Chemical Cues Induced from Fly-Oviposition Mediate the Host-Seeking Behaviour of Fopius arisanus (Hymenoptera: Braconidae), an Effective Egg Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae), within a Tritrophic Context
Abstract
:1. Introduction:
2. Material and Methods
2.1. Parasitoids (F. arisanus)
2.2. Fly Colony
2.3. Fruit Materials
2.4. Behavioural Assays
2.5. Experimental Design
2.5.1. Experiment 1: Behavioural Responses to Diverse Fruit Species with Different Treatments
2.5.2. Experiment 2: Behavioural Responses to Fruits Infested for Varying Duration
2.5.3. Experiment 3: Behavioural Responses to Different Fruits Species with Different Levels of Mechanical Damage
2.5.4. Experiment 4: Behavioural Responses to Mango Fruits Sprayed with Different Insecticides
2.5.5. Experiment 5: Which Factor Plays a More Important Role in F. arisanus Host Location
2.6. Data Analysis
3. Results
3.1. Experiment 1: Behavioural Responses to Diverse Fruit Species with Different Treatments
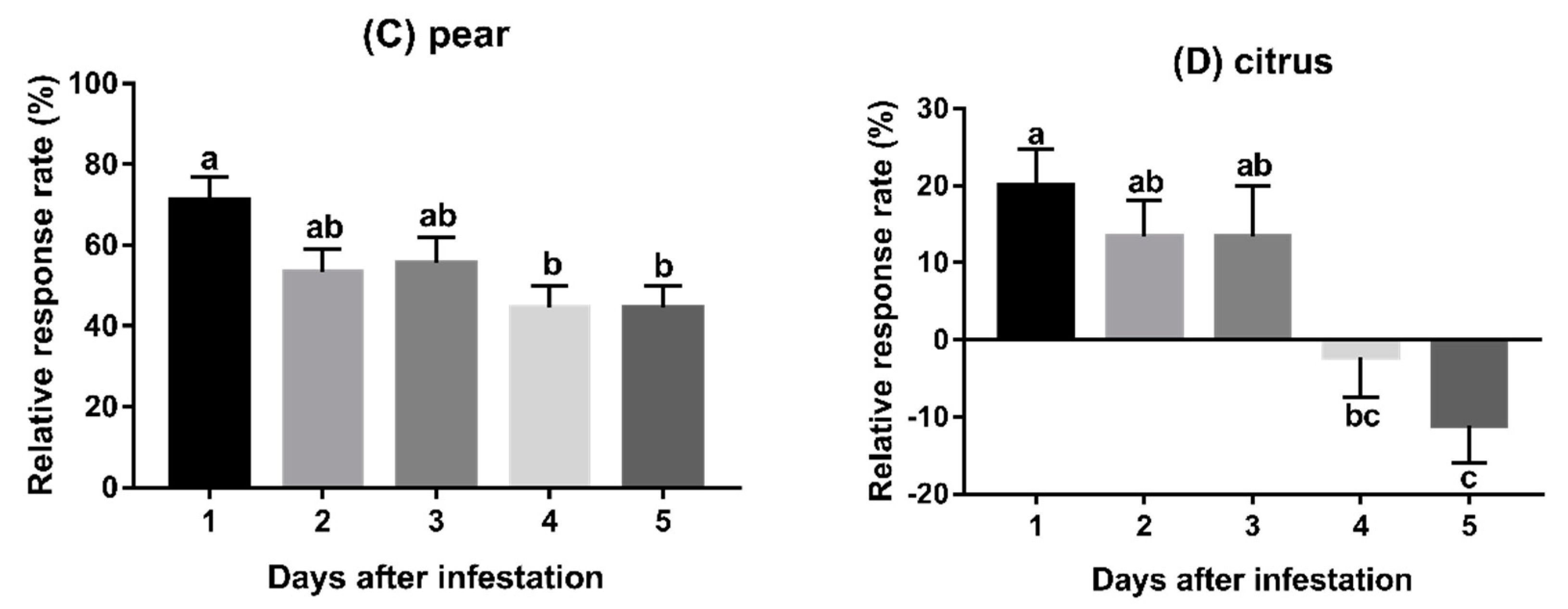
3.2. Experiment 2: Behavioural Responses to Fruits Infested for Varying Duration
3.3. Experiment 3: Behavioural Responses to Different Fruit Species with Different Levels of Damage
3.4. Experiment 4: Behavioural Responses to Mango Fruits Sprayed with Different Insecticides
3.5. Experiment 5: Which Factor Plays a More Important Role in F. arisanus Host Location?
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allwood, A.; Chinajariyawong, A.; Kritsaneepaiboon, S.; Drew, R.; Hamacek, E.; Hancock, D.; Hengsawad, C.; Jipanin, J.; Jirasurat, M.; Krong, C.K. Host plant records for fruit flies (Diptera: Tephritidae) in Southeast Asia. Raffles Bull. Zool. 1999, 47, 1–92. [Google Scholar]
- Vargas, R.I.; Leblanc, L.; Putoa, R.; Eitam, A. Impact of introduction of Bactrocera dorsalis (Diptera: Tephritidae) and classical biological control releases of Fopius arisanus (Hymenoptera: Braconidae) on economically important fruit flies in French Polynesia. J. Econ. Entomol. 2007, 100, 670–679. [Google Scholar] [CrossRef]
- Wu, J.; Feng, X.; Huang, P.; You, M. Entry, infestation, and control of three economically important pests in fruits and vegetables imported from Taiwan. J. Biosaf. 2019, 28, 177–180. [Google Scholar]
- CABI. Bactrocera dorsalis. In Invasive Species Compendium; CAB International: Wallingford, UK, 2018. [Google Scholar]
- Ji, Q.; Bi, K.; Chen, J. Response of egg-pupal parasitoid Fopius arisanus (Sonan) to infochemicals from the host eggs’ surface of Bactrocera dorsalis (Hendel). J. Asia-Pac. Entomol. 2016, 19, 1151–1157. [Google Scholar] [CrossRef]
- Zhang, R.; He, S.; Chen, J. Monitoring of Bactrocera dorsalis (Diptera: Tephritidae) resistance to Cyantraniliprole in the south of China. J. Econ. Entomol. 2014, 107, 1233–1238. [Google Scholar] [CrossRef]
