Polyphenol-Rich Purple Corn Pericarp Extract Adversely Impacts Herbivore Growth and Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corn Dry Milling for Pericarp Recovery
2.2. Pericarp Extract Preparation
2.3. Quantification of Anthocyanins, Tannins, and Total Polyphenols
2.4. Insect Colony
2.5. Artificial Diet
2.6. Plant Material
2.7. Detailed Experimental Design
2.8. Bioassays
2.8.1. Egg Hatching
2.8.2. First Instar Survival
2.8.3. Mass
2.8.4. Mass Gain
2.8.5. Feeding Behavior
2.8.6. Ethovision
2.8.7. Time to Pupate
2.8.8. Diet Switch Assay
2.8.9. Spray Experiment
2.8.10. Data Analysis
3. Results
3.1. Quantification of Anthocyanins, Tannins, and Total Polyphenols
3.2. Bioassays
3.2.1. Effects of Purple Corn Pericarp Extract on Egg Hatching and First Instar Mortality
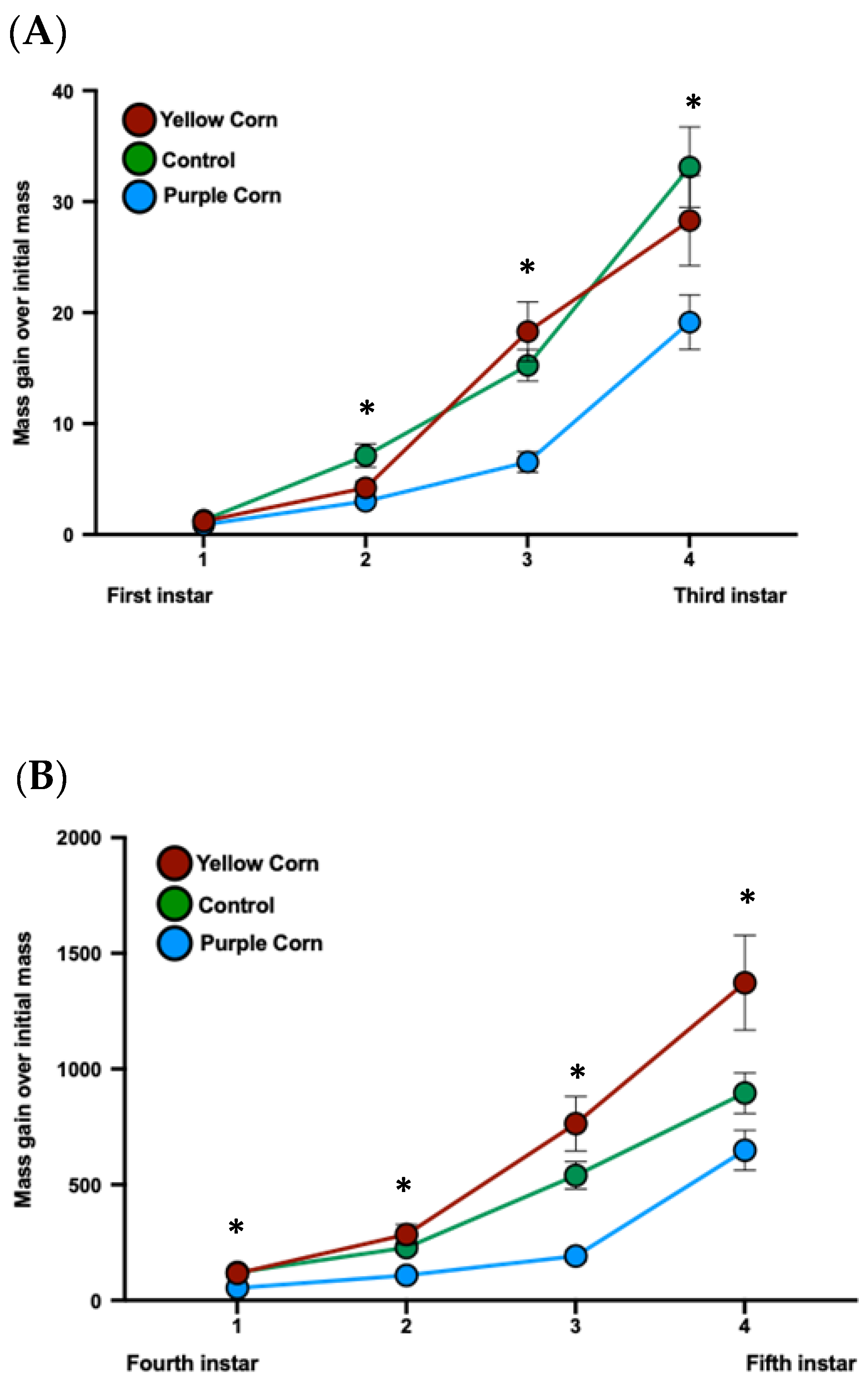
3.2.2. Effects of Purple Corn Pericarp Extract on Larval Mass
3.2.3. Effects of Purple Corn Pericarp Extract on Larval Mass Gain
3.2.4. Effects of Purple Corn Pericarp Extract on Larval Feeding Preference
3.2.5. Ethovision
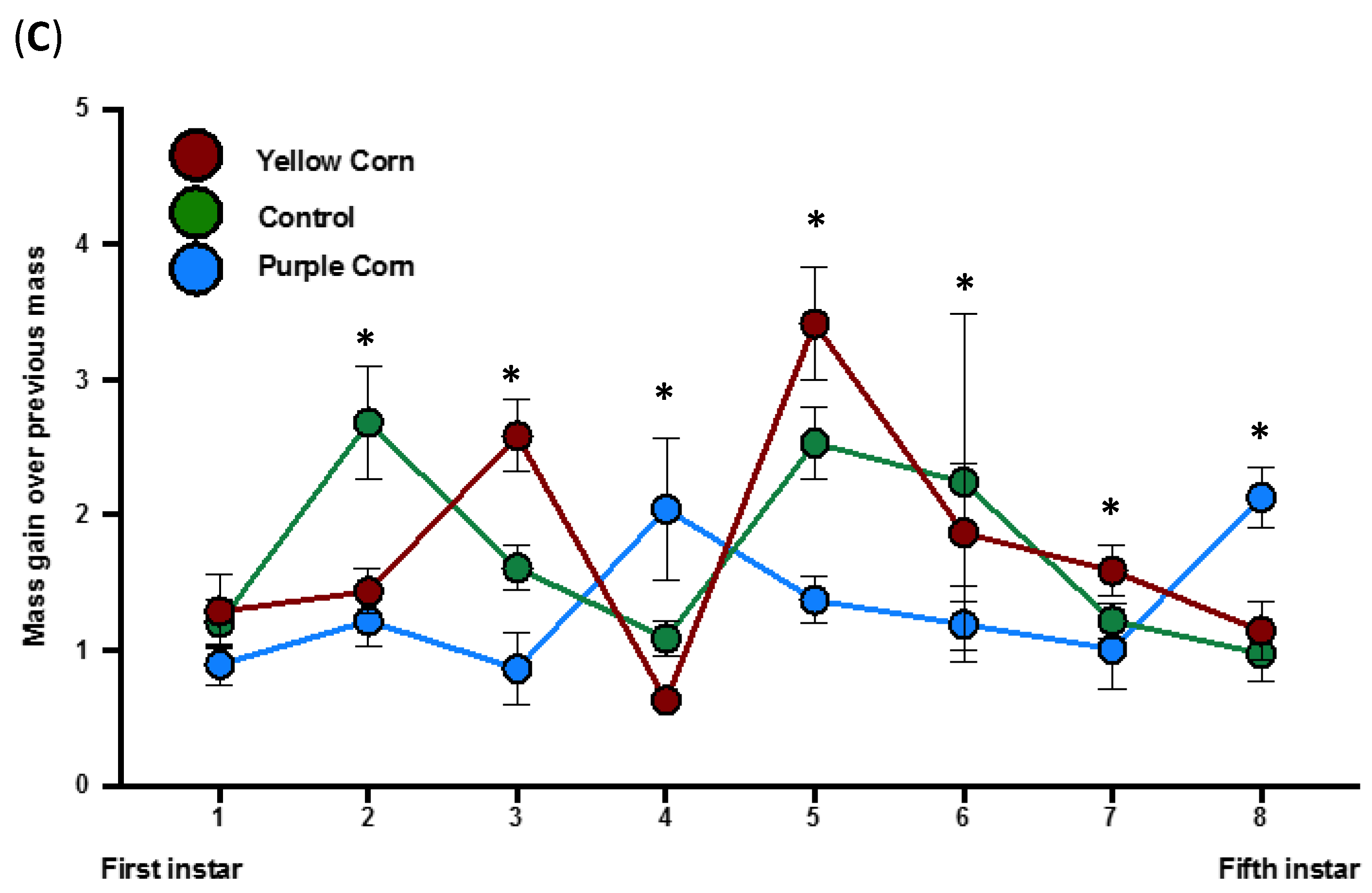
3.2.6. Effects of Purple Corn Pericarp Extract on Caterpillar Survival Throughout the Larval Stages
3.2.7. Effects of Purple Corn Pericarp Extract on Pupation Time
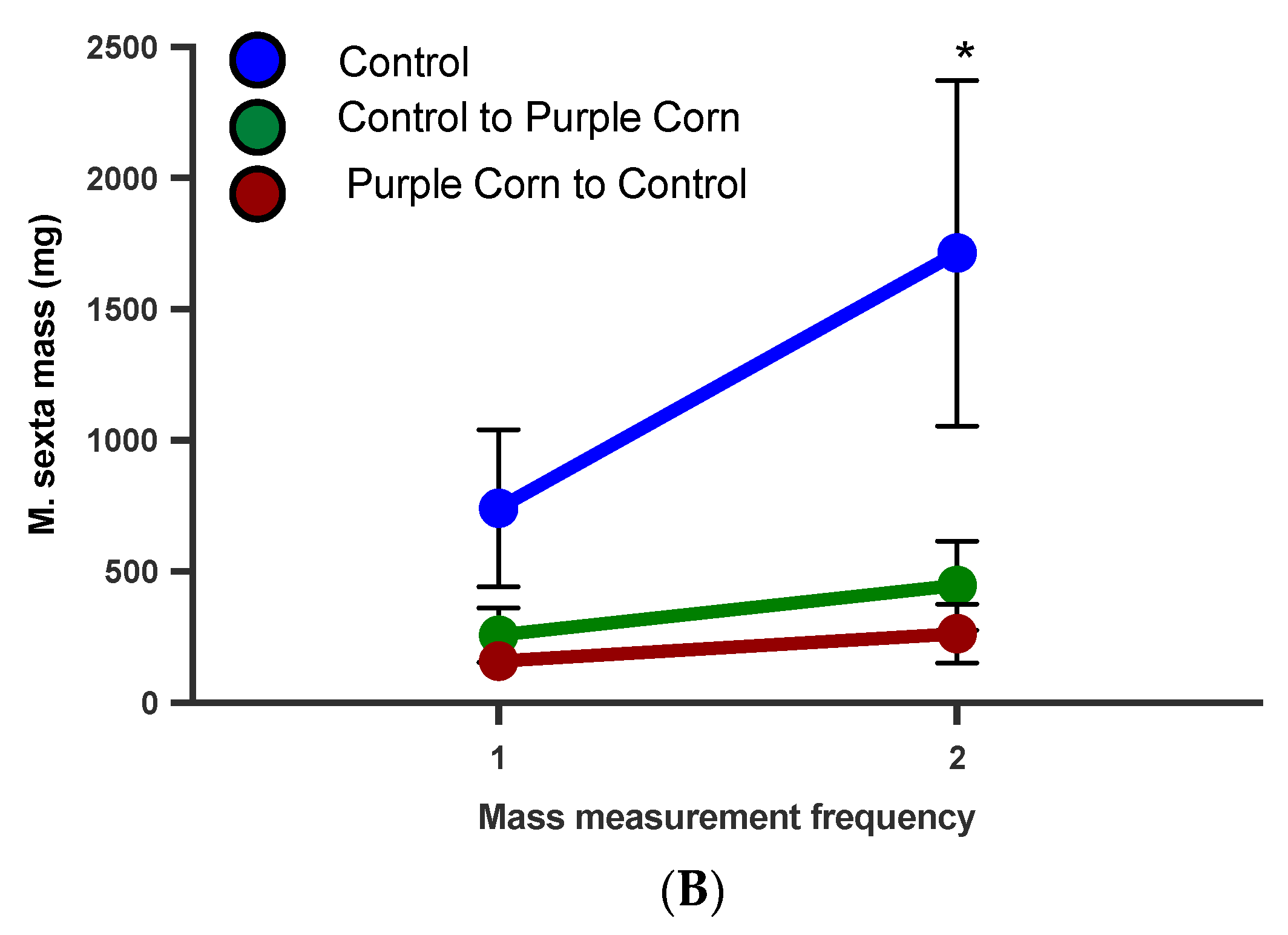
3.2.8. M. sexta Mass on Switched Diet Experiment
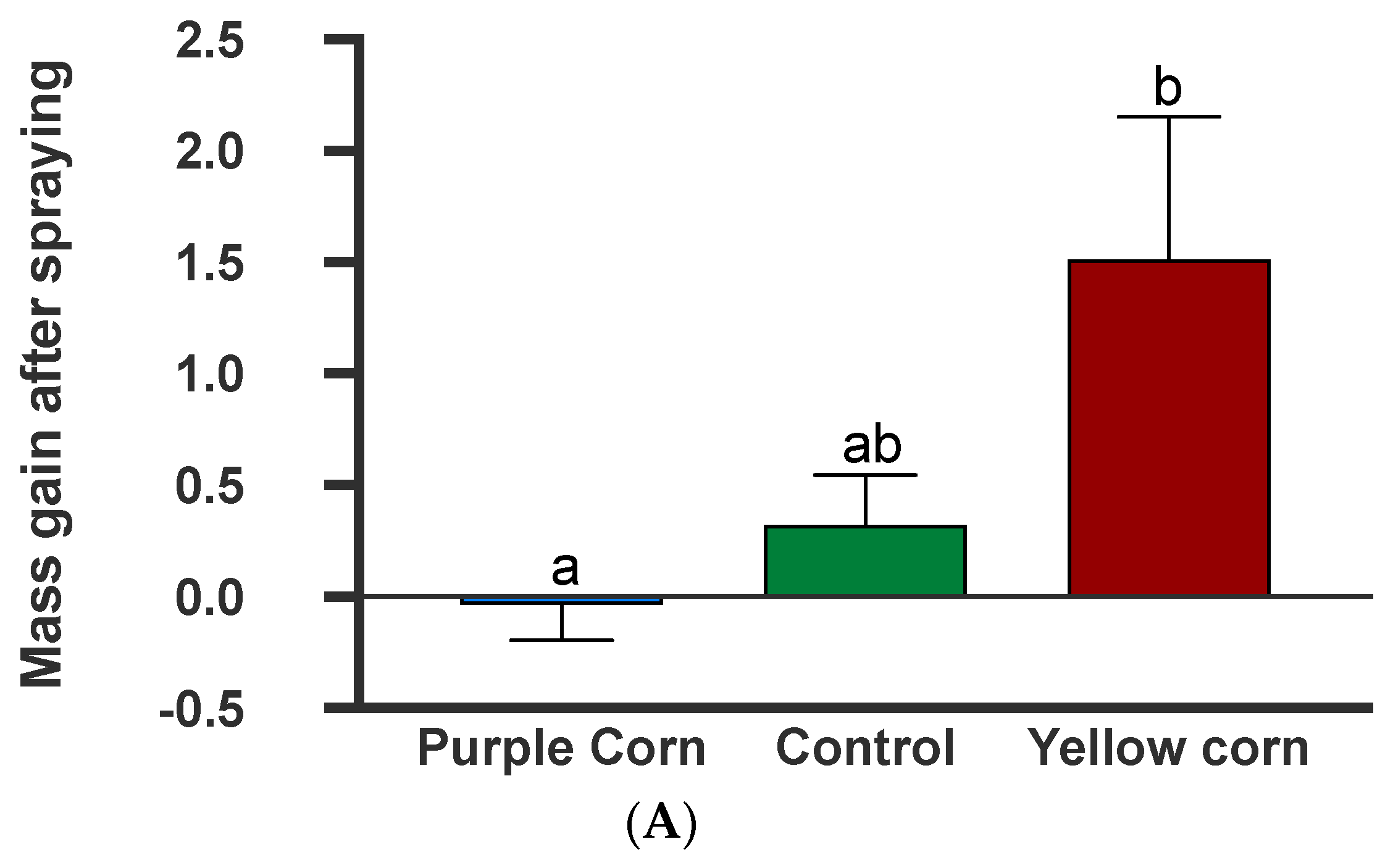
3.2.9. Effects of Plant-Sprayed Purple Corn Pericarp Extract on Larval Mass Gains and Frass Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Theis, N.; Lerdau, M. The Evolution of Function in Plant Secondary Metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Koul, O.; Jain, M.P.; Sharma, V.K. Growth inhibitory and antifeedant activity of extracts from Melia dubia to Spodoptera litura and Helicoverpa armigera larvae. Indian J. Exp. Biol 2000, 38, 63–68. [Google Scholar] [PubMed]
- Ayasse, M. Floral Scent and Pollinator Attraction in Sexually Deceptive Orchids. Biol. Flor. Scent 2006, 219–241. [Google Scholar]
- Dobson, H. Relationship between Floral Fragrance Composition and Type of Pollinator. Biol. Flor. Scent 2006, 147–198. [Google Scholar]
