Two Roles for the Tenebrio molitor Relish in the Regulation of Antimicrobial Peptides and Autophagy-Related Genes in Response to Listeria monocytogenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. T. molitor Rearing
2.2. Sample Collection after Immune Challenge with L. monocytogenes
2.3. RNA Extraction, cDNA Synthesis and qRT-PCR
2.4. Double-Stranded RNA TmRelish Synthesis
2.5. Mortality Assay of TmRelish-Knockdown T. molitor Larvae Upon L. monocytogenes Infection
2.6. Effect of TmRelish Gene Silencing on AMP Expression After Bacterial Insult
2.7. TmRelish RNAi and Regulation of Autophagy-related Genes in Response to L. monocytogenes
2.8. Statistical Analysis
3. Results
3.1. Expression and Induction of TmRelish Transcripts Upon L. monocytogenes Challenge
3.2. Effect of TmRelish Gene Knockdown on T. molitor Larval Mortality after L. monocytogenes Infection
3.3. Effect of TmRelish RNAi on AMP Genes Expression in Response to L. monocytogenes
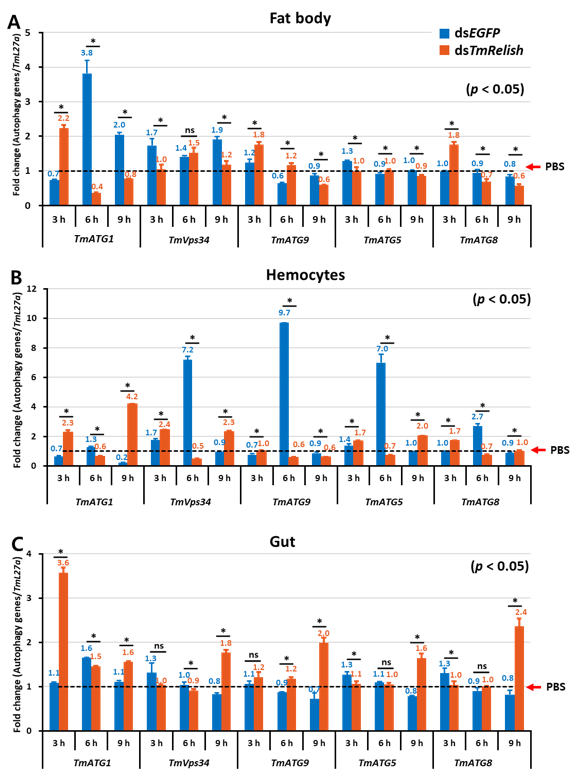
3.4. TmRelish Gene Contribution to the Regulation of Autophagy-Related Genes in Response to L. monocytogenes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.; Ren, M.; Liu, X.; Xia, H.; Chen, K. Peptidoglycan recognition proteins in insect immunity. Mol. Immunol. 2019, 106, 69–76. [Google Scholar] [CrossRef]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune defenses of a beneficial pest: The mealworm beetle, Tenebrio molitor. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Ferrandon, D.; Imler, J.-L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Yano, T.; Aggarwal, K.; Lim, J.-H.; Ueda, K.; Oshima, Y.; Peach, C.; Erturk-Hasdemir, D.; Goldman, W.E.; Oh, B.-H. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 2006, 7, 715. [Google Scholar] [CrossRef]
- Yano, T.; Mita, S.; Ohmori, H.; Oshima, Y.; Fujimoto, Y.; Ueda, R.; Takada, H.; Goldman, W.E.; Fukase, K.; Silverman, N.; et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat. Immunol. 2008, 9, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takehana, A.; Katsuyama, T.; Yano, T.; Oshima, Y.; Takada, H.; Aigaki, T.; Kurata, S. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc. Natl. Acad. Sci. USA 2002, 99, 13705–13710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaechter, M. Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Osanai, A.; Sashinami, H.; Asano, K.; Li, S.-J.; Hu, D.-L.; Nakane, A. Mouse peptidoglycan recognition protein PGLYRP-1 plays a role in the host innate immune response against Listeria monocytogenes infection. Infect. Immun. 2011, 79, 858–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tindwa, H.; Patnaik, B.; Kim, D.; Mun, S.; Jo, Y.; Lee, B.; Lee, Y.; Kim, N.; Han, Y. Cloning, characterization and effect of TmPGRP-LE gene silencing on survival of Tenebrio molitor against Listeria monocytogenes infection. Int. J. Mol. Sci. 2013, 14, 22462–22482. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Weiss, L.M.; Orlofsky, A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J. Biol. Chem. 2009, 284, 1694–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Lee, Y.S.; Kang, S.S.; Han, Y.S. Molecular cloning and characterization of autophagy-related gene TmATG8 in Listeria-invaded hemocytes of Tenebrio molitor. Dev. Comp. Immunol. 