- Cai, P.M.; Gu, X.H.; Yao, M.Y.; Zhang, H.H.; Huang, J.; Idrees, A.; Ji, Q.E.; Chen, J.H.; Yang, J.Q. The optimal age and radiation dose for Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) eggs as hosts for mass-reared Fopius arisanus (Sonan) (Hymenoptera: Braconidae). Biol. Control 2017, 108, 89–97. [Google Scholar] [CrossRef]
- Vargas, R.I.; Leblanc, L.; Harris, E.J.; Manoukis, N.C. Regional suppression of Bactrocera fruit flies (Diptera: Tephritidae) in the Pacific through biological control and prospects for future introductions into other areas of the world. Insects 2012, 3, 727–742. [Google Scholar] [CrossRef] [Green Version]
- Rousse, P.; Harris, E.J.; Quilici, S. Fopius arisanus, an egg-pupal parasitoid of Tephritidae. Overview. Biocontrol News Info. 2005, 26, 59–69. [Google Scholar]
- Vargas, R.I.; Stark, J.D.; Banks, J.; Leblanc, L.; Manoukis, N.C.; Peck, S. Spatial dynamics of two oriental fruit fly (Diptera: Tephritidae) parasitoids Fopius arisanus and Diachasmimorpha longicaudata (Hymenoptera: Braconidae) in a guava orchard in Hawaii. Environ. Entomol. 2013, 42, 888–901. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.M.; Hong, J.F.; Wang, C.; Yang, Y.C.; Zhang, Q.W.; Ji, Q.E.; Chen, J.H. Radiation of Bactrocera dorsalis (Diptera: Tephritidae) eggs to improve the mass rearing of Diachasmimorpha longicaudata (Hymenoptera: Braconidae). J. Econ. Entomol. 2018, 111, 1157–1164. [Google Scholar] [CrossRef]
- Yang, J.Q.; Cai, P.M.; Chen, J.; Zhang, H.H.; Wang, C.; Xiang, H.J.; Wu, J.; Yang, Y.C.; Chen, J.H.; Ji, Q.E.; et al. Interspecific competition between Fopius arisanus and Psyttalia incisi (Hymenoptera: Braconidae), parasitoids of Bactrocera dorsalis (Diptera: Tephritidae). Biol. Control 2018, 121, 183–189. [Google Scholar] [CrossRef]
- Bautista, R.C.; Harris, E.J. Effect of fruit substrates on parasitization of tephritid fruit flies (Diptera) by the parasitoid Biosteres arisanus (Hymenoptera: Braconidae). Environ. Entomol. 1996, 25, 470–475. [Google Scholar] [CrossRef]
- Rousse, P.; Chiroleu, F.; Domerg, C.; Quilici, S. Naive Fopius arisanus females respond mainly to achromatic cues. Biol. Control 2007, 43, 41–48. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Ramadan, M.M.; Ekesi, S. In and out of Africa: parasitoids used for biological control of fruit flies. In Fruit Fly Research and Development in Africa-Towards a Sustainable Management Strategy to Improve Horticulture; Springer: Cham, Switzerland, 2016; pp. 325–368. [Google Scholar]
- Geng, J. Study on mass-rearing of Fopius arisanus and biological control effects on Bactrocera dorsalis in orchards. Ph.D. Thesis, Fujian Agriculture and Forestry University. Fuzhou, China, 2009. [Google Scholar]