- Runyon, J.B. Volatile Chemical Cues Guide Host Location and Host Selection by Parasitic Plants. Science 2006, 313, 1964–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turlings, T.C.J.; Hiltpold, I.; Rasmann, S. The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil 2012, 358, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Kariyat, R.R.; Mauck, K.E.; Moraes, C.M.D.; Stephenson, A.G.; Mescher, M.C. Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol. Lett. 2012, 15, 301–309. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Scanlon, S.R.; Moraski, R.P.; Stephenson, A.G.; Mescher, M.C.; Moraes, C.M.D. Plant inbreeding and prior herbivory influence the attraction of caterpillars (Manduca sexta) to odors of the host plant Solanum carolinense (Solanaceae). Am. J. Bot. 2014, 101, 376–380. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Raya, C.E.; Chavana, J.; Cantu, J.; Guzman, G.; Sasidharan, L. Feeding on glandular and non-glandular leaf trichomes negatively affect growth and development in tobacco hornworm (Manduca sexta) caterpillars. Arthropod Plant Interact. 2019, 13, 321–333. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Wittstock, U.; Gershenzon, J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 2002, 5, 300–307. [Google Scholar] [CrossRef]
- Harborne, J.B. Role of Secondary Metabolites in Chemical Defence Mechanisms in Plants. In Ciba Foundation Symposium 154—Bioactive Compounds from Plants Novartis Foundation Symposia; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 126–139. [Google Scholar]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Campbell, S.A.; Thaler, J.S.; Kessler, A. Plant chemistry underlies herbivore-mediated inbreeding depression in nature. Ecol. Lett. 2012, 16, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lev-Yadun, S.; Gould, K.S. Role of Anthocyanins in Plant Defence. In Anthocyanins; Springer: New York, NY, USA, 2008; pp. 22–28. [Google Scholar]
- Andersen, Ø.M.; Jordheim, M. Anthocyanins. In Flavonoids: Chemistry, Biochemistry and Applications; Anderson, O.M., Markham, K.R., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Adler, L.S.; Karban, R.; Strauss, S.Y. Direct and Indirect Effects of Alkaloids on Plant Fitness Via Herbivory and Pollination. Ecology 2001, 82, 2032–2044. [Google Scholar] [CrossRef]
- Irwin, R.E.; Adler, L.S.; Brody, A.K. The Dual Role of Floral Traits: Pollinator Attraction and Plant Defense. Ecology 2004, 85, 1503–1511. [Google Scholar] [CrossRef]
