1. Introduction
Siricidae is a small group of species, in which individuals are relatively large with a clean body surface and easily identifiable morphological features. One of the most striking features of Siricidae is what appears to be incredible variation in wing venation, including the appearance or the disappearance of veins symmetrically or asymmetrically on either wing. Such variation is very rarely seen in other Hymenoptera, a group where wing veins are important for classification [
1]. The wing characteristics of Siricidae are unstable and seldom used in taxonomic studies. In general, the classification and identification of this group is based mainly on the structure of the thorax and abdomen. However, these structures are very similar in closely related species; thus, it is difficult to accurately identify species in some cases. Interestingly, geometrics, as a new classification method, has been recently applied in many classification studies of Hymenoptera [
2].
It is well known that insect wing shape can exhibit a high heritability in nature [
3,
4]. Thus, wing morphology is of primary importance to entomologists studying systematics. Since the 1970s, several investigators have used the two-dimensional characteristics of insect wings to advance the fields of systematics and phylogeny [
5,
6]. Geometric morphometrics utilizes powerful and comprehensive statistical procedures to analyze the variations in shape using either homologous landmarks or outlines of the structure [
6,
7,
8], and it is currently considered to be the most rigorous morphometric method. Wings are excellent in studies that define morphological variations because they are nearly two-dimensional and their venation provides many well-defined morphological landmarks [
9]. For instance, vein intersections are easily identifiable, which enables the general shape of the wing to be captured [
10]. The use of geometric morphometrics to study wing venation has also been useful in insect identification at the individual level [
11], in differentiation between sibling species [
12,
13], and in delimitation among genera. However, thismethodology has not yet been applied in studies of woodwasps.
The wood-boring wasp,
Sirex noctilio Fabricius (Hymenoptera: Siricidae), is an invasive pest of numerous species of pine tree (
Pinus spp.) worldwide and most of the destruction from
S. noctilio is in commercial plants [
14,
15]. In August 2013, woodwasps were detected as a pest of Mongolian pine (
Pinus sylvestris var.
mongolica) in the Duerbote Mongolian Autonomous County, Heilongjiang Province, China [
16]. In 2016, two morphologically similar woodwasps were found to damage
P. sylvestris var.
mongolica in Jinbaotun forest farm, Inner Mongolia, causing a lot of pine forests to weaken and die. After morphological comparison and molecular identification, the two woodwasps were
S. noctilio and
S. nitobei [
17]. Each have two pairs of large, transparent-film wings with visible mesh veins. As the insect wing is a planar structure, it is relatively easy to acquire two-dimensional images, and it is difficult to unintentionally distort the structure. Unfortunately, it is rather difficult to identify
S. noctilio and
S. nitobei wing vein characteristics with the naked eye, and these two species display unstable vein patterns, which means that the use of geometric morphometrics is appropriate [
18].
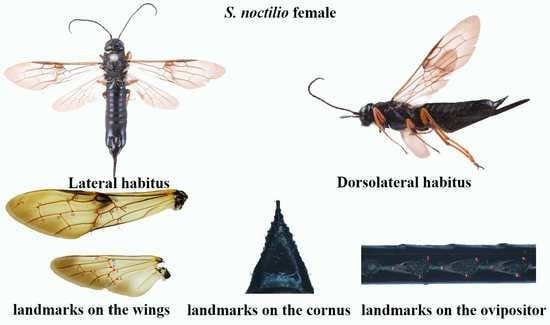
In previous studies, the pits on the ventral portion (lancet) of the ovipositor have been consistently used as an identifying structure. The lancets of the ovipositor independently slide back and forth to move the egg and to penetrate wood. This characteristic was used for the first time by Kjellander (1945) to segregate females of
S. juvencus from those of
S. noctilio. Furthermore, the size, shape, and number of pits on the ovipositors can be used as distinguishing features for the identification of most species [
15]. This also holds true for
Sirex, in which the most important distinguishing characteristics on the ovipositor are pits located from the base to approximately the middle of the lancet, although the apical teeth segments usually do not show distinct differences [
15]. Another striking diagnostic feature is the large hornlike projection, called the cornus, on the last abdominal segment of the females. The cornus is thought to help the larvae pack the frass in the tunnel. The cornus varies in shape (the shape of the female cornus does not vary with size for most species), although their distinguishing features remain poorly characterized. These difficulties underline the need for further studies to clarify the taxonomy of woodwasps, either by searching for new morphological characteristics with clear distinguishing variations or applying alternative effective methods to provide a basis for studying flight and reproductive behavior.