2015, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Noh, M.Y.; Kim, D.H.; Kim, I.; Han, Y.S.; Lee, Y.S.; Lee, B.L.; Kim, N.J. Depletion of autophagy-related genes Atg3 and Atg5 in Tenebrio molitor leads to decreased survivability against an intracellular pathogen, Listeria monocytogenes. Arch. Insect Biochem. Physiol. 2015, 88, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, Y.H.; Patnaik, B.B.; Park, K.B.; Tindwa, H.; Seo, G.W.; Chandrasekar, R.; Lee, Y.S.; Han, Y.S. Cloning, expression analysis, and RNA interference study of a HORMA domain containing autophagy-related gene 13 (ATG13) from the coleopteran beetle, Tenebrio molitor. Front. Physiol. 2015, 6, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.; Hiroyasu, A.; Guzman, R.M.; Roberts, S.A.; Goodman, A.G. Analysis of Drosophila STING reveals an evolutionarily conserved antimicrobial function. Cell Rep. 2018, 23, 3537–3550. [Google Scholar] [CrossRef]
- Petersen, A.J.; Katzenberger, R.J.; Wassarman, D.A. The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics 2013, 194, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Nandy, A.; Lin, L.; Velentzas, P.D.; Wu, L.P.; Baehrecke, E.H.; Silverman, N. The NF-κB Factor Relish Regulates Atg1 Expression and Controls Autophagy. Cell Rep. 2018, 25, 2110–2120. [Google Scholar] [CrossRef] [Green Version]
- Kurata, S. Peptidoglycan recognition proteins in Drosophila immunity. Dev. Comp. Immunol. 2014, 42, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Kurata, S. Extracellular and intracellular pathogen recognition by Drosophila PGRP-LE and PGRP-LC. Int. Immunol. 2010, 22, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Balfour, C.E.; Carmichael, L. The light reactions of the meal worm (Tenebrio molitor Linn). Am. J. Psychol. 1928, 40, 576–584. [Google Scholar] [CrossRef]
- Park, J.B.; Choi, W.H.; Kim, S.H.; Jin, H.J.; Han, Y.S.; Kim, N.J. Developmental characteristics of Tenebrio molitor larvae (Coleoptera: Tenebrionidae) in different instars. Int. J. Ind. Entomol. 2014, 28, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, H.; Buxdorf, K.; Shapira, I.; Ein-Gedi, S.; Zvi, M.M.-B.; Fridman, E.; Moshelion, M.; Levy, M. LogSpin: A simple, economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC Res. Notes 2012, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chung, T.J.; Park, C.W.; Hahn, Y.; Chung, J.H.; Lee, B.L.; Han, D.M.; Jung, Y.H.; Kim, S.; Lee, Y. Structure and Expression of the Tenecin 3 Gene inTenebrio molitor. Biochem. Biophys. Res. Commun. 1996, 218, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.-B.; Kim, C.-H.; Lee, H.; Kwon, H.-M.; Park, J.-W.; Ryu, J.-H.; Kurokawa, K.; Ha, N.-C.; Lee, W.-J.; Lemaitre, B. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J. Biol. Chem. 2009, 284, 19474–19481. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; HONG, S.Y.; OH, J.E.; YOON, J.H.; LEE, B.L.; MOON, H.M. Identification and characterization of the antimicrobial peptide corresponding to C-terminal β-sheet domain of tenecin 1, an antibacterial protein of larvae of Tenebrio molitor. Biochem. J. 1998, 334, 99–105. [Google Scholar] [CrossRef]
- Chae, J.-H.; Kurokawa, K.; So, Y.-I.; Hwang, H.O.; Kim, M.-S.; Park, J.-W.; Jo, Y.-H.; Lee, Y.S.; Lee, B.L. Purification and characterization of tenecin 4, a new anti-Gram-negative bacterial peptide, from the beetle Tenebrio molitor. Dev. Comp. Immunol. 2012, 36, 540–546. [Google Scholar] [CrossRef]