- Barber, N.A.; Adler, L.S.; Theis, N.; Hazzard, R.V.; Kiers, E.T. Herbivory reduces plant interactions with above- and belowground antagonists and mutualists. Ecology 2012, 93, 1560–1570. [Google Scholar] [CrossRef] [Green Version]
- Gorden, N.L.S.; Adler, L.S. Abiotic conditions affect floral antagonists and mutualists of Impatiens capensis (Balsaminaceae). Am. J. Bot. 2013, 100, 679–689. [Google Scholar] [CrossRef]
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental Regulation of Methyl Benzoate Biosynthesis and Emission in Snapdragon Flowers. Plant Cell 2000, 12, 949. [Google Scholar] [CrossRef] [Green Version]
- Pott, M.B.; Pichersky, E.; Piechulla, B. Evening specific oscillations of scent emission, SAMT enzyme activity, and SAMT mRNA in flowers of Stephanotis floribunda. J. Plant Physiol. 2002, 159, 925–934. [Google Scholar] [CrossRef] [Green Version]
- Schnepp, J.; Dudareva, N. Floral Scent: Biosynthesis, Regulation and Genetic Modifications. Annu. Plant Rev. Online 2018, 240–257. [Google Scholar]
- Kumar, S. Plant secondary metabolites (PSMs) of Brassicaceae and their role in plant defense against insect herbivores—A review. J. Appl. Nat. Sci. 2017, 9, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Balasundaram, A.; Ragupathy, R.; Sankar, S.; Thiyagarajan, M.; Ravi, L.; Karuppasamy, R.; Veerappapillai, S. Investigation of Phytocompounds and Computational Approach for the Evaluation of Therapeutic Properties of Ethanolic Leaf Extract of Callistemon citrinu. Int. J. Pharm. Sci. Rev. Res. 2016, 37, 110–116. [Google Scholar]
- Lattanzio, V. Phenolic Compounds: Introduction. Nat. Prod. 2013, 1543–1580. [Google Scholar]
- Zobrel, A.M.; Brown, S.A. Coumarins in the interactions between the plant and its environment. Allelopath. J. 1995, 2, 9–20. [Google Scholar]
- Pourcel, L.; Routaboul, J.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Silva, T.M.S.; dos Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.S.; da Silva, G.S.; de Novais, J.S.; dos Santos, F.D.A.R.; Camara, C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Marles, M.; Ray, H.; Gruber, M.Y. New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 2003, 64, 367–383. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Dixon, R.A. Proanthocyanidin biosynthesis—still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [Green Version]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Bernays, E.; Driver, G.C.; Bilgener, M. Herbivores and Plant Tannins. In Advances in Ecological Research; Academic Press: New york, NY, USA, 1989; Volume 19, pp. 263–302. [Google Scholar]
