs in Ips typographus (Insecta: Coleoptera)—Fine structure of antennal sensilla. Protoplasma 1982, 111, 206–214. [Google Scholar] [CrossRef]
- Monteforti, G.; Angeli, S.; Petacchi, R.; Minnocci, A. Ultrastructural characterization of antennal sensilla and immunocytochemical localization of a chemosensory protein in Carausius morosus Brünner (Phasmida: Phasmatidae). Arthropod Struct. Dev. 2002, 30, 195–205. [Google Scholar] [CrossRef]
- Faucheux, M.J. The larviform females of Malacogaster nigripes Schaufuss 1867. Antennal sensilla: Persistence and significance of larval characteristics (Coleoptera: Elateridae: Agrypninae: Drilini). Bull. Soc. Sci. Nat. Ouest Fr. (N.S.) 2016, 38, 287–310. [Google Scholar]
- Faucheux, M.J. La sensille campaniforme des insectes: La vraie et les fausses. Bull. Soc. Sci. Nat. Ouest Fr. (N.S.) 2019, 41, 155–159. [Google Scholar]
- Nurme, K.; Merivee, E.; Must, A.; Di Giulio, A.; Muzzi, M.; Williams, I.; Mänd, M. Bursty spike trains of antennal thermo- and bimodal hygro-thermoreceptor neurons encode noxious heat in elaterid beetles. J. Therm. Biol. 2018, 72, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Stange, G.; Stowe, S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc. Res. Tech. 1999, 47, 416–427. [Google Scholar] [CrossRef] [Green Version]
- McIver, S.B. Mechanoreception. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: London, UK, 1985; Volume 6, pp. 71–132. [Google Scholar]
- Ritcey, G.M.; McIver, S.B. External morphology of antennal sensilla of four species of adult flea beetles (Coleoptera: Chrysomelidae: Alticinae). Int. J. Insect Morphol. Embryol. 1990, 19, 141–153. [Google Scholar] [CrossRef]
- Fischer, D.C.; Kogan, M. Chemoreceptors of adult Mexican bean beetles. External morphology and role in food preference. Entomol. Exp. Appl. 1986, 40, 3–12. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Ann. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Faucheux, M.J.; Mason, F. Les antennes des Némotélinés (Insecta: Diptera: Stratiomyidae). I: Nemotelus Geoffroy et Cluninemotelus Mason. Bull. Soc. Sci. Nat. Ouest Fr. (N.S.) 2000, 22, 49–70. [Google Scholar]
- Faucheux, M.J. Sensilles auricilliformes multipores sur l’antenne de l’adulte femelle de Liposcelis decolor (Pearman 1925) (Psocoptera: Troctomorpha: Liposcelididae). Bull. Soc. Sci. Nat. Ouest Fr. (N.S.) 2012, 34, 233–242. [Google Scholar]
- Gurjeva, E.L. A review of the Palearctic species of the genus Agriotes Esch. (Coleoptera, Elateridae). Entomol. Obozr. 1972, 51, 859–877. [Google Scholar]
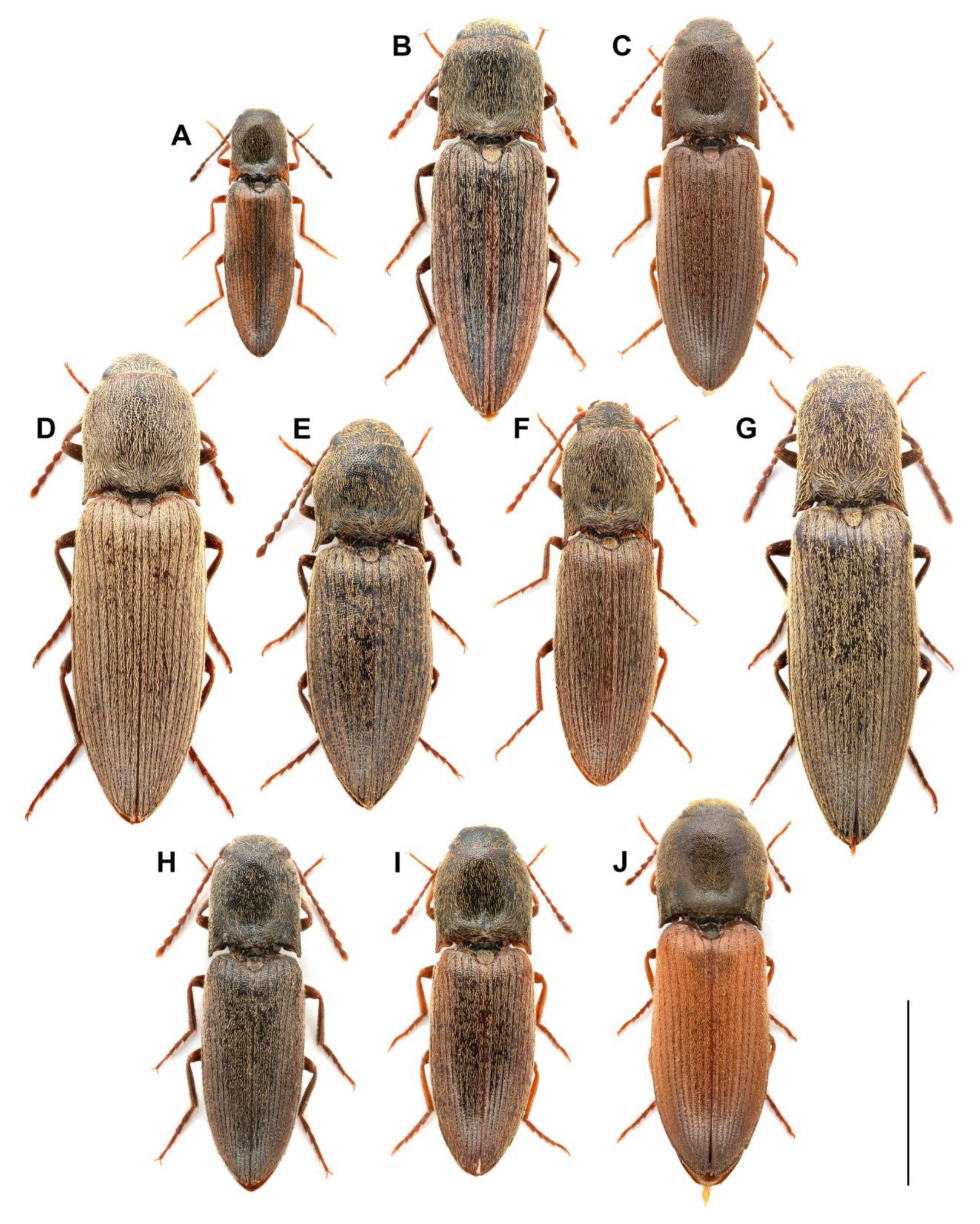










