Flight Muscle and Wing Mechanical Properties are Involved in Flightlessness of the Domestic Silkmoth, Bombyx mori
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Rearing
2.2. Measuring the Flight Apparatus
2.2.1. Body Mass, Flight Muscle Mass, and Flight Muscle Ratio
2.2.2. Wing Shape, Wing Area, Wing Loading and Wing Mechanical Properties
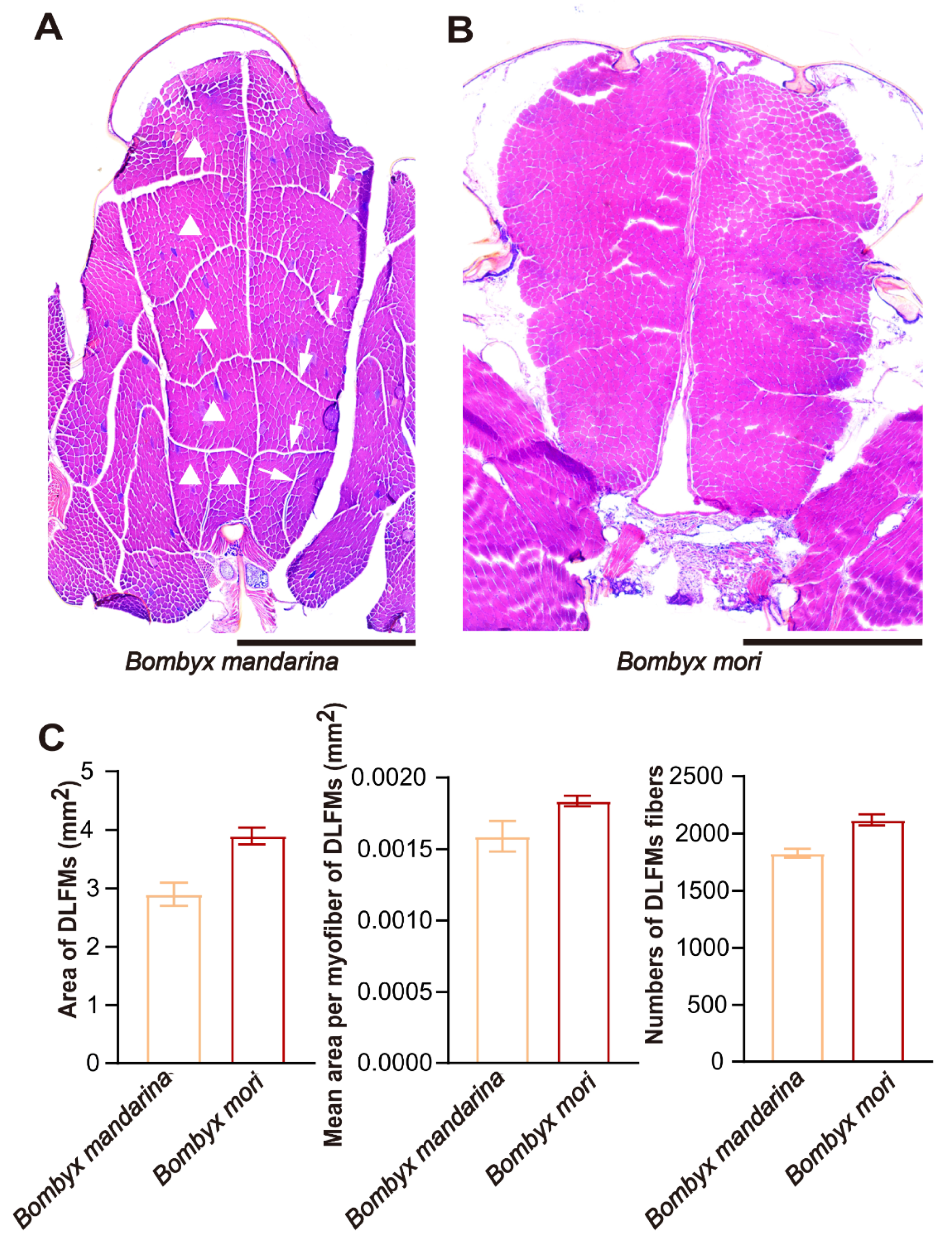
2.3. Microscopy of Flight Muscle
2.4. Statistical Analysis
3. Results
3.1. Population Divergence of Morphological Traits
3.2. Body Mass, Wing Loading, and Wing Mechanical Properties
3.3. Flight Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Mitterboeck, T.F.; Liu, S.; Adamowicz, S.J.; Fu, J.; Zhang, R.; Song, W.; Meusemann, K.; Zhou, X. Positive and relaxed selection associated with flight evolution and loss in insect transcriptomes. GigaScience 2017, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, M.F.; Bradler, S.; Maxwell, T. Loss and recovery of wings in stick insects. Nature 2003, 421, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Wollaston, T.V. Insecta Maderensia: Being an Account of the Insects of the Islands of the Madeiran Group; John Van Voorst: London, UK, 1854; p. 634. [Google Scholar]
- Wagner, D.L.; Liebherr, J.K. Flightlessness in insects. Trends Ecol. Evol. 1992, 7, 216–220. [Google Scholar] [CrossRef]
- Roff, D.A. The evolution of flightlessness: Is history important? Ecol. Evol. 1994, 8, 639–657. [Google Scholar] [CrossRef]
- Altizer, S.; Davis, A.K. Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evol. Int. J. Org. Evol. 2010, 64, 1018–1028. [Google Scholar] [CrossRef]
- Arbas, E.A. Thoracic morphology of a flightless mexican grasshopper, Barytettix psolus: Comparison with the locust, Schistocerca gregaria. J. Morphol. 1983, 176, 141–153. [Google Scholar] [CrossRef]
- Liu, S.P.; Wipfler, B.; Niitsu, S.; Beutel, R.G. The thoracic anatomy of the male and female winter moth Nyssiodes lefuarius (lepidoptera: Geometridae) and evolutionary changes in the thorax of moths and butterflies. Org. Divers. Evol. 2017, 17, 565–594. [Google Scholar] [CrossRef]
- Marden, J.H. Maximum lift production during takeoff in flying animals. J. Exp. Biol. 1987, 130, 235–258. [Google Scholar]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–230. [Google Scholar] [CrossRef] [Green Version]
- Betts, C.R.; Wootton, R.J. Wing shape and flight behavior in butterflies (lepidoptera, papilionoidea and hesperioidea)—A preliminary-analysis. J. Exp. Biol. 1988, 138, 271–288. [Google Scholar]
- DeVries, P.J.; Penz, C.M.; Hill, R.I. Vertical distribution, flight behaviour and evolution of wing morphology in morpho butterflies. J. Anim. Ecol. 2010, 79, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.B.; Luo, H.X.; Song, J.L.; Lu, X.Y. Force production and asymmetric deformation of a flexible flapping wing in forward flight. J. Fluids Struct. 2013, 36, 149–161. [Google Scholar] [CrossRef]
- Nakata, T.; Liu, H. Aerodynamic performance of a hovering hawkmoth with flexible wings: A computational approach. Proc. R. Soc. B Biol. Sci. 2012, 279, 722–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wootton, R.J. Support and deformability in insect wings. J. Zool. 1981, 193, 447–468. [Google Scholar] [CrossRef]