Geometric morphometrics [
6,
19,
20] overcomes the shortcomings of conventional morphological analysis and focuses on the topological information of the organic form [
18]. In addition, as it is not affected by various factors, such as size and shape, this method has the potential to be more widely used in the identification of insects, resulting in automatic insect recognition system that is continually updated and improved [
3]. In taxonomy and other fields, genetics and morphometrics are complementary tools that are often used to understand the origins of phenotypic differences. The application of marker points in biology can be divided into three categories [
21]. In this study, we focused on two of these categories, namely (i) the common points that can be accurately found on each specimen based on the anatomical features, which is a mathematical point supported by substantial evidence between homologous subjects [
22], such as structural intersections (the basis for marking points on wings); and (ii) the mathematical point for homologous subjects, which is supported by geometrical rather than histological evidence, such as depressed or convex points (the basis for marking points on ovipositors and cornus). Platts analysis was used to superimpose the marker points and minimize the deviation of the marker points. In the same coordinate system, the influence of non-morphological factors in morphological information analysis was eliminated, and the average contour of each population was obtained. Thus, in the present study, we applied landmark-based geometric morphometrics to quantify and analyze wing, cornus, and ovipositor morphologies of two
Sirex species that have not been previously characterized. We explored the similarities between these species to strengthen the available quantitative research data that form the basis of species identification and to provide new insights for automatic insect identification systems.
4. Discussion
In recent years, we have witnessed monumental improvements in geometric morphometrics, which have enhanced the study of insect morphology. In general, these methods separate species shape from size and primarily focus on the shape as the key morphological characteristic, as few variables can reveal morphological differences between similar species. Geometric measurement methods, together with relevant mathematical models (e.g., principal component analysis, canonical variate analysis, and cluster analysis), can be used to acquire information on the morphology, genetic differentiation, development, and behavior of insects. When competing for resources on the same part of the host plant, the morphological structure of the two woodwasps may occur adaptive genetic variation. The use of geometric morphometrics allowed us to explain morphological similarities and differences between the two Sirex species, of which thin-spline analysis plots showed average contour distortion, and the length of the stick demonstrated the change size.
Compared with conventional classification methods, geometric survey methods can identify subtle morphological differences in insects [
33]. To distinguish the two species from the traditional taxonomic morphology, we mainly distinguish the color of the male’s abdomen and the female’s leg. However, there is intraspecific variation in color patterns on the legs, abdomen and antennae, for example, females of
Sirex californicus,
S. nitidus, and
S. noctilio each have pale and dark leg color morphs [
34,
35]. The results of relative warp analysis can show differences in the classification of intraspecies and interspecies [
36]. In studies of morphology, the small unit on the wing is usually independent unit in the shape change, and has a certain genetic basis. Thus, different insects have different wing types and wing vein structures [
3]. However, in similar species, these differences may be indistinguishable, with minor variations in the direction and branching of veins [
37]. The shapes and vein profiles of insect wings contain valuable information, although it is difficult to understand the effects of behavioral and environmental factors on morphological variations using conventional classification methods [
33].
Insect flight involves the first two branches of the radius vein and the thicker area of the wing film [
38]), which is gradually reduced from the base to the end of the wing.
S. noctilio woodwasps have a variable flight behavior, which relates to initial body size [
39]. The body size of the
Sirex also varies in different regions, and the two woodwasps cannot be distinguished simply by it.
Sirex species mainly use carbohydrates for fuel during flight. The dry weight of
S. noctilio is significantly larger than
S. nitobei in this study. Therefore, the wing variations of both
Sirex species in this study might explain the differences in flight ability and behavior. In addition, the ovipositor, an appendage through which females deposit eggs, plays important roles in sensing the microenvironment and initiating the laying of eggs. In this study, we observed differences in forewing and ovipositor shape, which were due to developmental plasticity. These findings provide a strong basis for further research on flight behavior and ovipositor function in various species.
In this study, we identified and classified two
Sirex species, although the lack of inclusion of other Siricidae species was a major limitation. When species cannot be immediately identified by their appearance, landmark points can be easily extracted and analyzed. We anticipate that additional landmark points, such as those of the head, chest, and other parts, will be used in future studies [
40]. Geographic distance is one of the key factors of population differentiation for widespread species. It is generally believed that the more geographically separated populations have less chance of gene exchange, resulting in morphological differences between populations.
S. noctilio has invaded many areas in China, next we can collect samples from different regions for analysis of different geographical populations. At the same time, this paper provides basic data for the automatic recognition system of insects. Further identification of
Sirex species will be provided. In addition, insects have a short life cycle and a fast response to the environment. Using geometric morphology can accurately analyze small changes in morphological structures and possible evolutionary trends in a short period of time.