Abstract
Western flower thrips (WFT), Frankliniella occidentalis (Pergande), is a highly invasive pest which is harmful to many cash crops globally and resistant to various insecticides. Entomopathogenic fungi (EPF), as biological control agents, have demonstrated a good control effect on WFT. The aim of this study was to evaluate the synergistic and pathogenicity efficacy of the fungal strain Metarhizium flavoviride WSWL51721 when distributed with diatomaceous earth (DE) and the active ingredient imidacloprid using four bioassay methods against adult and second instar larvae of WFT. The data of the four bioassays have been fitted to the time–concentration–mortality (TCM) model. The corrected mortality ranges of WFT adults were 75–100%, 82.69–100%, 78.85–100%, and 92.31–100%, and the corrected mortality ranges of WFT second instar larvae were 72.22–100%, 85.19–100%, 77.77–100%, and 100% in the four bioassays at concentrations of 1.2 × 106 to 1.2 × 108 conidia/mL, respectively. At 1.2 × 108 conidia/mL, assays 2 (M. flavoviride with DE), 3 (M. flavoviride with imidacloprid), and 4 (M. flavoviride with DE and imidacloprid) had the shortest median lethal time (LT50), compared with that of assay 1 (M. flavoviride alone) for adults at 2.26 d, 2.06 d, and 1.53 d, and second instar larvae at 2.45 d, 1.70 d, and 1.41 d, respectively. The median lethal concentration (LC50) in the four bioassays decreased within 3–10 days of inoculation. On the third day, it was found that the lowest median lethal concentrations in assays 2, 3, and 4 were 1.58 × 107, 1.13 × 107, and 3.39 × 106 conidia/mL, respectively, which were significantly different from that in assay 1 for the adults. For the second instar larvae, assays 2, 3, and 4 also had the lowest lethal concentrations and were significantly different from those of assay 1. There were significant differences in sporulation between adults and second instar larvae under the four bioassays. Our results indicate that assays 2 (M. flavoviride with DE), 3 (M. flavoviride with imidacloprid), and 4 (M. flavoviride with DE and imidacloprid) demonstrate synergistic effects on the control of both adult and second instar larvae of WFT under laboratory conditions.
1. Introduction
Western flower thrips (WFT), Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), is an invasive pest in agroforestry worldwide, impacting many economically important greenhouse-grown crops, such as ornamental flowers, vegetables, fruits, and other various plants through their piercing-sucking mouthparts, causing direct feeding damage and transmission of several destructive plant viruses which have caused significant economic losses in many open-field and greenhouse crops [1,2,3,4,5,6].
Currently, due to the wide suitable area for WFT, they pose a huge threat to agriculture and forestry crops. Therefore, chemical insecticides have been mainly applied to prevent and control WFT; however, due to the unreasonable and intensive use of various insecticides, WFT have become resistant to various insecticides, such as organophosphorus, organochlorine, carbamate, pyrethroids, and spinosad. Thus, the management of WFT has become a complex task [2,7,8,9,10]. Moreover, due to the high toxicity of chemical insecticides, problems with large residual amounts of chemicals and a long residual period in marketable cash crops, contamination of the environment, threats to human health, toxicity to beneficial non-target organisms, species displacement, and destruction of IPM systems [3,11,12,13,14], finding reliable biological control methods that can protect the ecological environment and control the population of WFT effectively and continuously, as well as being compatible with other components, has become an important area of research for the integrated control of WFT [3,13,15].
Entomopathogenic fungi (EPF) are biological control agents with insecticidal activities against a variety of agricultural insect pests worldwide [16,17,18,19]. Most importantly, they do not pollute the environment, are harmless to humans and animals, have an ecologically wide distribution, and target a variety of host species, demonstrating a broad spectrum of insecticidal effectiveness and strong pathogenicity against many insect pests in the agricultural field. Consequently, they have been widely used and studied throughout the world [17]. There are seven species of entomopathogenic fungi which have been successfully applied for the control of WFT, including Lecanicillium lecanii (Zimmermann) [20], Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Ascomycota) [6,21,22,23,24], Metarhizium anisopliae (Metschnikoff) Sorokin (Hyphomycetes: Deuteromycotina) [3,9,10,12], Metarhizium brunneum (Metschnikoff) Sorokin [25], Metarhizium flavoviride (Gams and Rozsypal) (syn. Metarhizium anisopliae var. Acridum, pro parte) (Hyphomycetes: Deuteromycotina) [26], Neozygites parvispora (Zygomycotina: Entomophthorales) [27], and Isaria (Paecilomyces) fumosorosea (Wize) Brown and Smith [6,10], among which the fungal pathogens B. bassiana and M. anisopliae have been the most studied for the control of WFT. In addition, as the essential oils (EOs) of botanical insecticides have similar control efficacies on pests as EPF, there have also been many studies on EOs in controlling WFT [28,29].
Diatomaceous earth (DE) belongs to inert dusts, a large class of substances with stable properties which do not easily produce chemical reactions. DE has a good ability to absorb esters, and so it is a potential natural insecticide [30,31,32]. There are numerous reports, both domestic and foreign, which have indicated that DE has broad prospects for controlling a wide range of store-product pests, such as Tribolium confusum (J du Val), Tribolium castaneum (Herbst), Acanthoscelides obtectus (Say), Cryptolestes ferrugineus (Stephens), Sitophilus granarius (L.), and Rhyzopertha dominica (F.) [15,33,34,35,36], as well as a variety of agricultural pests, such as Solenopsis invicta (Buren), Gryllidae (Bolívar), and Leptinotarsa decemlineata (Say) [37,38]. The insecticidal principle of DE is mainly to adsorb the cuticular lipids from the targeted insects; the tiny DE particles are trapped by the exposed cuticle surface of an insect, which causes various kinds of physical or mechanical damage and loosens the cuticle, causing loss of water and resulting in death [39,40,41,42,43].
EPF have been demonstrated to control different insect pests, but have a relatively slow effect compared with chemical insecticides, as all fungal agents have a latent period in their host after infecting the target pest [44,45,46,47]. In addition, a potential disadvantage of EPF is their relatively short shelf life, compared to conventional chemical insecticides [6]. Further, when the pest population density is high, it is difficult to control the pest density below an economic threshold with only the use of a single fungal agent [12]. Therefore, combining fungal biocontrol agents with adjuvants and other insecticides with good compatibility is an effective and reliable solution to improve the pest control effect [15,18,32,39,48]. Numerous studies have reported that the combination of EPF with insecticides [44,48,49,50], DE [34,39,51,52], and with insecticides and DE have significantly improved their pest control efficacies [15,32,33,35]. For example, Nian et al. [49] demonstrated that combining I. fumosorosea with sub-lethal doses of the insecticides beta-cypermethrin and bacillus thuringiensis caused synergistic effects against Plutella xylostella larvae under laboratory conditions. In another study, Wakil et al. [32] demonstrated that combining B. bassiana with the neonicotinoid insecticide thiamethoxam and DE led to synergistic effects against Rhyzopertha dominica under laboratory conditions. At present, there are two main strategies for the biological control of WFT: (1) EPF combined with predatory mites [12,13,20,53]; and (2) EPF combined with insecticides [3,6,9,10]. For example, Wu et al. [13] demonstrated that the combination of B. bassiana and the predatory mite Neoseiulus barkeri improved WFT control in greenhouse-grown cucumbers; similarly, Jacobson et al. [53] showed that the simultaneous use of Amblyseius cucumeris and B. bassiana improved WFT control in cucumbers. In addition, Ansari et al. [9] demonstrated that M. anisopliae combined with sub-lethal doses of conventional insecticides (imidacloprid and fipronil) had a better control effect on WFT than the individual fungal biocontrol agents.
However, the synergistic effects between M. flavoviride and insecticides and DE against WFT have not been reported. Therefore, in this study, we first evaluated the compatibility of three commonly used chemical insecticides with M. flavoviride, and the best compatible insecticide was selected to combine treatment with M. flavoviride and DE, which was analyzed through the use of the complex but robust method of time–concentration–mortality responses, instead of simple concentration–mortality responses, to discern the virulence of different bioassays in adults and second instar larvae of WFT under laboratory conditions.
2. Materials and Methods
2.1. Rearing Protocols for WFT
A number of WFT (Frankliniella occidentalis) were collected from rose petals in greenhouses of the Dounan Flower Planting Base in Kunming, Yunnan province (24°90′ N, 102°79′ E), in 2018. WFT were maintained as described by Ansari et al. [9], with slight modifications. Briefly, thrips demes were continuously reared on sterilized kidney bean pods (Phaseolus vulgaris L.) in tissue culture bottles (9.1 cm in height, 6.9 cm in diameter; Xuzhou Hualian Glass Products Co., Ltd., China) and covered with a fine mesh to allow for ventilation. These bottles were kept at 25 ± 1 ℃, 65 ± 5% RH, and with a L 14 h:D 10 h photoperiod in growth chambers (RG-300, Guangzhou Kenton Apparatus Co., Ltd., China). Coeval healthy thrips cohorts were obtained by rearing adults on fresh and healthy kidney bean pods for oviposition. After three days, the eggs laid on the pods were transferred to fresh ventilated tissue culture bottles. The first instar larvae hatched from the eggs 2 days later. The second instar larvae were collected three days post-eclosion. The second instar larvae and adults were collected from the tissue culture bottles for experimental use.
2.2. Fungal Strains and Preparation
The fungal strain WSWL51721 of Metarhizium flavoviride was originally isolated from a naturally infected cadaver of an unknown Coleoptera adult in the Xishuangbanna National Nature Reserve in Yunnan Province in 2017 and maintained at 4 ℃ in the Insect Fungi Laboratory of the College of Plant Protection, Yunnan Agricultural University. The strain was cultured on Sabouraud Dextrose Agar (SDA) medium at 25 ± 1 ℃, 75% RH, and under conditions of 16 h light/8 h dark. After seven days, a large number of conidia were harvested from the Petri plates by gently scraping the surface of the SDA medium, which were suspended in sterile aqueous 0.05% TWEEN® 80 (Beijing Solaibao Technology Co. Ltd., China). Conidial suspensions were quantified using a Neubauer Hemocytometer (Shanghai Anxin Optical Instrument Manufacturing Co., Ltd., China) and the experimental concentration was adjusted to 1.2 × 108 conidia/mL using sterile 0.05% TWEEN® 80. Conidial suspensions were diluted to 1.2 × 106 conidia/mL for subsequent experiments. The viability of the conidia was assessed according to the method of Goettel and Inglis, demonstrating that the percentage germination was >90% [54].