- Wootton, R.J. Functional morphology of insect wings. Annu. Rev. Entomol. 1992, 37, 113–140. [Google Scholar] [CrossRef]
- Mountcastle, A.M.; Combes, S.A. Wing flexibility enhances load-lifting capacity in bumblebees. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130531. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Huang, Q.; Deng, X.; Sane, S.P. Aerodynamic effects of flexibility in flapping wings. J. R. Soc. Interface 2010, 7, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Dudley, R.; Srygley, R. Flight physiology of neotropical butterflies: Allometry of airspeeds during natural free flight. J. Exp. Biol. 1994, 191, 125–139. [Google Scholar]
- Wang, Y.; Hu, Y.; He, D.; Chen, S.; Li, S.; Lan, D.; Ren, P.; Lin, Z.; Liu, Y. Contribution of both positive selection and relaxation of selective constraints to degeneration of flyability during geese domestication. PLoS ONE 2017, 12, e0185328. [Google Scholar] [CrossRef] [Green Version]
- Provine, R.R.; Strawbridge, C.L.; Harrison, B.J. Comparative analysis of the development of wing-flapping and flight in the fowl. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 1984, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Guo, Y.; Zhang, Z.; Li, D.; Xuan, Z.; Li, Z.; Dai, F.; Li, Y.; Cheng, D.; Li, R.; et al. Complete resequencing of 40 genomes reveals domestication events and genes in silkworm (Bombyx). Science 2009, 326, 433–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underhill, A.P. Current issues in chinese neolithic archaeology. J. World Prehistory 1997, 11, 103–160. [Google Scholar] [CrossRef]
- Yu, H.S.; Shen, Y.H.; Yuan, G.X.; Hu, Y.G.; Xu, H.E.; Xiang, Z.H.; Zhang, Z. Evidence of selection at melanin synthesis pathway loci during silkworm domestication. Mol. Biol. Evol. 2011, 28, 1785–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Tong, X.; Zuo, W.; Luan, Y.; Gao, R.; Han, M.; Xiong, G.; Gai, T.; Hu, H.; Dai, F.; et al. Qtl analysis of cocoon shell weight identifies bmrpl18 associated with silk protein synthesis in silkworm by pooling sequencing. Sci. Rep. 2017, 7, 17985. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.K.; Thomas, C.D.; Blakeley, D.S. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia 1999, 121, 165–170. [Google Scholar] [CrossRef]
- Jantzen, B.; Eisner, T. Hindwings are unnecessary for flight but essential for execution of normal evasive flight in lepidoptera. Proc. Natl. Acad. Sci. USA 2008, 105, 16636–16640. [Google Scholar] [CrossRef] [Green Version]
- Pal, S. Mechanical properties of biological materials. In Design of Artificial Human Joints & Organs; Springer: New York, NY, US, 2014; pp. 23–40. [Google Scholar]
- Lomakin, J.; Huber, P.A.; Eichler, C.; Arakane, Y.; Kramer, K.J.; Beeman, R.W.; Kanost, M.R.; Gehrke, S.H. Mechanical properties of the beetle elytron, a biological composite material. Biomacromolecules 2011, 12, 321–335. [Google Scholar] [CrossRef]