- Somavat, P.; Kumar, D.; Singh, V. Techno-economic feasibility analysis of blue and purple corn processing for anthocyanin extraction and ethanol production using modified dry grind process. Ind. Crop. Prod. 2018, 115, 78–87. [Google Scholar] [CrossRef]
- Lawrence, W.J.; Price, J.R.; Robinson, G.M.; Robinson, R. The distribution of anthocyanins in flowers, fruits and leaves. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1939, 230, 149–178. [Google Scholar]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-B.; Hashimoto, F.; Shimizu, K.; Sakata, Y. Anthocyanins from red flowers of Camellia cultivar ‘Dalicha.’. Phytochemistry 2008, 69, 3166–3171. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Singh, V.; de Mejia, E.G.; Somavat, P. Effect of sulfur dioxide and lactic acid in steeping water on the extraction of anthocyanins and bioactives from purple corn pericarp. Cereal Chem. 2019, 96, 575–589. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- FAO. Traditional High Andean Cuisine: Allin Mikuy/Sumak Mikuy, 1st ed.; Food and Agriculture Organization of the United Nations: Santiago de Chile, Chile, 2013. [Google Scholar]
- Fernandes, I.; Faria, A.; Calhau, C.; Freitas, V.D.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Johnson, M.H.; Wallig, M.; Vital, D.A.L.; de Mejia, E.G. Alcohol-free fermented blueberry–blackberry beverage phenolic extract attenuates diet-induced obesity and blood glucose in C57BL/6J mice. J. Nutr. Biochem. 2016, 31, 45–59. [Google Scholar] [CrossRef]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory Effects of Wild Blueberry Anthocyanins and Other Flavonoids on Biomarkers of Acute and Chronic Inflammation in Vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef]
- Lee, D.W.; Brammeier, S.; Smith, A.P. The Selective Advantages of Anthocyanins in Developing Leaves of Mango and Cacao. Biotropica 1987, 19, 40. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L. The Ecophysiology of Foliar Anthocyanin. Bot. Rev. 2003, 69, 149–161. [Google Scholar] [CrossRef]
- Freeman. An Overview of Plant Defenses against Pathogens and Herbivores. Plant Health Instr. 2008. [Google Scholar]