2.3. DE and Insecticide Formulations
The diatomaceous earth (DE) formulation used in this experiment was Puliangtai® (Shanxi Keli Science and Technology Co., Ltd., China), which contained 85% amorphous silicon dioxide. Three insecticides commonly used in the control of WFT were selected: The commercial emulsifiable concentrate of Abamectin, containing 18 g/L of active ingredient (Henan Yongguan Qiaodi Agricultural Technology Co., Ltd., China); the commercial wettable granule formulation of Imidacloprid, containing 700 g/L of active ingredient (Bayer CropScience Limited, China); and the commercial wettable powder formulation of Buprofezin, containing 250 g/L of active ingredient (Jiangsu Yangzhou Suling Pesticide Chemical Co., Ltd., China) were used in the experiments.
2.4. Radial Hyphal Growth Test in the Presence of Insecticides
Imidacloprid, buprofezin, and avermectin were tested at three dose rates to evaluate their inhibitory effects on the radial growth of M. flavoviride. The fungal strain culture was performed as described in Section 2.2. After autoclaving, when the SDA medium temperature dropped to 45 ℃, but was not yet solidified and would not affect the pesticide activity [19], each different concentration of pesticide prepared with sterile distilled water was added to the culture medium for homogenization, and then poured into the Petri dishes for solidification. Next, 4 mm diameter fungal strain cores were taken from the M. flavoviride cultures and placed upside down in the middle of Petri dishes containing SDA medium with 0 (control), 10, 50, and 100 ppm of imidacloprid, buprofezin, and avermectin solution, respectively. The potential inhibitory effects of the insecticides on radial hyphal growth were determined as described by Russell et al. [48]. Briefly, surface radial growth was measured for 10 days on alternate days. Two radii at right angles to each other drawn on the bottom of each dish were measured using an Electronic Digital Caliper (Qingdao Meijite Precision Tools Co., Ltd., China). Each treatment was conducted three times.
2.5. Conidia Production Measure in vitro in the Presence of Insecticides
The fungal strain conidia production was measured using a slight modification of the technique described by Russell et al. [48]. Briefly, imidacloprid, buprofezin, and avermectin were incorporated into SDA medium at concentrations of 0 (control), 10, 50, and 100 ppm. Six replicate Petri dishes per treatment were inoculated with 4 mm diameter cores of fungal strain from the M. flavoviride cultures, then maintained at 25 ± 1 ℃ with a 16 h light (L):8 h dark (D) photoperiod. After ten days, three 4 mm diameter cores, from the center point of each colony to 1/2 of the edge distance, were taken from each Petri dish and added separately to 2 ml microcentrifuge tubes containing 1 ml of sterile 0.05% TWEEN® 80. Conidia suspensions from each core were quantified using a Neubauer Hemocytometer to estimate conidia per cm2. The experiment was replicated three times.
2.6. Bioassays
In the bioassays, imidacloprid was selected and applied at 50 ppm, based on radial hyphal growth and conidia production of fungal strain in the presence of insecticides according to Section 2.4 and Section 2.5, while diatomaceous earth was tested at 200 ppm. First, the virulence effects of diatomaceous earth, imidacloprid, and diatomaceous earth combined with imidacloprid were examined for the control of adults, and second instar larvae of WFT (F. occidentalis) were tested. Secondly, the fungal strain was tested at the two concentrations of 1.2 × 106 and 1.2 × 108 conidia/mL. Imidacloprid and diatomaceous earth were tested at 50 ppm and 200 ppm, respectively. Four bioassays, as described in Table 1, were performed using immersion for the adults and the second instar larvae of WFT. For inoculation, the adults and second instar larvae were individually immersed in various concentrations of fungus alone, as well as fungus + insecticide, fungus + diatomaceous earth, and fungus + diatomaceous earth + insecticide combinations for 10 s. Excess suspension was then removed by filter paper, and 20 adults and second instar larvae were carefully transferred to a sterile glass insect tube (10.0 cm height, 3.0 cm diameter) using a fine brush; fresh kidney bean pods served as a food source. All treated adults and second instar larvae were kept in glass insect tubes (10.0 cm height, 3.0 cm diameter) and sealed with 200 mesh gauze and rubber bands to prevent the WFT from escaping during the treatments.

Table 1.
Combinations of Metarhizium flavoviride, diatomaceous earth, and imidacloprid against the adults and second instar larvae of WFT (F. occidentalis) in efficacy studies.
The test insects were incubated at 25 ± 1 ℃ and 65 ± 5% RH with a L 14 h:D 10 h photoperiod in growth chambers. The experiment was repeated three times. The mortality of adults and second instar larvae were observed daily for 10 days, and fresh untreated pods were added every two days. Dead adults and second instar larvae were collected from all treatment groups, the WFT carcasses were surface disinfected with 70% ethanol for 2–3 min, followed by three washings in sterile distilled water, and were placed on moist sterilized filter paper in the same Petri dishes, sealed with parafilm, and incubated at 25 ± 1 °C and 75 ± 5% RH for 3–5 days to promote fungal hyphal growth on the surface of the carcass and to further confirm that death was caused by mycosis using a light microscope (LEICA-M205FA, Leica, Germany).
2.7. Quantification of Spore Production on Mycotized WFT Carcasses
All M. flavoviride-sporulating adults and second instar larvae from Section 2.6 in the four bioassays were cultured in petri dishes lined with moist, sterilized filter paper for 12 days. Fungal growth on the carcass surface was observed every day in order to ensure that fungal sporulation had occurred on the surface of all WFT carcasses [48]. Twenty adults and second instar larvae from each of the above treatments were separately transferred to a 1.5 mL sterile centrifuge tube supplemented with 1 mL of sterile 0.05% TWEEN® 80, followed by shaking on an orbital shaker for 10 min [55]. The amount of conidia on each insect was estimated by counting the total number of conidia using a Neubauer Hemocytometer. Each treatment was repeated three times.
2.8. Statistical Analysis
Radial hyphal growth and conidia production of M. flavoviride in the presence of insecticides, as well as the data of number of conidia on the carcass, were analyzed by one-way ANOVA. Means were compared using Tukey’s HSD test at a 5% level of significance. All data were analyzed using the Data Processing System (DPS) software, version 14.0 [56], as well as the SPSS statistical software, version 20.0.
As the Time–Concentration–Mortality (TCM) model can combine time and dose effects into a single model, the interactions of time and dose effects can be examined to reflect the integrity and objectivity of bioassay data. Therefore, the data from the different bioassays (i.e., 1–4) were analyzed using the TCM model to determine the interactions between M. flavoviride and the adults and second instar larvae of WFT [57,58,59]. Briefly, the adults and second instar larvae of WFT mortality (qij), called the conditional mortality probability, caused by a given conidial suspension concentration of M. flavoviride (di) at specific time intervals [tj−1, tj] (i.e., day 1, day 2, …, day 10 after immersion treatment) were corrected with background mortalities (mortality was only treated with sterilized 0.05% TWEEN® 80) observed from the controls of each assay. This was then fitted to the conditional TCM model qij = 1 − exp[−exp(γj + β log10(di))] by approaching a maximum likelihood equation , where nij represents the number of adults and second instar larvae of WFT surviving at time tj−1 after an inoculation dose di, and rij represents the number of adults and second instar larvae of WFT caused by M. flavoviride infection by the time interval [tj−1, tj]. This conditional modelling of the β and γj estimates were used to determine the cumulative TCM relationships in the form of pij = 1 − exp[−exp(τj + βlog10(di))], which were obtained with the cumulative time effect of τj = ln(). The parameter estimates of β and τj, as well as their variances and covariances, were finally used to calculate the lethal concentrations (LC), including LC50 and LC90 (the conidial concentrations of M. flavoviride causing 50 or 90% of the adults and second instar larvae of WFT to die, respectively) as a function of time after immersion treatment and the lethal times (LT), including LT50 and LT90 (the lethal time for a given concentration of M. flavoviride to cause 50 or 90% mortality of the adults and second instar larvae of WFT) as a function of the conidial concentration.