| Code | Species | Sex | Geographic Origin |
|---|---|---|---|
| Ag01 | A. acuminatus | M | Hungary, Vas county, Sárvár |
| Ag02 | A. acuminatus | M | Hungary, Szabolcs-Szatmár county, Tákos |
| Ag03 | A. acuminatus | M | Hungary, Somogy county, Kaposvár |
| Ag04 | A. acuminatus | F | Greece, Ioannina regional unit, Metsovo |
| Ag05 | A. acuminatus | F | Greece, Ioannina regional unit, Metsovo |
| Ag06 | A. acuminatus | F | Hungary, Tolna county, Bátaapáti |
| Ag07 | A. lineatus | M | Romania, Sălaj county, Huta |
| Ag08 | A. lineatus | M | Hungary, Baranya county, Sumony |
| Ag09 | A. lineatus | M | Hungary, Hajdú-Bihar county, Nagyhegyes |
| Ag10 | A. lineatus | F | Hungary, Budapest |
| Ag11 | A. medvedevi | M | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag12 | A. medvedevi | M | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag13 | A. medvedevi | M | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag14 | A. medvedevi | M | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag15 | A. medvedevi | F | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag16 | A. medvedevi | F | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag17 | A. medvedevi | F | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag18 | A. modestus | M | Hungary, Pest county, Tatárszentgyörgy |
| Ag19 | A. modestus | M | Hungary, Pest county, Tatárszentgyörgy |
| Ag20 | A. modestus | F | Hungary, Pest county, Tatárszentgyörgy |
| Ag21 | A. obscurus | M | Czech Republic, Moravia, Olomouc |
| Ag22 | A. obscurus | M | Czech Republic, Moravia, Olomouc |
| Ag23 | A. obscurus | M | Czech Republic, Moravia, Olomouc |
| Ag24 | A. obscurus | M | Hungary, Pest county, Tahitótfalu |
| Ag25 | A. obscurus | F | Romania, Sălaj county, Huta |
| Ag26 | A. obscurus | F | Hungary, Borsod-Abaúj-Zemplén county, Szögliget |
| Ag41 | A. paludum | M | Greece, Corinthia regional unit, Kato Sinikia Trikala |
| Ag42 | A. paludum | F | Greece, Grevena regional unit, Kipuro |
| Ag27 | A. pilosellus | M | Romania, Sălaj county, Aghireș |
| Ag28 | A. pilosellus | M | Hungary, Pest county, Kemence |
| Ag29 | A. pilosellus | F | Hungary, Győr-Moson-Sopron county, Sopron |
| Ag30 | A. rufipalpis | M | Hungary, Heves county, Hort |
| Ag32 | A. rufipalpis | M | Albania, Shkodër county, Omarë |
| Ag31 | A. rufipalpis | F | Albania, Shkodër county, Omarë |
| Ag33 | A. sputator | M | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag34 | A. sputator | M | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag35 | A. sputator | F | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag36 | A. sputator | F | Hungary, Bács-Kiskun county, Dunatetétlen |