- Williams, L.A.D.; Mansingh, A. Pesticidal potentials of tropical plants—I. Insecticidal activity in leaf extracts of sixty plants. Int. J. Trop. Insect Sci. 1993, 14, 697–700. [Google Scholar] [CrossRef]
- Zounos, A.K.; Allan, E.J.; Mordueluntz, A.J. Bioactive compounds from neem tissue cultures and screening against insects. Pestic. Sci. 1999, 55, 497–500. [Google Scholar] [CrossRef]
- Sampson, B.J.; Tabanca, N.; Kirimer, N.; Demirci, B.; Baser, K.H.C.; Khan, I.A.; Spiers, J.M.; Wedge, D.E. Insecticidal activity of 23 essential oils and their major compounds against adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera). Pest Manag. Sci. 2005, 61, 1122–1128. [Google Scholar] [CrossRef]
- Moreira, M.D.; Picanço, M.C.; Barbosa, L.C.D.A.; Guedes, R.N.C.; Campos, M.R.D.; Silva, G.A.; Martins, J.C. Plant compounds insecticide activity against Coleoptera pests of stored products. Pesqui. Agropecuária Bras. 2007, 42, 909–915. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C. Azadirachta indica (neem): A plant of multiple biological and pharmacological activities. Phytochem. Rev. 2009, 8, 601–620. [Google Scholar] [CrossRef]
- Aljaghthmi, O.; Heba, H.; Zeid, I.A. Bioactive Compounds Extracted from Mangrove Plants (Avicennia marina and Rhizophora mucronata): An Overview. Pathophysiology 2018. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Kariyat, R.R.; Balogh, C.M.; Moraski, R.P.; Moraes, C.M.D.; Mescher, M.C.; Stephenson, A.G. Constitutive and herbivore-induced structural defenses are compromised by inbreeding in Solanum carolinense (Solanaceae). Am. J. Bot. 2013, 100, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Portman, S.L.; Kariyat, R.R.; Johnston, M.A.; Stephenson, A.G.; Marden, J.H. Cascading effects of host plant inbreeding on the larval growth, muscle molecular composition, and flight capacity of an adult herbivorous insect. Funct. Ecol. 2014, 29, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Kariyat, R.R.; Hardison, S.B.; Moraes, C.M.D.; Mescher, M.C. Plant spines deter herbivory by restricting caterpillar movement. Biol. Lett. 2017, 13, 20170176. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Somavat, P.; Singh, V.; Chatham, L.; de Mejia, E.G. A comparative study of anthocyanin distribution in purple and blue corn coproducts from three conventional fractionation processes. Food Chem. 2017, 231, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Somavat, P. Evaluation and modification of processing tech-niques for recovery of anthocyanins from colored corn. Agricultural and Biological Engineering. Ph.D. Thesis, University of Illinois, Urbana-Champaign, IL, USA, 2017. [Google Scholar]