- Qiao, L.; Li, Y.; Xiong, G.; Liu, X.; He, S.; Tong, X.; Wu, S.; Hu, H.; Wang, R.; Hu, H.; et al. Effects of altered catecholamine metabolism on pigmentation and physical properties of sclerotized regions in the silkworm melanism mutant. PLoS ONE 2012, 7, e42968. [Google Scholar] [CrossRef]
- Mayhew, P.J. Why are there so many insect species? Perspectives from fossils and phylogenies. Biol. Rev. 2007, 82, 425–454. [Google Scholar] [CrossRef]
- Wang, W.; Kidd, B.J.; Carroll, S.B.; Yoder, J.H. Sexually dimorphic regulation of the wingless morphogen controls sex-specific segment number in Drosophila. Proc. Natl. Acad. Sci. USA 2011, 108, 11139–11144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.E.; Zwaan, B.J.; Brakefield, P.M. Evolution of sexual dimorphism in the lepidoptera. Annu. Rev. Entomol. 2011, 56, 445–464. [Google Scholar] [CrossRef]
- Oliver, J.C.; Monteiro, A. On the origins of sexual dimorphism in butterflies. Proc. R. Soc. B Biol. Sci. 2011, 278, 1981–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berwaerts, K.; Van Dyck, H.; Aerts, P. Does flight morphology relate to flight performance? An experimental test with the butterfly Pararge aegeria. Funct. Ecol. 2002, 16, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ning, J.G.; Ren, H.L.; Zhang, P.F.; Zhao, H.Y. The function of resilin in honeybee wings. J. Exp. Biol. 2015, 218, 2136–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Ren, H.; Rajabi, H.; Zhao, H.; Ning, J.; Gorb, S. Structure, properties and functions of the forewing-hindwing coupling of honeybees. J. Insect Physiol. 2019, 118, 103936. [Google Scholar] [CrossRef]
- Walker, S.M.; Thomas, A.L.; Taylor, G.K. Deformable wing kinematics in the desert locust: How and why do camber, twist and topography vary through the stroke? J. R. Soc. Interface 2009, 6, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Young, J.; Walker, S.M.; Bomphrey, R.J.; Taylor, G.K.; Thomas, A.L. Details of insect wing design and deformation enhance aerodynamic function and flight efficiency. Science 2009, 325, 1549–1552. [Google Scholar] [CrossRef]
- Rajabi, H.; Gorb, S.N. How do dragonfly wings work? A brief guide to functional roles of wing structural components. Int. J. Odonatol. 2020, 23, 23–30. [Google Scholar] [CrossRef]
- Snelling, E.P.; Seymour, R.S.; Matthews, P.G.D.; White, C.R. Maximum metabolic rate, relative lift, wingbeat frequency and stroke amplitude during tethered flight in the adult locust Locusta migratoria. J. Exp. Biol. 2012, 215, 3317–3323. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.F.; Lighton, J.R.B. Oxygen-sensitive flight metabolism in the dragonfly Erythemis simplicicollis. J. Exp. Biol. 1998, 201, 1739–1744. [Google Scholar] [PubMed]
- Casey, T.M.; May, M.L.; Morgan, K.R. Flight energetics of euglossine bees in relation to morphology and wing stroke frequency. J. Exp. Biol. 1985, 116, 271–289. [Google Scholar]