3. Results
3.1. Radial Hyphal Growth in the Presence of Insecticides
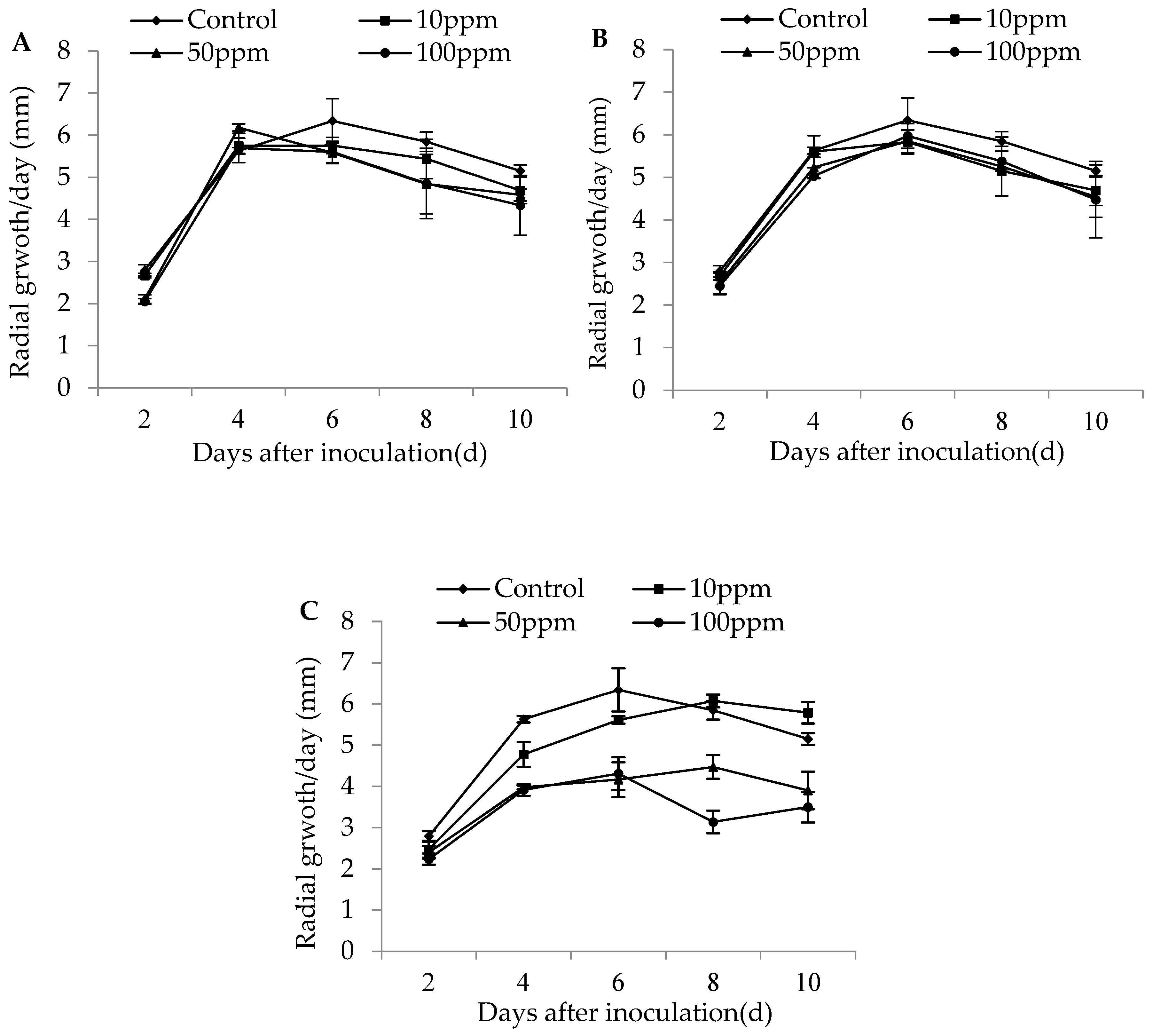
The colony growth of M. flavoviride after 2 days’ inoculation on SDA plates was significantly affected by different dose rates of imidacloprid (D2: F3,11 = 16.19, p = 0.0009 < 0.01), but had no significant effect on the colony growth of the strain during other inoculation days (D4: F3,11 = 1.51, p = 0.29; D6: F3,11 = 0.49, p = 0.69; D8: F3,11 = 0.65, p = 0.61; D10: F3,11 = 0.72, p = 0.57). No significant effect was found for the dose rates of buprofezin on the colony growth of M. flavoviride on SDA plates for any inoculation day (D2: F3,11 = 0.69, p = 0.58; D4: F3,11 = 1.62, p = 0.26; D6: F3,11 = 0.44, p = 0.73; D8: F3,11 = 0.41, p = 0.75; D10: F3,11 = 0.32, p = 0.81). The colony growth of M. flavoviride on SDA plates at 4, 6, 8, and 10 days of inoculation was significantly affected by different dose rates of abamectin (D2: F3,11 = 2.09, p = 0.18; D4: F3,11 = 21.90, p = 0.0003 < 0.01; D6: F3,11 = 7.11, p = 0.01; D8: F3,11 = 31.41, p = 0.0001 < 0.01; D10: F3,11 = 10.45, p = 0.004 < 0.01). The three different dose rates of imidacloprid and buprofezin did not significantly affect the colony growth of M. flavoviride inoculated on SDA plates, indicating that the two insecticides had good compatibility with M. flavoviride. The differences of the daily growth rate of colony diameter (in mm) for M. flavoviride by adding low (10 ppm) and medium (50 ppm) dose rates of imidacloprid were not significantly different from those in the control, and the highest radial growths were observed to be 5.76 ± 0.19 mm and 6.18 ± 0.09 mm after days 6 and 4 of incubation, respectively. The differences in radial hyphal growth of M. flavoviride by adding low and medium dose rates of buprofezin (10 and 50 ppm, respectively) were not significantly different from those of the control, where the highest radial growths were 5.83 ± 0.28 mm and 5.85 ± 0.27 mm after 6 days of incubation (Figure 1).
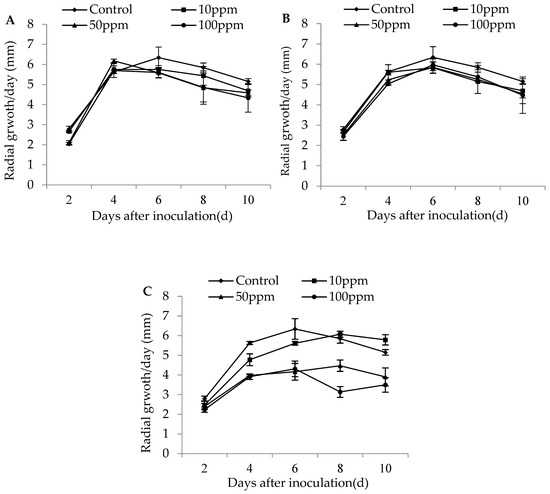
Figure 1.
Radial growth rate (mm/day ± SE) of M. flavoviride on SDA added with three insecticides: (A) 10, 50, and 100 ppm Imidacloprid; (B) 10, 50, and 100 ppm Buprofezin; and (C) 10, 50, and 100 ppm Abamectin.
3.2. Conidia Production Measure in vitro in the Presence of Insecticides
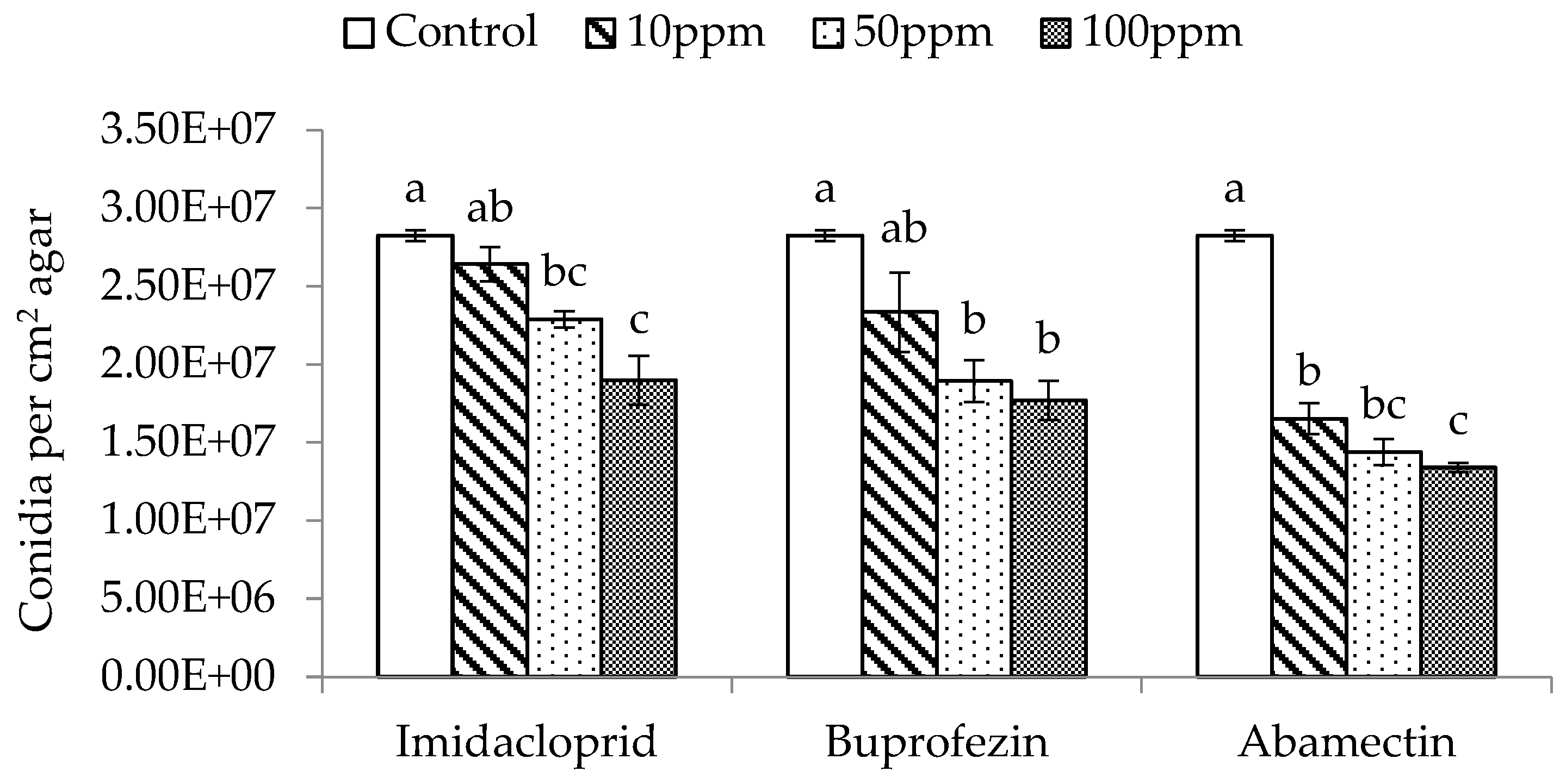
The three different dose rates (10, 50, and 100 ppm) of imidacloprid, buprofezin, and abamectin significantly affected the spore production of M. flavoviride inoculated on SDA plates, compared with the control (F3,11 = 16.60, p = 0.0009 < 0.01; F3,11 = 9.25, p = 0.0056 < 0.01; F3,11 = 99.71, p = 0.0001 < 0.01). The spore production of M. flavoviride on SDA plates decreased with an increase of dose rate in all three insecticides, and was significantly affected by the three insecticides at low-, medium-, and high-dose rates (10, 50, and 100 ppm) as well (Figure 2). The spore production of M. flavoviride on SDA plates after adding imidacloprid, buprofezin, and abamectin at the low dose rate was 2.64 × 107 ± 1.08 × 106, 2.34 × 107 ± 2.54 × 106, and 1.65 × 107 ± 9.92 × 105, respectively; these were significantly different (F 2,8 = 8.96, p = 0.02 < 0.05). The spore production of M. flavoviride at the medium dose rate of imidacloprid, buprofezin, and abamectin was 2.29 × 107 ± 5.14 × 105, 1.89 × 107 ± 1.33 × 106, and 1.44 × 107 ± 8.32 × 105, respectively; these were significantly different (F2,8 = 19.92, p = 0.0022 < 0.01). The spore production of M. flavoviride at the high dose rate of imidacloprid, buprofezin, and abamectin was 1.90 × 107 ± 1.57 × 106, 1.77 × 107 ± 1.26 × 106, and 1.34 × 107 ± 2.95 × 105, respectively; these were significantly different (F2,8 = 6.25, p = 0.0341 < 0.05).
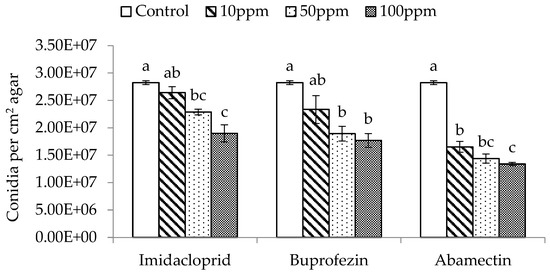
Figure 2.
Mean (± SE) conidia production per cm2 on SDA with added insecticides Imidacloprid, Buprofezin, and Abamectin at 10, 50, and 100 ppm. The same letters above the bar indicate no significant difference (Tukey’s HSD at p = 0.05).