- Patent Number: WO2017015539A1, Patentscope.wipo.int. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017015539 (accessed on 26 January 2020).
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [PubMed]
- Rausch, K.D.; Pruiett, L.E.; Wang, P.; Xu, L.; Belyea, R.L.; Tumbleson, M.E. Laboratory Measurement of Yield and Composition of Dry-Milled Corn Fractions Using a Shortened, Single-Stage Tempering Procedure. Cereal Chem. J. 2009, 86, 434–438. [Google Scholar] [CrossRef]
- Heck, C.I.; Schmalko, M.; de Mejia, E.G. Effect of Growing and Drying Conditions on the Phenolic Composition of Mate Teas (Ilex paraguariensis). J. Agric. Food Chem. 2008, 56, 8394–8403. [Google Scholar] [CrossRef]
- Burns, R.E. Method for Estimation of Tannin in Grain Sorghum1. Agron. J. 1907, 63, 511–512. [Google Scholar] [CrossRef]
- Mira, A.; Bernays, E.A. Trade-offs in host use by Manduca sexta: Plant characters vs natural enemies. Oikos 2002, 97, 387–397. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Weisbrod, A.V. Genetic variation in response of the gypsy moth to aspen phenolic glycosides. Biochem. Syst. Ecol. 1991, 19, 97–103. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. Ecology and Behavior of First Instar Larval Lepidoptera. Annu. Rev. Entomol. 2002, 47, 361–393. [Google Scholar] [CrossRef] [PubMed]
- Nitao, J.K.; Johnson, K.S.; Scriber, J.M.; Nair, M.G. Magnolia virginiana Neolignan compounds as chemical barriers to swallowtail butterfly host use. J. Chem. Ecol. 1992, 18, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Kaur, A.; Saraf, I.; Singh, I.P.; Kaur, S. Effect of crude extracts and purified compounds of Alpinia galanga on nutritional physiology of a polyphagous lepidopteran pest. Spodoptera Litura Fabr. Ecotoxicol. Environ. Saf. 2019, 168, 324–329. [Google Scholar] [CrossRef]
- Koul, O.; Shankar, J.S.; Mehta, N.; Taneja, S.C.; Tripathi, A.K.; Dhar, K.L. Bioefficacy of crude extracts of Aglaiaspecies (Meliaceae) and some active fractions against lepidopteran larvae. J. Appl. Entomol. 1997, 121, 245–248. [Google Scholar] [CrossRef]