- Zappia, M.P.; Frolov, M.V. E2f function in muscle growth is necessary and sufficient for viability in Drosophila. Nat. Commun. 2016, 7, 10509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah, L.B.; Davison, C.; Fan, S.J.; Morris, J.F.; Goberdhan, D.C.; Wilson, C. Fine-tuning of pi3k/akt signalling by the tumour suppressor pten is required for maintenance of flight muscle function and mitochondrial integrity in ageing adult Drosophila melanogaster. PLoS ONE 2015, 10, e0143818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonbauer, C.; Distler, J.; Jahrling, N.; Radolf, M.; Dodt, H.U.; Frasch, M.; Schnorrer, F. Spalt mediates an evolutionarily conserved switch to fibrillar muscle fate in insects. Nature 2011, 479, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Deslauriers, A.R.; Holt, N.C.; Eaton, C.E. Resistance to radial expansion limits muscle strain and work. Biomech. Modeling Mechanobiol. 2017, 16, 1633–1643. [Google Scholar] [CrossRef] [Green Version]
- Maas, H.; Huijing, P.A. Myofascial force transmission in dynamic muscle conditions: Effects of dynamic shortening of a single head of multi-tendoned rat extensor digitorum longus muscle. Eur. J. Appl. Physiol. 2005, 94, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Street, S.F. Lateral transmission of tension in frog myofibers: A myofibrillar network and transverse cytoskeletal connections are possible transmitters. J. Cell. Physiol. 1983, 114, 346–364. [Google Scholar] [CrossRef]





| Population | Origin | Sex | N | FMR | Wing Loading (mg/mm2) | Forewing Area (mm2) | Body Mass (mg) | FMM (mg) | Aspect Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Wild Silkmoth | Wild-caught | M | 20 | 0.31 (±0.03) | 0.75 (±0.08) | 246.98 (±30.73) | 184.35 (±30.97) | 55.75 (±6.32) | 1.99 (±0.09) |
| F | 13 | 0.14 (±0.02) | 1.32 (±0.21) | 293.47 (±49.23) | 385.85 (±85.40) | 54.08 (±9.89) | 1.98 (±0.17) | ||
| Domestic Silkmoth | J106 | M | 25 | 0.28 (±0.03) | 0.92 (±0.17) | 232.01 (±23.27) | 213.76 (±40.83) | 58.88 (±7.19) | 2.04 (±0.09) |
| F | 30 | 0.11 (±0.01) | 1.67 (±0.26) | 280.38 (±26.40) | 464.13 (±59.42) | 51.67 (±4.90) | 1.99 (±0.11) | ||
| 872 | M | 25 | 0.22 (±0.03) | 1.33 (±0.23) | 245.41 (±25.99) | 322.92 (±43.67) | 71.44 (±8.65) | 2.09 (±0.08) | |
| F | 17 | 0.10 (±0.01) | 2.36 (±0.38†) | 267.15 (±17.88†) | 617.12 (±73.24) | 64.35 (±6.61) | 2.04 (±0.14†) | ||
| Dazao | M | 32 | 0.22 (±0.02) | 1.56 (±0.28) | 192.63 (±19.43) | 298.41 (±44.19) | 63.81 (±5.93) | 1.98 (±0.09) | |
| F | 33 | 0.09 (±0.01) | 2.57 (±0.31) | 215.56 (±24.49) | 549.24 (±52.26) | 51.73 (±4.24) | 1.84 (±0.09) |
| Measurements | Factors | Df | F Values | p Values |
|---|---|---|---|---|
| Body Mass | Sex | 1 | 903.720 | <0.001 |
| Population | 3 | 80.771 | <0.001 | |
| Population × sex | 3 | 4.314 | =0.006 | |
| Flight muscle Mass | Sex | 1 | 50.855 | <0.001 |
| Population | 3 | 35.499 | <0.001 | |
| Population × sex | 3 | 4.683 | =0.004 | |
| Forewing Area | Sex | 1 | 68.719 | <0.001 |
| Population | 3 | 62.361 | <0.001 | |
| Population × sex | 3 | 3.317 | =0.021 | |
| Aspect Ratio (wing shape) | Sex | 1 | 14.286 | <0.001 |
| Population | 3 | 19.067 | <0.001 | |
| Population × sex | 3 | 3.146 | =0.026 | |
| Wing Loading | Sex | 1 | 450.410 | <0.001 |
| Population | 3 | 162.878 | <0.001 | |
| Population × sex | 3 | 7.310 | <0.001 | |
| Flight Muscle Ratio | Sex | 1 | 2082.351 | <0.001 |