3.3. Bioassays
3.3.1. Effect of Different Bioassays on Mortalities with Adults and Second Instar Larvae of WFT
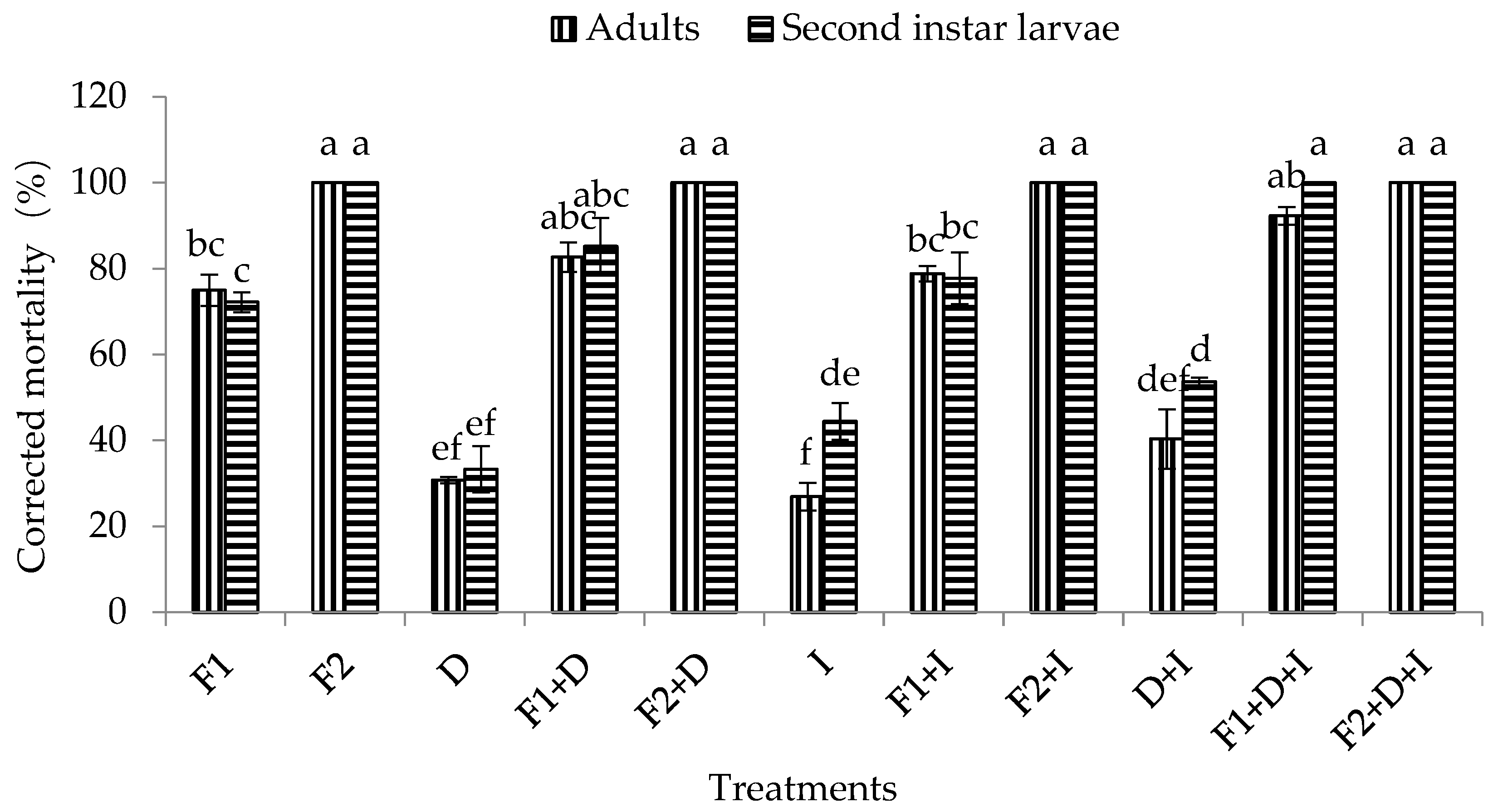
It was observed that DE, imidacloprid, and the combination of DE and imidacloprid had certain pathogenicity in the adults and second instar larvae of WFT, with corrected mortality rates in adults of 30.77%, 26.92%, and 40.38%, respectively, and corrected mortality rates in second instar larvae of 33.33%, 44.44%, and 53.70%, respectively. Moreover, the corrected mortality of the second instar larvae of WFT under the combined treatment with DE and imidacloprid was significantly higher than that of adults (F1,5 = 122.18, p = 0.0004 < 0.01) (Figure 3).
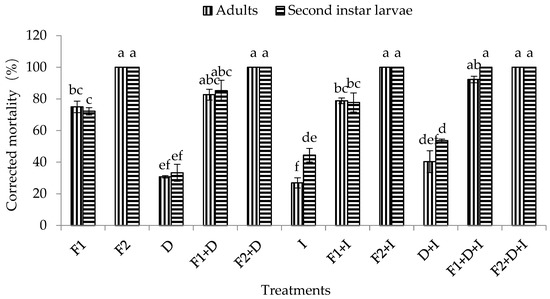
Figure 3.
Effect of conidia concentrations of M. flavoviride associated with DE and imidacloprid on the mortality of adults and second instar larvae of WFT (F. occidentalis). Bars with the same letter above the bar are not significantly different (Tukey’s HSD at p = 0.05). Error bars denote SE values. D = DE at 200 ppm, F1 = M. flavoviride at 1.2 × 106 conidia/mL, F2 = M. flavoviride at 1.2 × 108 conidia/mL, I = imidacloprid at 50 ppm, D + I = DE at 200 ppm + imidacloprid at 50 ppm, D + F1 = DE at 200 ppm + M. flavoviride at 1.2 × 106 conidia/mL, D + F2 = DE at 200 ppm + M. flavoviride at 1.2 × 108 conidia/mL, I + F1 = imidacloprid at 50 ppm + M. flavoviride at 1.2 × 106 conidia/mL, I + F2 = imidacloprid at 50 ppm + M. flavoviride at 1.2 × 108 conidia/mL, D + I + F1 = DE at 200 ppm + imidacloprid at 50 ppm + M. flavoviride at 1.2 × 106 conidia/mL, D + I +F2 = DE at 200 ppm + imidacloprid at 50 ppm + M. flavoviride at 1.2 × 108 conidia/mL.
The mortality of adults and second instar larvae of WFT, in response to various conidia concentrations of the M. flavoviride conidia alone (assay 1); together with DE at 200 ppm (assay 2); with imidacloprid at 50 ppm (assay 3); or with DE and imidacloprid (assay 4) after 10 days of inoculation are illustrated in Figure 3. The mortality of adults and second instar larvae of WFT observed in the four different bioassays were concentration- and time-dependent, regardless of whether M. flavoviride was used alone or in combination with DE or imidacloprid (or even the fungus in combination with DE and imidacloprid). Furthermore, the corrected mortality under the four different bioassays increased with conidial concentrations. The final corrected mortality rates of adults and second instar larvae of WFT after 10 days of inoculation ranged from 75–100% and 72.22–100% in assay 1; 82.69–100% and 85.19–100% in assay 2; 78.85–100% and 77.77–100% in assay 3; and 92.31–100% and 100% in assay 4 (combination of M. flavoviride with DE and imidacloprid) (Figure 3). At a low concentration (1.2 × 106 conidia/mL) of M. flavoviride, the corrected mortality rates of adults and second instar larvae of WFT were 75% and 72.22% in assay 1 (M. flavoviride alone); 82.69% and 85.19% in assay 2 (M. flavoviride with DE); 78.85% and 77.77% in assay 3 (M. flavoviride with imidacloprid); and 92.31% and 100% in assay 4 (M. flavoviride with DE and imidacloprid). The results showed that the mortalities were all significantly different from that in assay 4 (Adults: F3,11 = 6.74, p = 0.01 < 0.05; Larvae: F3,11 = 6.70, p = 0.01 < 0.05). However, at a high concentration (1.2 × 108 conidia/mL) of M. flavoviride, the corrected mortality rates of adults and second instar larvae of WFT in assays 1 (M. flavoviride alone), 2 (M. flavoviride with DE), and 3 (M. flavoviride with imidacloprid) were not significantly different from that in assay 4 (M. flavoviride with DE and imidacloprid).
3.3.2. Fitted TCM Relationships
The observed responses of the adults and second instar larvae of WFT from assays 1–4 fitted the TCM model well, with an accepted homogeneity fit based on the Hosmer–Lemeshow statistic C (p ≥ 0.05) (Table 2 and Table 3), to the adults and second instar larvae of WFT: (C = 3.73, df = 8, p = 0.88 for assay 1; C = 4.79, df = 8, p = 0.78 for assay 2; C = 12.38, df = 8, p = 0.14 for assay 3; C = 11.96, df = 8, p = 0.15 for assay 4) and (C = 2.26, df = 8, p = 0.97 for assay 1; C = 4.67, df = 7, p = 0.70 for assay 2; C = 15.49, df = 8, p = 0.05 for assay 3; C = 7.11, df = 8, p = 0.53 for assay 4), respectively. In addition, the t-statistics of all time-effect parameters estimated were significant (p < 0.0001); that is, the standard error was extremely small relative to the parameter estimates, indicating that the dose-effect and time-effect in the test strain M. flavoviride were extremely significant.

Table 2.
Virulence parameters of M. flavoviride WSWL51721 against adult WFT.

Table 3.
Virulence parameters of M. flavoviride WSWL51721 against WFT second instar larvae.
The estimated parameters (β) for the effect of M. flavoviride concentration were 0.53, 0.57, 0.92, and 1.06 for the adults, and 0.46, 0.46, 1.04, and 0.64 for the second instar larvae of WFT in assay 1 (M. flavoviride alone), assay 2 (M. flavoviride with DE), assay 3 (M. flavoviride with imidacloprid), and assay 4 (M. flavoviride with DE and imidacloprid), respectively; which indicated that the pathogenicity of M. flavoviride integrated with DE and imidacloprid against adult WFT (assay 4) was greater than those in assays 1–3, and that the pathogenicity of M. flavoviride integrated with imidacloprid against the second instar larvae of WFT (assay 3) was greater than that of assays 1, 2, and 4. This result further indicates that M. flavoviride combined with DE and imidacloprid exerted significantly greater control efficacy against WFT adults; however, M. flavoviride combined with imidacloprid exerted significantly greater control efficacy against the second instar larvae of WFT. The parameters for the conditional time effects (γj) peaked at γ6 for the WFT adults, indicating that the highest mortality was at 6 days after treatment in assay 1. The real maximum mortality peaked at 4 and 8 days, and 4 and 7 days after treatment in assays 2 and 3, respectively. In assay 4, the highest mortality peaked at 7 days after treatment. However, the parameters for the conditional time effects (γj) of the second instar larvae of WFT reached the highest mortality at 6 and 8 days after the inoculation treatment in assay 1. The true highest mortality peaks were obtained at 6, and 3 and 7 days after inoculation treatment in assays 2 and 3, respectively. In assay 4, the peak real highest mortality was achieved at 7, 9, and 10 days after inoculation treatment.