| Ag37 | A. ustulatus | M | Czech Republic, Moravia, Olomouc |
| Ag38 | A. ustulatus | M | Hungary, Pest county, Vác |
| Ag39 | A. ustulatus | F | Albania, Shkodër county, Lisi i Locit |
| Ag40 | A. ustulatus | F | Hungary, Heves county, Kerecsend |
| Sensillum Type | Length (µm) | Basal Width (µm) | Pores |
|---|---|---|---|
| chaeticum C1 | 75.6 ± 4.8 | 3.3 ± 0.3 | no pore |
| chaeticum C2 | 87.8 ± 2.4 | 4.2 ± 0.4 | terminal pore? |
| trichodeum | 24.6 ± 3.9 | 2.3 ± 0.2 | wall pores? |
| basiconicum B1 | 10.9 ± 1.7 | 2.1 ± 0.3 | wall pores |
| basiconicum B2 | 11.5 ± 2.3 | 2.0 ± 0.1 | wall pores |
| basiconicum B3 | 10.1 ± 0.9 | 1.8 ± 0.2 | wall pores |
| basiconicum B4 | 6.2 ± 0.8 | 1.6 ± 0.2 | wall pores |
| basiconicum B5 | 5.8 ± 0.7 | 1.8 ± 0.3 | wall pores? |
| basiconicum B6 | 9.7 ± 0.6 | 1.9 ± 0.1 | wall pores |
| basiconicum B7 | 5.1 ± 0.3 | 1.8 ± 0.4 | wall pores |
| basiconicum B8 | 27.3 ± 0.1 | 12.5 ± 0.1 | wall pores? |
| basiconicum B9 | 9.9 ± 0.3 | 2.2 ± 0.2 | wall pores? |
| dome-shaped D1 | 5.1 ± 0.4 | 5.8 ± 0.4 | wall pores |
| dome-shaped D2 | 3.8 ± 0.3 | 3.6 ± 0.5 | wall pores |
| Böhm sensillum | 8.7 ± 2.3 | 1.8 ± 0.3 | no pore |
| Sensilla | Antennomere | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | |
| SC1 | + | + | + | + | + | + | + | + | + | + | + |
| SC2 | + | + | + | + | + | + | + | + | + | + | + |
| ST | - | - | - | + | + | + | + | + | + | + | + |
| SB1 | - | - | - | + | + | + | + | + | + | + | + |
| SB2 | - | - | - | + | + | + | + | + | + | + | + |
| SB3 | - | - | - | - | - | - | - | - | - | +1 | + |
| SB4 | - | - | - | - | - | - | +2 | + | + | + | + |
| SB5 | - | - | - | - | - | - | - | - | - | +3 | + |
| SB6 | - | - | - | - | - | - | - | - | - | - | + |
| SB7 | - | - | - | + | +4 | +5 | +6 | + | + | + | + |
| SB8 | - | - | - | - | - | - | - | +7 | - | - | - |
| SB9 | - | - | - | - | - | - | - | - | +8 | +8 | +8 |
| SD1 | - | - | - | + | + | +9 | + | +10 | + | + | + |
| SD2 | - | - | - | - | - | - | - | - | - | - | +11 |
| Sca | - | + | - | - | - | - | - | - | - | - | - |
| BS | + | + | - | - | - | - | - | - | - | - | - |
| Species | Code | Sex | SC1 | SC2 | ST | SB1–9 | SD1–2 | SCa | BS |
|---|---|---|---|---|---|---|---|---|---|
| A. acuminatus | Ag01 | M | 24.0% | 4.3% | 24.6% | 38.4% | 1.3% | 0.1% | 7.3% |
| Ag02 | M | 25.8% | 4.3% | 23.9% | 37.2% | 1.3% | 0.1% | 7.4% | |
| Ag03 | M | 24.7% | 4.4% | 26.5% | 35.8% | 1.2% | 0.1% | 7.3% | |
| Ag04 | F | 39.6% | 4.4% | 6.4% | 41.9% | 1.3% | 0.1% | 6.3% | |