- Jo, Y.H.; Park, S.; Park, K.B.; Noh, M.Y.; Cho, J.H.; Ko, H.J.; Kim, C.E.; Patnaik, B.B.; Kim, J.; Won, R. In silico identification, characterization and expression analysis of attacin gene family in response to bacterial and fungal pathogens in Tenebrio molitor. Entomol. Res. 2018, 48, 45–54. [Google Scholar] [CrossRef]
- Kim, D.H.; Noh, M.Y.; Park, K.B.; Jo, Y.H. Expression profiles of two thaumatin-like protein (TmTLP) genes in responses to various micro-organisms from Tenebrio molitor. Entomol. Res. 2017, 47, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Noh, M.Y.; Jo, Y.H. Identification and sequence analysis of two thaumatin-like protein (TmTLP) genes from Tenebrio molitor. Entomol. Res. 2016, 46, 354–359. [Google Scholar] [CrossRef]
- Keshavarz, M.; Jo, Y.H.; Patnaik, B.B.; Park, K.B.; Ko, H.J.; Kim, C.E.; Edosa, T.T.; Lee, Y.S.; Han, Y.S. Tm Relish is required for regulating the antimicrobial responses to Escherichia coli and Staphylococcus aureus in Tenebrio molitor. Sci. Reports 2020, 10, 1–18. [Google Scholar]
- Vilcinskas, A. Evolutionary plasticity of insect immunity. J. Insect. Physiol. 2013, 59, 123–129. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.C.; Morey, A.T.; Castanheira, G.M.; Bocate, K.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Costa, I.N.; Mora-Montes, H.M. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, E.-K.; Yuk, J.-M.; Shin, D.-M.; Sasakawa, C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front. Immunol. 2013, 4, 97. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, X.; Huang, J.; Chen, M.; An, J. Structural Insights into the Preferential Binding of PGRP-SAs from Bumblebees and Honeybees to Dap-Type Peptidoglycans Rather than Lys-Type Peptidoglycans. J. Immunol. 2019, 202, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Li, F.-H.; Dong, B.; Wang, B.; Luan, W.; Zhang, X.-J.; Zhang, L.-S.; Xiang, J.-H. Molecular cloning, characterization and expression analysis of a putative C-type lectin (Fclectin) gene in Chinese shrimp Fenneropenaeus chinensis. Mol. Immunol. 2007, 44, 598–607. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Zhang, H.; Zheng, P.; Zhao, J.; Qiu, L.; Zhang, Y.; Song, L. Molecular cloning and expression of a Relish gene in Chinese mitten crab Eriocheir sinensis. Int. J. Immunogenet. 2010, 37, 499–508. [Google Scholar] [CrossRef]
- Tzou, P.; Ohresser, S.; Ferrandon, D.; Capovilla, M.; Reichhart, J.-M.; Lemaitre, B.; Hoffmann, J.A.; Imler, J.-L. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 2000, 13, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Altincicek, B.; Hain, T.; Domann, E.; Vilcinskas, A.; Chakraborty, T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl. Env. Microbiol. 2010, 76, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, B.E.; Dionne, M.S.; Schneider, D.S.; Freitag, N.E. Exploration of host–pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell. Microbiol. 2003, 5, 901–911. [Google Scholar] [CrossRef]
- Meister, S.; Kanzok, S.M.; Zheng, X.-l.; Luna, C.; Li, T.-R.; Hoa, N.T.; Clayton, J.R.; White, K.P.; Kafatos, F.C.; Christophides, G.K. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2005, 102, 11420–11425. [Google Scholar] [CrossRef] [Green Version]