The fitted parameters γj were different between the developmental stages of F. occidentalis, with differences among the bioassay methods. This indicates that the time-specific effects and biocontrol potential of M. flavoviride at the tested conidial concentrations also varied with the different bioassay methods.
3.3.3. Lethal Concentrations (LC50 and LC90) of M. flavoviride against the Adults and Second Instar Larvae of WFT under Different Bioassays
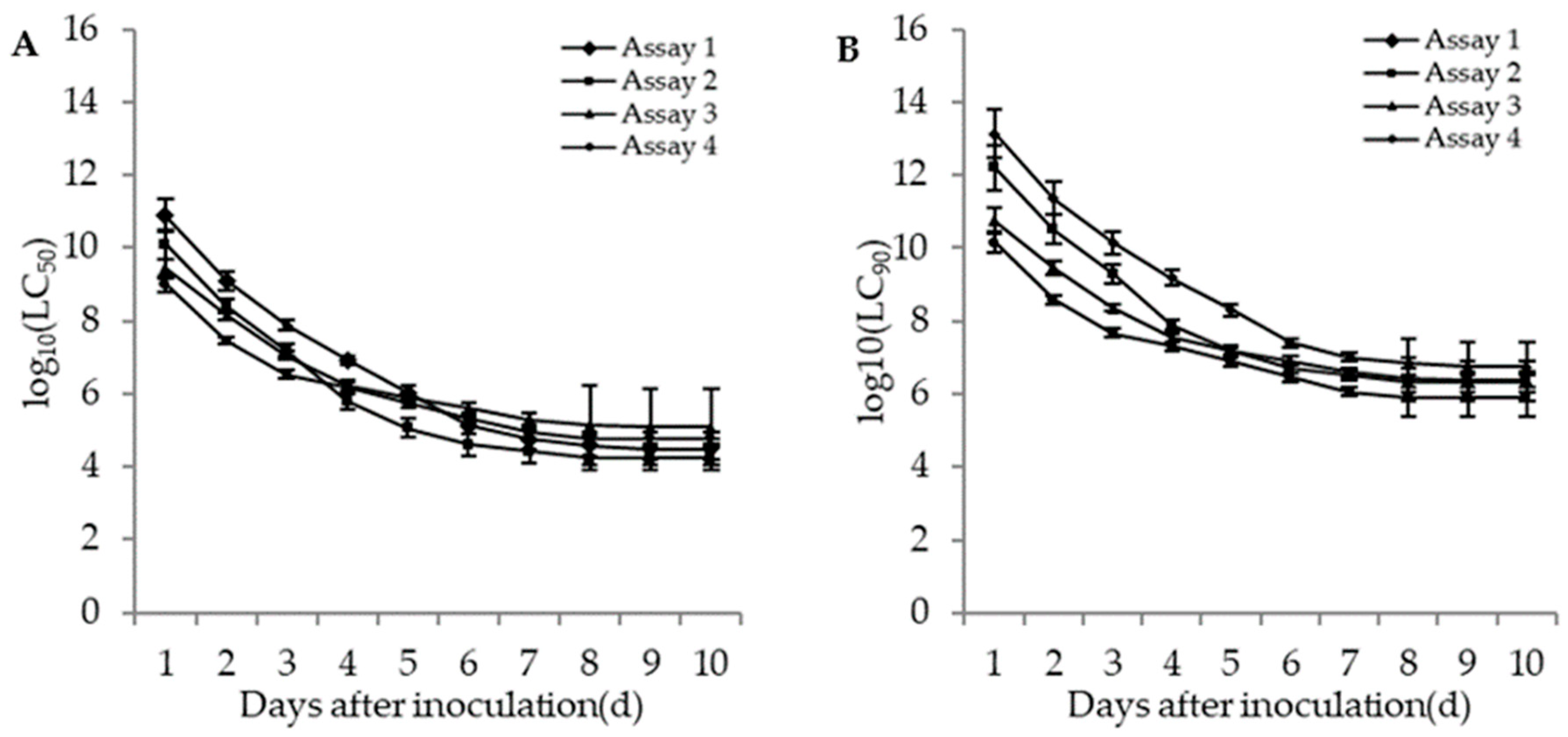
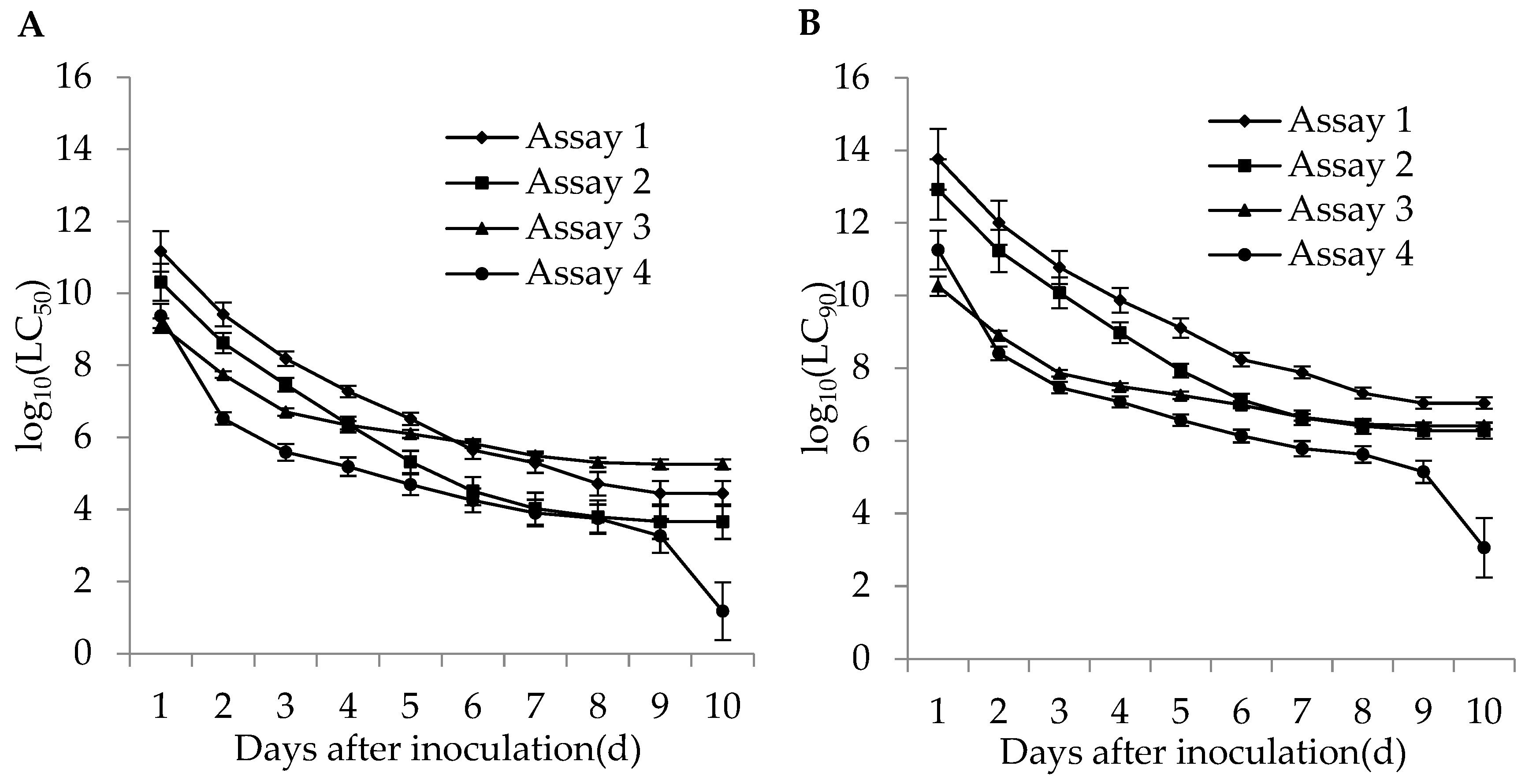
The lethal concentrations (LC50 and LC90) of the adults and second instar larvae of WFT were estimated based on the TCM model in different bioassays. The lethal concentrations in different bioassays for the adults and second instar larvae of WFT decreased with an increase of incubation time (Figure 4 and Figure 5). The more insecticides (imidacloprid and DE) that were contained in the fungal concentrations, the lower the estimated LC50. For the adult WFT, the estimated LC50 values between days 3 and 10 in the four bioassays were 7.56 × 107–3.06 × 104 conidia/mL in assay 1, 1.58 × 107–1.73 × 104 conidia/mL in assay 2, 1.13 × 107–1.20 × 105 conidia/mL in assay 3, and 3.39 × 106–6.07 × 104 conidia/mL in assay 4; further, the LC90 values in the four bioassays were estimated to be 1.40 × 1010–5.65 × 106 conidia/mL in assay 1, 2.00 × 109–2.19 × 106 conidia/mL in assay 2, 2.31 × 108–2.46 × 106 conidia/mL in assay 3, and 4.61 × 107–8.27 × 105 conidia/mL in assay 4. For the second instar larvae WFT, between days 3 and 10, the LC50 values in the four bioassays were estimated to be 1.53 × 108–2.79 × 104 conidia/mL in assay 1, 2.90 × 107–4.61 × 103 conidia/mL in assay 2, 5.06 × 106–1.80 × 105 conidia/mL in assay 3, and 3.88 × 105–15.01 conidia/mL in assay 4; similarly, the LC90 values in the four bioassays were estimated to be 5.99 × 1010–1.09 × 107 conidia/mL in assay 1, 1.19 × 1010–1.90 × 106 conidia/mL in assay 2, 7.28 × 107–2.59 × 106 conidia/mL in assay 3, and 2.95 × 107–1.14 × 103 conidia/mL in assay 4.
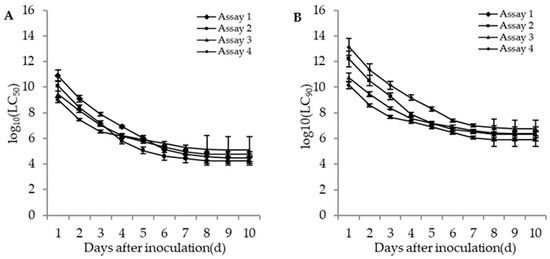
Figure 4.