| Ag05 | F | 38.2% | 4.4% | 6.6% | 43.2% | 1.3% | 0.1% | 6.2% | |
| Ag06 | F | 39.3% | 4.5% | 5.9% | 42.6% | 1.3% | 0.1% | 6.3% | |
| A. lineatus | Ag07 | M | 46.3% | 2.8% | 20.7% | 25.1% | 0.9% | <0.1% | 4.3% |
| Ag08 | M | 45.4% | 3.2% | 19.3% | 26.6% | 1.0% | <0.1% | 4.5% | |
| Ag09 | M | 45.9% | 3.0% | 20.4% | 25.4% | 0.9% | <0.1% | 4.4% | |
| Ag10 | F | 54.6% | 3.4% | 3.4% | 32.9% | 1.1% | <0.1% | 4.5% | |
| A. medvedevi | Ag11 | M | 51.6% | 2.3% | 17.5% | 23.6% | 0.8% | <0.1% | 4.3% |
| Ag12 | M | 52.0% | 2.2% | 18.1% | 22.5% | 0.9% | <0.1% | 4.3% | |
| Ag13 | M | 51.4% | 2.3% | 17.7% | 23.3% | 0.9% | <0.1% | 4.4% | |
| Ag14 | M | 51.8% | 2.3% | 18.1% | 22.4% | 1.0% | <0.1% | 4.4% | |
| Ag15 | F | 59.2% | 2.7% | 2.9% | 30.2% | 0.9% | <0.1% | 4.1% | |
| Ag16 | F | 58.7% | 2.5% | 3.2% | 30.5% | 0.9% | <0.1% | 4.2% | |
| Ag17 | F | 60.2% | 2.4% | 3.0% | 29.2% | 0.9% | <0.1% | 4.2% | |
| A. modestus | Ag18 | M | 60.6% | 2.0% | 14.5% | 18.6% | 0.7% | <0.1% | 3.5% |
| Ag19 | M | 59.8% | 2.1% | 13.9% | 19.8% | 0.8% | <0.1% | 3.6% | |
| Ag20 | F | 65.3% | 2.3% | 4.7% | 23.3% | 0.9% | <0.1% | 3.4% | |
| A. obscurus | Ag21 | M | 49.8% | 2.4% | 20.5% | 22.6% | 1.0% | <0.1% | 3.7% |
| Ag22 | M | 50.9% | 2.3% | 20.4% | 21.7% | 1.0% | <0.1% | 3.7% | |
| Ag23 | M | 51.0% | 2.2% | 20.4% | 21.4% | 1.1% | <0.1% | 3.9% | |
| Ag24 | M | 47.3% | 2.3% | 19.9% | 25.7% | 1.0% | <0.1% | 3.8% | |
| Ag25 | F | 60.9% | 2.7% | 4.6% | 27.0% | 1.0% | <0.1% | 3.7% | |
| Ag26 | F | 59.4% | 2.6% | 4.8% | 28.2% | 1.1% | <0.1% | 3.9% | |
| A. paludum | Ag41 | M | 45.7% | 3.1% | 20.8% | 25.2% | 1.0% | <0.1% | 4.4% |
| Ag42 | F | 54.9% | 3.3% | 4.4% | 32.3% | 0.8% | <0.1% | 4.2% | |
| A. pilosellus | Ag27 | M | 41.0% | 2.1% | 19.3 % | 32.9% | 0.8% | <0.1% | 4.0% |
| Ag28 | M | 39.7% | 2.1% | 19.3% | 34.2% | 0.7% | <0.1% | 4.0% | |
| Ag29 | F | 49.6% | 2.5% | 3.8% | 39.4% | 0.7% | <0.1% | 4.0% | |
| A. rufipalpis | Ag30 | M | 46.4% | 2.8% | 16.7% | 28.4% | 1.0% | <0.1% | 4.7% |
| Ag32 | M | 48.7% | 2.6% | 15.3% | 27.7% | 1.0% | <0.1% | 4.7% | |
| Ag31 | F | 51.2% | 3.0% | 3.9% | 36.5% | 0.8% | <0.1% | 4.5% | |
| A. sputator | Ag33 | M | 46.3% | 2.6% | 18.8% | 27.1% | 0.8% | <0.1% | 4.3% |
| Ag34 | M | 45.9% | 2.5% | 19.2% | 27.3% | 0.8% | <0.1% | 4.3% | |
| Ag35 | F | 55.7% | 3.1% | 3.5% | 32.5% | 0.8% | <0.1% | 4.4% | |
| Ag36 | F | 51.8% | 2.9% | 3.2% | 37.0% | 0.8% | <0.1% | 4.3% | |
| A. ustulatus | Ag37 | M | 37.0% | 3.4% | 24.9% | 28.4% | 1.0% | <0.1% | 5.2% |
| Ag38 | M | 38.2% | 3.5% | 26.1% | 25.9% | 1.1% | <0.1% | 5.2% | |
| Ag39 | F | 46.0% | 4.2% | 6.9% | 36.3% | 1.0% | <0.1% | 5.4% | |