- Antonova, Y.; Alvarez, K.S.; Kim, Y.J.; Kokoza, V.; Raikhel, A.S. The role of NF-κB factor REL2 in the Aedes aegypti immune response. Insect. Biochem. Mol. Biol. 2009, 39, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Sagisaka, A.; Nakajima, Y.; Fujita, K.; Imanishi, S.; Yamakawa, M. Correlation of differential expression of silkworm antimicrobial peptide genes with different amounts of rel family proteins and their gene transcriptional activity. Biosci. Biotechnol. Biochem. 2009, 73, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Rao, X.-J.; Yi, H.-Y.; Lin, X.-Y.; Huang, X.-H.; Yu, X.-Q. Co-expression of Dorsal and Rel2 negatively regulates antimicrobial peptide expression in the tobacco hornworm Manduca sexta. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiemstra, P.S.; van den Barselaar, M.T.; Roest, M.; Nibbering, P.H.; van Furth, R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 1999, 66, 423–428. [Google Scholar] [CrossRef] [PubMed]
- López-Solanilla, E.; González-Zorn, B.; Novella, S.; Vázquez-Boland, J.A.; Rodríguez-Palenzuela, P. Susceptibility of Listeria monocytogenes to antimicrobial peptides. Fems Microbiol. Lett. 2003, 226, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Mraheil, M.A.; Silva, S.; Müller, D.; Cemic, F.; Hemberger, J.; Hain, T.; Vilcinskas, A.; Chakraborty, T. Anti-Listeria activities of Galleria mellonella hemolymph proteins. Appl. Env. Microbiol. 2011, 77, 4237–4240. [Google Scholar] [CrossRef] [Green Version]
- Chinchore, Y.; Gerber, G.F.; Dolph, P.J. Alternative pathway of cell death in Drosophila mediated by NF-κB transcription factor Relish. Proc. Natl. Acad. Sci. USA 2012, 109, E605–E612. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.; Amoyel, M.; Bergantinos, C.; De La Cova, C.; Schertel, C.; Basler, K.; Johnston, L. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 2014, 346, 1258236. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Chtarbanova, S.; Petersen, A.J.; Ganetzky, B. Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proc. Natl. Acad. Sci. USA 2013, 110, E1752–E1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.C.; Juhász, G.; Neufeld, T.P. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr. Biol. 2007, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.C.; Schuldiner, O.; Neufeld, T.P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 2004, 7, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Ma, L.; Guo, E.; Deng, X.; Ma, S.; Xia, Q.; Cao, Y.; Li, S. 20-hydroxyecdysone upregulates Atg genes to induce autophagy in the Bombyx fat body. Autophagy 2013, 9, 1172–1187. [Google Scholar] [CrossRef] [Green Version]
- Rich, K.A.; Burkett, C.; Webster, P. Cytoplasmic bacteria can be targets for autophagy. Cell. Microbiol. 2003, 5, 455–468. [Google Scholar] [CrossRef]
- Py, B.F.; Lipinski, M.M.; Yuan, J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy 2007, 3, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Dortet, L.; Mostowy, S.; Cossart, P. Listeria and autophagy escape: Involvement of InlK, an internalin-like protein. Autophagy 2012, 8, 132–134. [Google Scholar] [CrossRef] [Green Version]




| Primer | Sequence |
|---|---|
| TmRelish_qPCR_Fw | 5′-AGCGTCAAGTTGGAGCAGAT-3′ |
| TmRelish_qPCR_Rv | 5′-GTCCGGACCTCATCAAGTGT-3′ |
| TmRelish_Temp_Fw | 5′-TGTGGGAAGATTACGGGAAA-3′ |
| TmRelish_Temp_Rv | 5′-CAAATTGGCCACGATCTCTT-3′ |
| dsTmRelish_Fw | 5′-TAATACGACTCACTATAGGGTGACGTGCACCATCAATA-3′ |
| dsTmRelish_Rv | 5′-TAATACGACTCACTATAGGGTGCGTGTTTGGCCTTGAT-3′ |
| dsEGFP_Fw | 5′-TAATACGACTCACTATAGGGTACGTAAACGGCCACAAGTTC-3′ |