Curve trends in the logarithmic estimates of LC50 (A) and LC90 (B) (bars: SE) for assays 1 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL alone), 2 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL integrated with DE), 3 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL integrated with imidacloprid), and 4 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL integrated with DE and imidacloprid) against the adults of WFT (F. occidentalis) against days after inoculation.
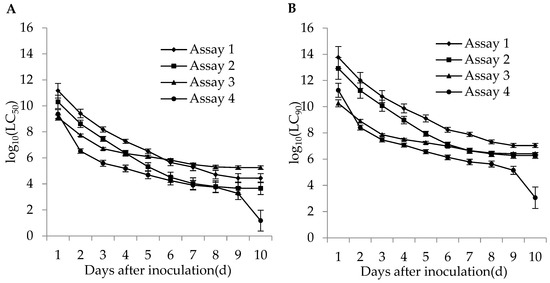
Figure 5.
Curve trends in the logarithmic estimates of LC50 (A) and LC90 (B) (bars: SE) for assays 1 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL alone), 2 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL integrated with DE), 3 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL integrated with imidacloprid), and 4 (M. flavoviride applied at 1.2 × 106 and 1.2 × 108 conidia/mL integrated with DE and imidacloprid) against the second instar larvae of WFT (F. occidentalis) against days after inoculation.
In conclusion, the estimated LC50 and LC90 values for the adults and second instar larvae of WFT in assay 4 (M. flavoviride with DE and imidacloprid) were lower than those in assays 1 (M. flavoviride alone), 2 (M. flavoviride with DE), and 3 (M. flavoviride with imidacloprid). It was also shown that the pathogenicity of M. flavoviride integrated with DE and imidacloprid on the adults and second instar larvae of WFT was higher than those of M. flavoviride alone, M. flavoviride with DE, and M. flavoviride with imidacloprid. Obviously, the lethal concentration trends highlight the dependence of the fungus, DE, and insecticide interactions on post-inoculation time.
3.3.4. Lethal Times (LT50 and LT90) of M. flavoviride against the Adults and Second Instar Larvae of WFT under Different Bioassays
The LT50 values were estimated by interpolation in the fitted TCM model of each bioassay method, which decreased with increasing M. flavoviride conidial concentration. This decrease (at a given fungal level) was significantly intensified by the combination of imidacloprid or DE (Table 4).

Table 4.
Lethal time values (LT50 and LT90) for the adults and second instar larvae of WFT infected by M. flavoviride WSWL51721 in four different bioassays.
For adults and second instar larvae of WFT, both LT50 and LT90 among assays 1–4 decreased with increasing fungal conidial concentration. The TCM model predicted the LT50 and LT90 values for high conidia concentrations in assays 1–4; however, for assays 1, 2, and 3 at lower conidia concentrations (1.2 × 106 conidia/mL), the LT90 could not be predicted as the mortality rate under them was less than 90%. For adults and second instar larvae of WFT, the lowest LT50 and LT90 values were estimated at the higher conidia concentration (1.2 × 108 conidia/mL) in assay 4 (M. flavoviride with DE and imidacloprid), which were 1.53 and 2.69 d, and 1.41 and 2.45 d, respectively. For adult WFT, the LT50 values were 2.82, 2.26, 2.06, and 1.53 d at the higher conidia concentration (1.2 × 108 conidia/mL) in assays 1–4, respectively. Similarly, for the second instar larvae of WFT, the LT50 values at the higher conidia concentration (1.2 × 108 conidia/mL) in assays 1–4 were 3.11, 2.45, 1.70, and 1.41 d, respectively. The above results show that the virulence of assay 1 (M. flavoviride alone) was lower than that in assays 2 (M. flavoviride with DE), 3 (M. flavoviride with imidacloprid), and 4 (M. flavoviride with DE and imidacloprid), which further indicates that the entomopathogenic fungus combined with imidacloprid or DE could improve the control effect on adults and second instar larvae of WFT.
3.4. Conidia Production from WFT Carcasses
Mycelium and conidia of M. flavoviride WSWL51721 were detected on WFT carcasses by fluorescence microscopy. Then, 20 adults and the second instar larvae of WFT were selected from the four different bioassays. The sporulated M. flavoviride on adults and second instar larvae were cultured in petri dishes lined with moist, sterilized filter paper for 12 days.
The average number of newly produced conidia found on infected WFT adults after incubation varied over the ranges (3.63 ± 0.18) × 105–(5.28 ± 0.13) × 105 conidia/adult treated with M. flavoviride alone (assay 1); (3.32 ± 0.04) × 105–(4.62 ± 0.04) × 105 conidia/adult treated with M. flavoviride plus DE (assay 2); (3.57 ± 0.19) × 105–(4.87 ± 0.12) × 105 conidia/adult treated with M. flavoviride plus imidacloprid (assay 3); and (3.60 ± 0.32) × 105–(5.17 ± 0.22) × 105 conidia/adult treated with M. flavoviride plus DE and imidacloprid (assay 4), with a significant effect of the different bioassays on overall new post-mortem conidiogenesis (F7,23 = 20.70, p < 0.01). Similarly, the mean number of newly produced conidia found on infected second instar larvae of WFT after incubation varied over the ranges (2.60 ± 0.17) × 105–(4.10 ± 0.35) × 105 conidia/larva treated with M. flavoviride alone (assay 1); (2.50 ± 0.06) × 105–(3.67 ± 0.09) × 105 conidia/larva treated with M. flavoviride plus DE (assay 2); (2.87 ± 0.19) × 105–(3.73 ± 0.09) × 105 conidia/larva treated with M. flavoviride plus imidacloprid (assay 3); and (2.77 ± 0.09) × 105–(3.50 ± 0.06) × 105 conidia/larva treated with M. flavoviride plus DE and imidacloprid (assay 4), with a highly significant effect of the different bioassays on quantitative conidiogenesis (F7,23 = 13.38, p < 0.01) (Table 5).

Table 5.
Conidia production from carcasses of adults and second instar larvae of WFT infected by M. flavoviride WSWL51721.
4. Discussion
Several studies on integrated pest management (IPM) have focused on the pathogenicity of B. bassiana and M. anisopliae, as well as the combination of B. bassiana with predatory mites or different insecticides against WFT [9,10,13,53]. However, there have been no studies on the effects of different combinations of EPF with adjuvants and compatible insecticides on WFT. In addition, no fungal candidate of M. flavoviride which has a good control effect on the WFT has been found. In our previous studies, it was found from many different fungal candidates that M. flavoviride WSWL51721 has good potential in controlling WFT adults, in which the mortality reached 82.65% at a concentration of 1.0 × 107 conidia/mL [26]. The compatibility levels between different chemical insecticides and EPF have been shown to be different [19]. We chose three chemical insecticides—imidacloprid, buprofezin, and abamectin—that have been commonly used to control WFT in the field. The influence of these three insecticides on the radial growth and conidial production rates of M. flavoviride were tested. The results showed that imidacloprid had good compatibility with M. flavoviride. Therefore, we conducted further studies to evaluate the efficacy of different combinations of M. flavoviride with DE and imidacloprid against adults and second instar larvae of WFT under laboratory conditions.
As a natural insecticide, DE has been applied to control many stored grain insect pests, achieving a good control effect [32,33,34,35,36]. However, there have been few reports on its use in the control of agricultural pests. Our results showed that DE has a certain control effect on the invasive insect WFT, which is similar to its control effect against invasive red fire ants, whose efficacy was lower than stored grain insect pests [32,35,37,38]. The differences in control effects were due to the fact that some insects are more sensitive to DE, due to their anatomical and physiological characteristics, and may also be related to differences in the cuticular composition of different insect species [34,37].
In this study, we found that the corrected mortalities of WFT adults and second instar larvae were 75% and 72.22%, respectively, at 10 days after inoculation of 1.2 × 106 conidia/mL in assay 1 (M. flavoviride alone); while the corrected mortality of WFT adults and second instar larvae was 100% at 10 days after inoculation with 1.2 × 108 conidia/mL, which is significantly higher than the results obtained for other pathogenic fungi of WFT [6,13,20,24]. However, the corrected mortalities of WFT adults and second instar larvae in assays 2 (M. flavoviride with DE), 3 (M. flavoviride with imidacloprid), and 4 (M. flavoviride with DE and imidacloprid) were higher than those in assay 1 (M. flavoviride alone), indicating that EPF combined with DE (assay 2), EPF combined with insecticide (assay 3), and EPF combined with DE and insecticide (assay 4) all had synergistic effects, thereby increasing the pathogenicity of EPF to WFT. These results were consistent with the studies reported by Athanassiou et al., in which a six-fold increase in adult mortality of T. confusum was observed when treated with B. bassiana and DE, compared with either B. bassiana or DE alone [15]. Similarly, Ashraf et al. clearly indicated that M. anisopliae combined with DE or thiamethoxam had a synergistic effect, compared to their individual efficacies, and was more effective in controlling four stored insect pests [35]. Likewise, Wakil et al. found that B. bassiana combined with DE or the neonicotinoid insecticide thiamethoxam increased the mortality of R. dominica adults, compared with individual treatments, and a significant additive effect was observed [32]. Similar synergistic interactions were observed when the tested fungi B. bassiana, M. anisopliae, and I. fumosorosea were combined with DE against Plodia interpunctella, Ephestia cautella, and Ephestia kuehniella [51]. Therefore, it is important to understand how a pathogen interacts with other insecticides and adjuvants. However, at present, little is known about the reasons for such synergism; it is generally believed that DE has unique physical properties: When it contacts insects, it will destroy the wax layer of the insect cuticles and cause water exudation from the body, which can provide favorable conditions for the attachment and germination of conidia, which can quickly penetrate the cuticle layer of the host insect body wall and then into the haemocoel, continuing to multiply and secrete a series of metabolites which, finally, leads to mycosis and death [39,40,41,42]. For neonicotinoid insecticides such as imidacloprid and thiamethoxam, when the target insect comes into contact with it, normal conduction of the central nervous system will be blocked, leading to rapid paralysis before death; this can assist the conidia in penetrating the weakened insects more easily. The fungi will eventually kill the weakened insect relatively quickly.