| Ag40 | F | 45.5% | 4.2% | 7.3% | 36.7% | 1.0% | <0.1% | 5.3% |
| This Study Agriotes | [26,27] Agriotes | [28,29] Limonius*, Melanotus** | [31] Tetrigus | [32] Elater | [33] Drilini |
|---|---|---|---|---|---|
| chaetica 1 | chaetica 1 | chaetica | chaetica 1,2,3 | chaetica | chaetica 1 |
| chaetica 2 | trichodea 1 | trichodea*, trichodea III** | trichodea 1 | trichodea 1 | chaetica 2 |
| trichodea | trichodea 2 | ––– | ––– | trichodea 2 | ––– |
| basiconica 1 | basiconica 1 | basiconica 1*, basiconica 2** | basiconica 1 | basiconica 1 | basiconica 1 |
| basiconica 2 | basiconica 2 | basiconica 2*, basiconica 1** | basiconica 2 | basiconica 2 | basiconica 2 |
| basiconica 3 | basiconica 3 | basiconica 3* | ––– | ––– | ––– |
| basiconica 4 | basiconica 4 | ––– | ––– | ––– | ––– |
| basiconica 5 | basiconica 5 | basiconica 5*, styloconica** | ––– | styloconica | ––– |
| basiconica 6 | basiconica 6 | ––– | ––– | ––– | ––– |
| basiconica 7 | basiconica 7 | basiconica 4* | coeloconica | grooved pegs | basiconica 7,8 |
| basiconica 8 | ––– | ––– | ––– | ––– | basiconica 16 |
| basiconica 9 | ––– | ––– | ––– | ––– | basiconica 10 |
| dome-shaped 1 | dome-shaped | campan. dome-shaped** | campan. | campan. | basiconica 14 |
| dome-shaped 2 | ––– | ––– | ––– | ––– | basiconica 13 |
| campaniformia | ––– | ––– | ––– | ––– | campan. |
| Böhm sensilla | chaetica 2 | Böhm sensilla or bristles** | Böhm’s bristles | Böhm sensilla | Böhm sensilla |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faucheux, M.J.; Németh, T.; Kundrata, R. Comparative Antennal Morphology of Agriotes (Coleoptera: Elateridae), with Special Reference to the Typology and Possible Functions of Sensilla. Insects 2020, 11, 137. https://doi.org/10.3390/insects11020137
Faucheux MJ, Németh T, Kundrata R. Comparative Antennal Morphology of Agriotes (Coleoptera: Elateridae), with Special Reference to the Typology and Possible Functions of Sensilla. Insects. 2020; 11(2):137. https://doi.org/10.3390/insects11020137
Chicago/Turabian StyleFaucheux, Michel J., Tamás Németh, and Robin Kundrata. 2020. "Comparative Antennal Morphology of Agriotes (Coleoptera: Elateridae), with Special Reference to the Typology and Possible Functions of Sensilla" Insects 11, no. 2: 137. https://doi.org/10.3390/insects11020137
APA StyleFaucheux, M. J., Németh, T., & Kundrata, R. (2020). Comparative Antennal Morphology of Agriotes (Coleoptera: Elateridae), with Special Reference to the Typology and Possible Functions of Sensilla. Insects, 11(2), 137. https://doi.org/10.3390/insects11020137