| Population | 3 | 95.981 | <0.001 | |
| Population × sex | 3 | 19.318 | <0.001 |
| Measurements | Males | Females | ||||
|---|---|---|---|---|---|---|
| Wild Silkmoth | Domestic Silkmoth | p Values | Wild Silkmoth | Domestic Silkmoth | p Values | |
| Body Mass (mg) | Wild silkmoth | J106 | =0.085 | Wild silkmoth | J106 | =0.002 |
| 872 | <0.001 | 872 | <0.001 | |||
| Dazao | <0.001 | Dazao | <0.001 | |||
| Flight Muscle Mass (mg) | Wild silkmoth | J106 | =0.455 | Wild silkmoth | J106 | =0.615 |
| 872 | <0.001 | 872 | <0.001 | |||
| Dazao | =0.001 | Dazao | =0.624 | |||
| Forewing Area (mm2) | Wild silkmoth | J106 | =0.182 | Wild silkmoth | J106 | =0.540 |
| 872 | =0.997 | 872 | =0.136 | |||
| Dazao | <0.001 | Dazao | <0.001 | |||
| Aspect Ratio (wing shape) | Wild silkmoth | J106 | =0.308 | Wild silkmoth | J106 | =0.979 |
| 872 | =0.004 | 872 | =0.523 | |||
| Dazao | =0.920 | Dazao | =0.005 | |||
| Wing Loading | Wild silkmoth | J106 | =0.035 | Wild silkmoth | J106 | =0.003 |
| 872 | <0.001 | 872 | <0.001 | |||
| Dazao | <0.001 | Dazao | <0.001 | |||
| Flight Muscle Ratio | Wild silkmoth | J106 | =0.008 | Wild silkmoth | J106 | <0.001 |
| 872 | <0.001 | 872 | <0.001 | |||
| Dazao | <0.001 | Dazao | <0.001 | |||
| Measurements | Wild Silkmoth | Means (± SD) | Domestic Silkmoth | Means (±SD) | p Values |
|---|---|---|---|---|---|
| Body Mass (mg) | Wild silkmoth (Female) | 385.85 (±85.40) | J106 (male) | 213.76 (±40.83) | <0.001 |
| 872 (male) | 322.92 (±43.67) | =0.003 | |||
| Dazao (male) | 298.41 (±44.19) | <0.001 | |||
| Wing Loading | Wild silkmoth (Female) | 1.32 (±0.21) | J106 (male) | 0.92 (±0.17) | <0.001 |
| 872 (male) | 1.33 (±0.23) | =0.999 | |||
| Dazao (male) | 1.56 (±0.28) | <0.010 | |||
| Flight Muscle Ratio | Wild silkmoth (Female) | 0.14 (±0.02) | J106 (male) | 0.28 (±0.03) | <0.001 |
| 872 (male) | 0.22 (±0.03) | <0.001 | |||
| Dazao (male) | 0.22 (±0.02) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; Liang, S.; Han, M.; Wu, C.; Song, J.; Li, C.; Wu, S.; He, S.; Ren, J.; Hu, H.; et al. Flight Muscle and Wing Mechanical Properties are Involved in Flightlessness of the Domestic Silkmoth, Bombyx mori. Insects 2020, 11, 220. https://doi.org/10.3390/insects11040220
Lu K, Liang S, Han M, Wu C, Song J, Li C, Wu S, He S, Ren J, Hu H, et al. Flight Muscle and Wing Mechanical Properties are Involved in Flightlessness of the Domestic Silkmoth, Bombyx mori. Insects. 2020; 11(4):220. https://doi.org/10.3390/insects11040220
Chicago/Turabian StyleLu, Kunpeng, Shubo Liang, Minjin Han, Chunman Wu, Jiangbo Song, Chunlin Li, Songyuan Wu, Songzhen He, Jianyu Ren, Hai Hu, and et al. 2020. "Flight Muscle and Wing Mechanical Properties are Involved in Flightlessness of the Domestic Silkmoth, Bombyx mori" Insects 11, no. 4: 220. https://doi.org/10.3390/insects11040220
APA StyleLu, K., Liang, S., Han, M., Wu, C., Song, J., Li, C., Wu, S., He, S., Ren, J., Hu, H., Shen, J., Tong, X., & Dai, F. (2020). Flight Muscle and Wing Mechanical Properties are Involved in Flightlessness of the Domestic Silkmoth, Bombyx mori. Insects, 11(4), 220. https://doi.org/10.3390/insects11040220