In time–concentration–response tests, the TCM modeling method has been applied in fungi–insect [60,61,62,63,64] and insecticide–insect bioassays [65,66,67]. In this study, the lethal times and concentrations in different bioassays were evaluated by inserting experimental data into the TCM model. We found that the lethal concentrations (LC50 and LC90) and lethal times (LT50 and LT90) for adult WFT treated with M. flavoviride plus DE and imidacloprid (assay 4) were lower than those of other bioassay methods (i.e., assays 1, 2, and 3), while the lethal concentrations and the lethal times of WFT second instar larvae in assay 4 were also lower than those of the bioassay methods (assays 1, 2, and 3). These results are in accordance with the above-mentioned results: the mortality of WFT adults and second instar larvae in assay 4 was higher than in the other bioassays (assays 1, 2, and 3), indicating that different combinations of EPF with DE and imidacloprid have different synergistic effects. Similarly, Kivett et al. clearly found that M. anisopliae combined with azadirachtin had synergistic effects on WFT larvae [6].
The carcasses caused by the infection of fungal agents play a very important role in the secondary recycling of infection, as the conidia produced on mycotized carcasses can be spread to other sites, which can infect the target insects and other pests, increasing the effective persistency of the fungal biocontrol agents [39,55,68]. Information on the post-mortem sporulation of carcasses after killing WFT by EPF had not been reported. In our study, we found that there was no significant difference in the sporulation of the carcasses between the four different bioassays of the WFT adults, indicating that DE and imidacloprid had no clear effect on the quantitative conidiogenesis of dead WFT adults. This may be due to the minimal amounts of imidacloprid and DE remaining on the surface of the WFT adults after inoculation, as well as passive or active removal of adjuvants on the cuticle by grooming and decomposition behaviors [55]. However, there was a significant difference in the sporulation of dead WFT second instar larvae in four different bioassays. We speculate that this may be related to the different components of the cuticle of WFT in different developmental stages.
5. Conclusions
In conclusion, our study showed that M. flavoviride WSWL5172 was not only highly virulent to adult WFT, but also to second instar larvae WFT under laboratory conditions. Most importantly, the combination of the fungal strain M. flavoviride WSWL5172 with DE and imidacloprid had a good control effect on the adult and second instar larvae of WFT, where the lethal time was decreased by half, compared with that of M. flavoviride WSWL5172 alone. Considering that the efficacy of EPF in the field is not as good as that indoors (as, in the field, EPF are affected by environmental factors, such as temperature, humidity, and light [17]), the combination of chemical insecticides or adjuvants with EPF will become the trend of IPM programs in future.
Author Contributions
W.G., B.C. and G.X. conceived the research. W.G., G.D. and B.C. conducted the experiments. W.G., B.C., G.X. and Z.L. analyzed the data and conducted the statistical analyses. W.G. wrote the manuscript. W.G., B.C., G.X., G.D. and L.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 31660537 and 31760519) and the National Key Research and Development Program of China (no. 2018YFD0200703).
Conflicts of Interest
The authors declare that there is no conflict of interest with regard to this work.
References
- Lewis, T. Pest thrips in perspective. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 1–13. [Google Scholar]
- Gao, Y.L.; Reitz, S.R.; Wang, J.; Xu, X.N.; Lei, Z.R. Potential of a strain of the entomopathogenic fungus Beauveria bassiana (Hypocreales: Cordycipitaceae) as a biological control agent against western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Biocontrol Sci. Technol. 2012, 22, 491–495. [Google Scholar] [CrossRef]
- Otieno, J.A.; Pallmann, P.; Poehling, H.M. The combined effect of soil-applied azadirachtin with entomopathogens for integrated management of western flower thrips. J. Appl. Entomol. 2015, 140, 174–186. [Google Scholar] [CrossRef]
- Morse, J.G.; Hoddle, M.S. Invasion Biology of Thrips. Annu. Rev. Entomol. 2006, 51, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.G.; Pappu, H.R. Tactics for management of thrips (Thysanoptera: Thripidae) and tomato spotted wilt virus in tomato. J. Econ. Entomol. 2004, 97, 1648–1658. [Google Scholar] [CrossRef]
- Kivett, J.M.; Cloyd, R.A.; Bello, N.M. Evaluation of Entomopathogenic Fungi Against the Western Flower Thrips (Thysanoptera: Thripidae) Under Laboratory Conditions. J. Entomol. Sci. 2016, 51, 274–291. [Google Scholar] [CrossRef]
- Espinosa, P.J.; Bielza, P.; Contreras, J.; Lacasa, A. Insecticide resistance in field populations of Frankliniella occidentalis (Pergande) in Murcia (south-east Spain). Pest Manag. Sci. 2002, 58, 967–971. [Google Scholar] [CrossRef]
- Wu, S.Y.; Tang, L.D.; Zhang, X.R.; Xing, Z.L.; Lei, Z.R.; Gao, Y.L. A decade of a thrips invasion in China: Lessons learned. Ecotoxicology 2018, 27, 1032–1038. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shah, F.A.; Whittaker, M.; Prasad, M.; Butt, T.M. Control of western flower thrips (Frankliniella occidentalis) pupae with Metarhizium anisopliae in peat and peat alternative growing media. Biol. Control 2007, 40, 293–297. [Google Scholar] [CrossRef]
- Ansari, M.A.; Brownbridge, M.; Shah, F.A.; Butt, T.M. Efficacy of entomopathogenic fungi against soil-dwelling life stages of western flower thrips, Frankliniella occidentalis, in plant-growing media. Entomol. Exp. Appl. 2008, 127, 80–87. [Google Scholar] [CrossRef]
- Jensen, S.E. Insecticide resistance in the western flower thrips, Frankliniella occidentalis. Integr. Pest Manag. Rev. 2000, 5, 131–146. [Google Scholar] [CrossRef]
- Saito, T.; Brownbridge, M. Compatibility of foliage-dwelling predatory mites and mycoinsecticides, and their combined efficacy against western flower thrips Frankliniella occidentalis. J. Pest Sci. 2018, 91, 1291–1300. [Google Scholar] [CrossRef]
- Wu, S.Y.; Gao, Y.L.; Xu, X.N.; Goettel, M.S.; Lei, Z.R. Compatibility of Beauveria bassiana with Neoseiulus barkeri for Control of Frankliniella occidentalis. J. Integr. Agric. 2015, 14, 98–105. [Google Scholar] [CrossRef]
- Mouden, S.; Sarmiento, K.F.; Klinkhamer, P.G.; Leiss, K.A. Integrated pest management in western flower thrips: Past, present and future. Pest Manag. Sci. 2017, 73, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Rumbos, C.I.; Sakka, M.K.; Vayias, B.J.; Stephou, V.K.; Nakas, C.N. Insecticidal effect of the combined application of spinosad, Beauveria bassiana and diatomaceous earth for the control of Tribolium confusum. Biocontrol Sci. Technol. 2016, 26, 809–819. [Google Scholar] [CrossRef]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Sohrabi, F.; Jamali, F.; Morammazi, S.; Saber, M.; Kamita, S.G. Evaluation of the compatibility of entomopathogenic fungi and two botanical insecticides tondexir and palizin for controlling Galleria mellonella L. (Lepidoptera: Pyralidae). Crop Prot. 2019, 117, 20–25. [Google Scholar] [CrossRef]
- Duarte, R.T.; Gonçalves, K.C.; Espinosa, D.J.L.; Moreira, L.F.; Bortoli, S.A.D.; Humber, R.A.; Polanczyk, R.A. Potential of Entomopathogenic Fungi as Biological Control Agents of Diamondback Moth (Lepidoptera: Plutellidae) and Compatibility with Chemical Insecticides. J. Econ. Entomol. 2016, 109, 594–601. [Google Scholar] [CrossRef]
- Vestergaard, S.; Gillespie, A.T.; Butt, T.M.; Schreiter, G.; Eilenberg, J. Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips, Frankliniella occidentalis. Biocontrol Sci. Technol. 1995, 5, 185–192. [Google Scholar] [CrossRef]
- Wu, S.Y.; Gao, Y.L.; Smagghe, G.; Xu, X.N.; Lei, Z.R. Interactions between the entomopathogenic fungus Beauveria bassiana and the predatory mite Neoseiulus barkeri and biological control of their shared prey/host Frankliniella occidentalis. Biol. Control 2016, 17, 43–51. [Google Scholar] [CrossRef]
- Ugine, T.A.; Wraight, S.P.; Brownbridge, M.; Sanderson, J.P. Development of a novel bioassay for estimation of median lethal concentrations (LC50) and doses (LD50) of the entomopathogenic fungus Beauveria bassiana against western flower thrips, Frankliniella occidentalis. J. Invertebr. Pathol. 2005, 89, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Lei, Z.R.; Reitz, S.R.; Wu, S.Y.; Gao, Y.L. Laboratory and Greenhouse Evaluation of a Granular Formulation of Beauveria bassiana for Control of Western Flower Thrips, Frankliniella occidentalis. Insects 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, L.M.; Mburu, D.M.; Masiga, D.; Ekesi, E. Selection of promising fungal biological control agent of the western flower thrips Frankliniella occidentalis (Pergande). Lett. Appl. Microbiol. 2012, 54, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wraight, S.P.; Ugine, T.A.; Ramos, M.E.; Sanderson, J.P. Efficacy of spray applications of entomopathogenic fungi against western flower thrips infesting greenhouse impatients under variable moisture conditions. Biol. Control 2016, 97, 31–47. [Google Scholar] [CrossRef]
- Ge, W.C.; Luo, W.C.; Chen, B.; Zhang, H.R.; Gu, X.H.; Zhang, L.M. Virulence of three hyphomycetes entomopathogenic fungi against the adult of Frankliniella occidentalis (Pergande). J. South. Agric. 2019, 50, 1735–1741. [Google Scholar]
- Montserrat, M.; Castañé, C.; Santamaria, S. Neozygites parvispora Zygomycotina: Entomophthorales causing an epizootic in Frankliniella occidentalis (Thysanoptera: Thripidae) on cucumber in Spain. J. Invertebr. Pathol. 1998, 71, 165–168. [Google Scholar] [CrossRef]
- Stepanycheva, E.; Petrova, M.; Chermenskaya, T.; Pavela, R. Fumigant effect of essential oils on mortality and fertility of thrips Frankliniella occidentalis Perg. Environ. Sci. Pollut. Res. 2019, 26, 30885–30892. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential oil compounds for thrips control—A review. Nat. Prod. Commun. 2008, 3, 1171–1182. [Google Scholar] [CrossRef]