- Bullangpoti, V.; Wajnberg, E.; Audant, P.; Feyereisen, R. Antifeedant activity of Jatropha gossypifolia and Melia azedarach senescent leaf extracts on Spodoptera frugiperda (Lepidoptera: Noctuidae) and their potential use as synergists. Pest Manag. Sci. 2012, 68, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kumar, R.; Upendrabhai, D.P.; Singh, I.P.; Kaur, S. Impact of sesquiterpenes fromInula racemose (Asteraceae) on growth, development and nutrition of Spodoptera litura (Lepidoptera: Noctuidae). Pest Manag. Sci. 2016, 73, 1031–1038. [Google Scholar] [CrossRef]
- Dastranj, M.; Borzoui, E.; Bandani, A.R.; Franco, O.L. Inhibitory effects of an extract from non-host plants on physiological characteristics of two major cabbage pests. Bull. Entomol. Res. 2017, 108, 370–379. [Google Scholar] [CrossRef]
- Diamond, S.E.; Kingsolver, J.G. Host plant quality, selection history and trade-offs shape the immune responses of Manduca sexta. Proc. R. Soc. B Biol. Sci. 2010, 278, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Brattsten, L.B. Enzymic adaptations in leaf-feeding insects to host-plant allelochemicals. J. Chem. Ecol. 1988, 14, 1919–1939. [Google Scholar] [CrossRef]
- Snyder, M.; Glendinning, J. Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. J. Comp. Physiol. A 1996, 179. [Google Scholar] [CrossRef]
- Slansky, F.; Feeny, P. Stabilization of the Rate of Nitrogen Accumulation by Larvae of the Cabbage Butterfly on Wild and Cultivated Food Plants. Ecol. Monogr. 1977, 47, 209–228. [Google Scholar] [CrossRef]
- Slansky, F.; Wheeler, G.S. Compensatory increases in food consumption and utilization efficiencies by velvet bean caterpillars mitigate impact of diluted diets on growth. Entomol. Exp. Et Appl. 1989, 51, 175–187. [Google Scholar] [CrossRef]
- Woods, H.A. Patterns and Mechanisms of Growth of Fifth Instar Manduca sexta Caterpillars Following Exposure to Low- or High-Protein Food during Early Instars. Physiol. Biochem. Zool. 1999, 72, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Dam, N.M.V.; Hermenau, U.; Baldwin, I.T. Instar-specific sensitivity of specialist Manduca sexta larvae to induced defences in their host plant Nicotiana attenuata. Ecol. Entomol. 2001, 26, 578–586. [Google Scholar]