| dsEGFP_Rv | 5′-TAATACGACTCACTATAGGGTTGCTCAGGTAGTGTTGTCG-3′ |
| TmTenecin-1_Fw | 5′-CAGCTGAAGAAATCGAACAAGG-3′ |
| TmTenecin-1_Rv | 5′-CAGACCCTCTTTCCGTTACAGT-3’ |
| TmTenecin-2_Fw | 5′-CAGCAAAACGGAGGATGGTC-3′ |
| TmTenecin-2_Rv | 5′-CGTTGAAATCGTGATCTTGTCC-3′ |
| TmTenecin-3_Fw | 5′-GATTTGCTTGATTCTGGTGGTC-3’ |
| TmTenecin-3_Rv | 5′-CTGATGGCCTCCTAAATGTCC-3′ |
| TmTenecin-4_Fw | 5′-GGACATTGAAGATCCAGGAAAG-3′ |
| TmTenecin-4_Rv | 5′-CGGTGTTCCTTATGTAGAGCTG-3′ |
| TmDefensin-1_Fw | 5′-AAATCGAACAAGGCCAACAC-3′ |
| TmDefencin-1_Rv | 5′-GCAAATGCAGACCCTCTTTC-3′ |
| TmDefensin-2_Fw | 5′-GGGATGCCTCATGAAGATGTAG-3′ |
| TmDefensin-2_Rv | 5′-CCAATGCAAACACATTCGTC-3′ |
| TmColeoptericin-1_Fw | 5′-GGACAGAATGGTGGATGGTC-3′ |
| TmColeoptericin-1_Rv | 5′-CTCCAACATTCCAGGTAGGC-3’ |
| TmColeoptericin-2_Fw | 5′-GGACGGTTCTGATCTTCTTGAT-3′ |
| TmColeoptericin-2_Rv | 5′-CAGCTGTTTGTTTGTTCTCGTC-3′ |
| TmAttacin-1a_Fw | 5′-GAAACGAAATGGAAGGTGGA-3′ |
| TmAttacin-1a_Rv | 5′-TGCTTCGGCAGACAATACAG-3′ |
| TmAttacin-1b_Fw | 5′-GAGCTGTGAATGCAGGACAA-3′ |
| TmAttacin-1b_Rv | 5′-CCCTCTGATGAAACCTCCAA-3′ |
| TmAttacin-2_Fw | 5′-AACTGGGATATTCGCACGTC-3′ |
| TmAttacin-2_Rv | 5′-CCCTCCGAAATGTCTGTTGT-3’ |
| TmCecropin-2_Fw | 5′-TACTAGCAGCGCCAAAACCT-3′ |
| TmCecropin-2_Rv | 5′-CTGGAACATTAGGCGGAGAA-3′ |
| TmThaumatin-like protein-1_Fw | 5′-CTCAAAGGACACGCAGGACT-3′ |
| TmThaumatin-like protein-1_Rv | 5′-ACTTTGAGCTTCTCGGGACA-3′ |
| TmThaumatin-like protein-2_Fw | 5′-CCGTCTGGCTAGGAGTTCTG-3′ |
| TmThaumatin-like protein-2_Rv | 5′-ACTCCTCCAGCTCCGTTACA-3′ |
| TmL27a_qPCR_Fw | 5′-TCATCCTGAAGGCAAAGCTCCAGT-3′ |
| TmL27a_qPCR_Rv | 5′-AGGTTGGTTAGGCAGGCACCTTTA-3′ |
| Autophagosome Protein Complex | Autophagy-Related Genes | Sequence |
|---|---|---|
| Initiation | TmATG1-qPCR-FwTmATG1-qPCR-Rv | 5′-TTGGCCGATTATCTCAACGC-3′5′-TTCATGGCGCCAGCTAATTG-3′ |
| Nucleation | TmVps34-qPCR-FwTmVps34-qPCR-Rv | 5′-AGCACCAAGGAGTTCCAGGAA-3′5′-ATGTTGCCGTTGTGTCTGTC-3′ |
| Recycling | TmATG9-qPCR-FwTmATG9-qPCR-Rv | 5′-AGTGCGAAAACGGCAAACTG-3′5′-ATGCTGCTCTGATTCTGCAC-3′ |
| Elongation | TmATG5-qPCR-FwTmATG5-qPCR-Rv | 5′-GGGCTGTGAATCGAAAGTTG-3′5′-GTTTTGCGGTGTCCATCTTC-3′ |
| Completion and extension | TmATG8-qPCR-FwTmATG8-qPCR-Rv | 5′-AAGATCCGCCGAAAGTATCC-3′5′-AACTGGCCGACTGTCAAATC-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshavarz, M.; Jo, Y.H.; Edosa, T.T.; Han, Y.S. Two Roles for the Tenebrio molitor Relish in the Regulation of Antimicrobial Peptides and Autophagy-Related Genes in Response to Listeria monocytogenes. Insects 2020, 11, 188. https://doi.org/10.3390/insects11030188
Keshavarz M, Jo YH, Edosa TT, Han YS. Two Roles for the Tenebrio molitor Relish in the Regulation of Antimicrobial Peptides and Autophagy-Related Genes in Response to Listeria monocytogenes. Insects. 2020; 11(3):188. https://doi.org/10.3390/insects11030188
Chicago/Turabian StyleKeshavarz, Maryam, Yong Hun Jo, Tariku Tesfaye Edosa, and Yeon Soo Han. 2020. "Two Roles for the Tenebrio molitor Relish in the Regulation of Antimicrobial Peptides and Autophagy-Related Genes in Response to Listeria monocytogenes" Insects 11, no. 3: 188. https://doi.org/10.3390/insects11030188
APA StyleKeshavarz, M., Jo, Y. H., Edosa, T. T., & Han, Y. S. (2020). Two Roles for the Tenebrio molitor Relish in the Regulation of Antimicrobial Peptides and Autophagy-Related Genes in Response to Listeria monocytogenes. Insects, 11(3), 188. https://doi.org/10.3390/insects11030188