- Ebeling, W. Sorptive dust for pest control. Annu. Rev. Entomol. 1971, 16, 123–158. [Google Scholar] [CrossRef]
- Michalaki, M.P.; Athanassiou, C.G.; Steenberg, T.; Buchelos, C.T. Effect of Paecilomyces fumosoroseus (Wise) Brown and Smith (Ascomycota: Hypocreales) alone or in combination with diatomaceous earth against Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Biol. Control 2007, 40, 280–286. [Google Scholar]
- Wakil, W.; Riasat, T.; Ashfaq, M. Residual efficacy of thiamethoxam, Beauveria bassiana (Balsamo) Vuillemin, and diatomaceous earth formulation against Rhyzopertha dominica F. (Coleoptera: Bostrychidae). J. Pest Sci. 2012, 85, 341–350. [Google Scholar] [CrossRef]
- Wakil, W.; Schmitt, T. Field trials on the efficacy of Beauveria bassiana, diatomaceous earth and Imidacloprid for the protection of wheat grains from four major stored grain insect pests. J. Stored Prod. Res. 2014, 64, 160–167. [Google Scholar] [CrossRef]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Howard, R.W. Diatomaceous earth increases the efficacy of Beauveria bassiana against Tribolium castaneum larvae and increases conidia attachment. J. Econ. Entomol. 2004, 97, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Farooq, M.; Shakeel, M.; Din, N.; Hussain, S.; Saeed, N.; Shakeel, Q.; Rajput, A. Influence of entomopathogenic fungus, Metarhizium anisopliae, alone and in combination with diatomaceous earth and thiamethoxam on mortality, progeny production, mycosis, and sporulation of the stored grain insect pests. Environ. Sci. Pollut. Res. 2017, 24, 28165–28174. [Google Scholar] [CrossRef]
- Nwaubani, S.I.; Opit, G.P.; Otitodun, G.O.; Adesida, M.A. Efficacy of two Nigeria-derived diatomaceous earths against Sitophilus oryzae (Coleoptera: Curculionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) on wheat. J. Stored Prod. Res. 2014, 59, 9–16. [Google Scholar] [CrossRef]
- Korunic, Z. Review Diatomaceous Earths, a Group of Natural Insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Brinkman, M.A.; Gardner, W.A. Use of diatomaceous earth and entomopathogen combinations against the red imported fire ant (Hymenoptera: Formicidae). Fla. Entomol. 2001, 84, 740–741. [Google Scholar] [CrossRef]
- Riasat, T.; Wakil, W.; Ashfaq, M.; Sahi, S.-T. Effect of Beauveria bassiana mixed with diatomaceous earth on mortality, mycosis and sporulation of Rhyzopertha dominica on stored wheat. Phytoparasitica 2011, 39, 325–331. [Google Scholar] [CrossRef]
- Vayias, B.J.; Athanassiou, C.G. Factors affecting the insecticidal efficacy of the diatomaceous earth formulation SilicoSec against adults and larvae of the confused flour beetle, Tribolium confusum Du Val (Coleoptera: Tenebrionidae). Crop Prot. 2004, 23, 565–573. [Google Scholar] [CrossRef]
- Vayias, B.J.; Athanassiou, C.G.; Kavallieratos, N.G.; Tsesmeli, C.D.; Buchelos, C.T. Persistence and efficacy of two diatomaceous earth formulations and a mixture of diatomaceous earth with natural pyrethrum against Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) on wheat and maize. Pest Manag. Sci. 2006, 62, 456–464. [Google Scholar] [CrossRef]
- Ziaee, M.; Khashaveh, A. Effect of five diatomaceous earth formulations against Tribolium castaneum (Coleoptera: Tenebrionidae), Oryzaephilus surinamensis (Coleoptera: Silvanidae) and Rhyzopertha dominica (Coleoptera: Bostrychidae). Insect Sci. 2007, 14, 359–365. [Google Scholar] [CrossRef]
- Ziaee, M.; Moharramipour, S.; Francikowski, J. The synergistic effects of Carum copticum essential oil on diatomaceous earth against Sitophilus granarius and Tribolium confusum. J. Asia Pac. Entomol. 2014, 17, 817–822. [Google Scholar] [CrossRef]
- Feng, M.G.; Pu, X.Y. Time-concentration-mortality modeling of the synergistic interaction of Beauveria bassiana and imidacloprid against Nilaparvata lugens. Pest Manag. Sci. 2005, 61, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Feng, M.G. Evaluation of the time-concentration-mortality responses of Plutella xylostella larvae to the interaction of Beauveria bassiana with a nereistoxin analogue insecticide. Pest Manag. Sci. 2006, 62, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.D.; Dun, Y.H.; Feng, M.G. Time and concentration dependent interactions of Beauveria bassiana with sublethal rates of imidacloprid against the aphid pests Macrosiphoniella sanborni and Myzus persicae. Ann. Appl. Biol. 2005, 146, 459–468. [Google Scholar] [CrossRef]
- Rivero-Borja, M.; Guzmán-Franco, A.W.; Rodríguez-Leyva, E.; Santillán-Ortega, C.; Pérez-Panduro, A. Interaction of Beauveria bassiana and Metarhizium anisopliae with chlorpyrifos ethyl and spinosad in Spodoptera frugiperda larvae. Pest Manag. Sci. 2018, 74, 2047–2052. [Google Scholar] [CrossRef]
- Russell, C.W.; Ugine, T.A.; Hajek, A.E. Interactions between imidacloprid and Metarhizium brunneum on adult Asian longhorned beetles (Anoplophora glabripennis (Motschulsky)) (Coleoptera: Cerambycidae). J. Invertebr. Pathol. 2010, 105, 305–311. [Google Scholar] [CrossRef]
- Nian, X.G.; He, Y.R.; Lu, L.H.; Zhao, R. Evaluation of the time-concentration-mortality responses of Plutella xylostella larvae to the interaction of Isaria fumosorosea with the insecticides beta-cypermethrin and Bacillus thuringiensis. Pest Manag. Sci. 2014, 71, 216–224. [Google Scholar] [CrossRef]
- Tang, J.F.; Liu, X.Y.; Ding, Y.C.; Jiang, W.J.; Xie, J.Q. Evaluation of Metarhizium anisopliae for rice planthopper control and its synergy with selected insecticides. Crop Prot. 2019, 121, 132–138. [Google Scholar] [CrossRef]
- Sabbour, M.M.; Abd-El-Aziz, S.E.; Sherief, M.A. Efficacy of three entomopathogenic fungi alone or in combination with diatomaceous earth modifications for the control of three pyralid moths in stored grains. J. Plant Prot. Res. 2012, 52, 359–363. [Google Scholar] [CrossRef]
- Arooni-Hesari, M.; Talaei-Hassanloui, R.; Sabahi, Q. Simultaneous Use of Entomopathogenic Fungus Beauveria bassiana and Diatomaceous Earth against the Larvae of Indian Meal Moth, Plodia interpunctella. Adv. Biosci. Biotechnol. 2015, 6, 501–507. [Google Scholar] [CrossRef]
- Jacobson, R.J.; Chandler, D.; Fenlon, J.; Russell, K.M. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci. Technol. 2001, 11, 391–400. [Google Scholar] [CrossRef]
- Goettel, S.; Inglis, G.D. Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Elsevier: Amsterdam, The Netherlands, 1997; pp. 213–249. [Google Scholar]
- Rodrigues, J.; Borges, P.R.; Fernandes, É.K.K.; Luz, C. Activity of additives and their effect in formulations of Metarhizium anisopliae s.l. IP 46 against Aedes aegypti adults and on post mortem conidiogenesis. Acta Trop. 2019, 193, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Feng, M.G. DPS Data Processing System for Practical Statistics; Science Press: Beijing, China, 2012. [Google Scholar]
- Nowierski, R.M.; Zheng, Z.; Jaronski, S.; Delgado, F.; Swearingen, W. Analysis and modeling of time-dose-mortality of Melanoplus sanguinipes, Locusta migratoria migratorioides, and Schistocerca gregaria (Orthoptera: Acrididae) from Beauveria, Metarhizium, and Paecilomyces isolates from Madagascar. J. Invertebr. Pathol. 1996, 67, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.G.; Liu, C.L.; Xu, J.H.; Xu, Q. Modeling and biological implication of time-dose-mortality data for the entomophthoralean fungus, Zoophthora anhuiensis, on the green peach aphid Myzus persicae. J. Invertebr. Pathol. 1998, 72, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Feng, M.G. The time-dose-mortality modeling and virulence indices for two entomophthoralean species, Pandora delphacis, and P. Neoaphidis, against the green peach aphid, Myzus persicae. Biol. Control 2000, 17, 29–34. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; He, S.Q.; Chen, B.; Su, Z.T.; Wang, W.Q.; Zhang, L.Y.; Li, Z.Y.; Xiao, G.L. Time-concentration-mortality response of potato tuber moth pupae to the biocontrol agent Cordyceps tenuipes. Biocontrol Sci. Technol. 2019, 29, 965–978. [Google Scholar] [CrossRef]
- Shi, W.B.; Zhang, L.; Feng, M.G. Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to three hypocrealean fungi as biocontrol agents. Biol. Control 2008, 46, 495–501. [Google Scholar] [CrossRef]
- Qiu, J.Z.; Song, F.F.; Mao, L.H.; Tu, J.; Guan, X. Time-dose-mortality data and modeling for the entomopathogenic fungus Aschersonia placenta against the whitefly Bemisia tabaci. Can. J. Microbiol. 2013, 59, 97–101. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; You, M.; Liu, B. Time-dose-mortality modelling and virulence indices for six strains of Verticillium lecanii against sweetpotato whitefly, Bemisia tabaci (Gennadius). J. Appl. Entomol. 2004, 128, 494–500. [Google Scholar] [CrossRef]
- Shan, L.T.; Feng, M.G. Evaluation of the biocontrol potential of various Metarhizium isolates against green peach aphid Myzus persicae (Homoptera: Aphididae). Pest Manag. Sci. 2010, 66, 669–675. [Google Scholar] [PubMed]
- Shi, Y.W.; Qin, X.W.; Wang, Z.Y.; Wang, M.; Liu, J.; Chen, Y.; Zhang, R.J.; Liu, Y.D. Analysis of the time-concentration-mortality response of Nilaparvata lugens (Hemiptera: Delphacidae) to Paichongding. J. Econ. Entomol. 2015, 108, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.; Eilenberg, J. Time-concentration mortality of Pieris brassicae (Lepidoptera: Pieridae) and Agrotis segetum (Lepidoptera: Noctuidae) larvae from different destruxins. Environ. Entomol. 2000, 29, 1041–1047. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Qin, X.W.; Yuan, F.H.; Zhang, R.J. Analysis of Time-and Concentration-Mortality Relationship of Nitenpyram Against Different Larval Stages of Nilaparvata lugens (Hemiptera: Delphacidae). J. Econ. Entomol. 2010, 103, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Wood, S.N.; Lomer, C.J. Biological control of locusts and grasshoppers using a fungal pathogen: The importance of secondary cycling. Proc. R. Soc. B Biol. Sci. 1995, 259, 265–270. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).