- Torres, P.; Avila, J.G.; Vivar, A.R.D.; García Ana, M.; Marín Juan, C.; Aranda, E.; Céspedes, C.L. Antioxidant and insect growth regulatory activities of stilbenes and extracts from Yucca periculosa. Phytochemistry 2003, 64, 463–473. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Molina, S.C.; Muñoz, E.; Lamilla, C.; Alarcon, J.; Palacios, S.M.; Carpinella, M.C.; Avila, J.G. The insecticidal, molting disruption and insect growth inhibitory activity of extracts from Condalia microphylla Cav. (Rhamnaceae). Ind. Crop. Prod. 2013, 42, 78–86. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I. Flavonols from Saffron Flower: Tyrosinase Inhibitory Activity and Inhibition Mechanism†. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef]
- Mcnamara, J.M.; Houston, A.I. State-dependent life histories. Nature 1996, 380, 215–221. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Hirai, N.; Nouhuys, S.V. Direct and trans-generational responses to food deprivation during development in the Glanville fritillary butterfly. Oecologia 2012, 171, 93–104. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple Corn (Zea mays L.) Phenolic Compounds Profile and Its Assessment as an Agent Against Oxidative Stress in Isolated Mouse Organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Orlando, P.; Browen, J.; Whelan, C. Co-adaptations of feeding behaviors and gut modulation as a mechanism of co-existence. Evol. Ecol. Res. 2009, 11, 541–560. [Google Scholar]
- Couture, J.J.; Mason, C.J.; Habeck, C.W.; Lindroth, R.L. Behavioral and morphological responses of an insect herbivore to low nutrient quality are inhibited by plant chemical defenses. Arthropod Plant Interact. 2016, 10, 341–349. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayal, M.; Somavat, P.; Rodriguez, I.; Thomas, T.; Christoffersen, B.; Kariyat, R. Polyphenol-Rich Purple Corn Pericarp Extract Adversely Impacts Herbivore Growth and Development. Insects 2020, 11, 98. https://doi.org/10.3390/insects11020098
Tayal M, Somavat P, Rodriguez I, Thomas T, Christoffersen B, Kariyat R. Polyphenol-Rich Purple Corn Pericarp Extract Adversely Impacts Herbivore Growth and Development. Insects. 2020; 11(2):98. https://doi.org/10.3390/insects11020098
Chicago/Turabian StyleTayal, Mandeep, Pavel Somavat, Isabella Rodriguez, Tina Thomas, Bradley Christoffersen, and Rupesh Kariyat. 2020. "Polyphenol-Rich Purple Corn Pericarp Extract Adversely Impacts Herbivore Growth and Development" Insects 11, no. 2: 98. https://doi.org/10.3390/insects11020098