- Groth, M.Z.; Loeck, A.E.; Nörnberg-Bernardi, S.D.D.; Nava, D.E. Biology of Fopius arisanus (Hymenoptera: Braconidae) in two species of fruit flies. J. Insect Sci. 2016, 16, e96. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Fatouros, N.E.; Dicke, M.; Mumm, R.; Meiners, T.; Hilker, M. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 2008, 19, 677–689. [Google Scholar] [CrossRef]
- Vinson, S.B. Host selection by insect parasitoids. Annu. Rev. Entomol. 1976, 21, 109–133. [Google Scholar] [CrossRef]
- Vet, L.E.M.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Hilker, M.; Meiners, T. Plants and insect eggs: How do they affect each other? Phytochemistry 2011, 72, 1612–1623. [Google Scholar] [CrossRef]
- Hilker, M.; Meiners, T. Early herbivore alert: insect eggs induce plant defense. J. Chem. Ecol. 2006, 32, 1379–1397. [Google Scholar] [CrossRef]
- Wang, X.G.; Messing, R.H. Foraging behavior andpatch time allocation by Fopius arisanus (Hymenoptera: Braconidae) an egg-larval parasitoid of Tephritid fruit flies. J. Insect Behav. 2003, 16, 593–612. [Google Scholar] [CrossRef] [Green Version]
- Liquido, N.J. Effect of ripeness and location of papaya fruits on the parasitization rates of Oriental fruit fly and melon fly (Diptera: Tephritidae) by braconid (Hymenoptera) parasitoids. Environ. Entomol. 1991, 20, 1732–1736. [Google Scholar] [CrossRef]
- Harris, E.J.; Bautista, R.C. Effects of fruit fly host, fruit species, and host egg to female parasitoid ratio on the laboratory rearing of Biosteres arisanus. Entomol. Exp. Appl. 1996, 79, 187–194. [Google Scholar] [CrossRef]
- Bautista, R.C.; Harris, E.J.; Vargas, R.I.; Jang, E.B. Parasitization of melon fly (Diptera: Tephritidae) by Fopius arisanus and Psyttalia fletcheri (Hymenoptera: Braconidae) and the effect of fruit substrates on host preference by parasitoids. Biol. Control 2004, 30, 156–164. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Sinzogan, A.A.; Bokonon-Ganta, A.H.; Karlsson, M.F. Host species and vegetable fruit suitability and preference by the parasitoid wasp Fopius arisanus. Entomol. Exp. Appl. 2017, 163, 70–81. [Google Scholar] [CrossRef]
- Altuzar, A.; Montoya, P.; Rojas, J.C. Response of Fopius arisanus (Hymenoptera: Braconidae) to fruit volatiles in a wind tunnel. Fla. Entomol. 2004, 87, 616–618. [Google Scholar] [CrossRef]
- Pérez, J.; Rojas, J.C.; Montoya, P.; Liedo, P.; Castillo, A. Anastrepha egg deposition induces volatiles in fruits that attract the parasitoid Fopius arisanus. B. Entomol. Res. 2013, 103, 318–325. [Google Scholar] [CrossRef]
- Pérez, J.; Rojas, J.C.; Montoya, P.; Liedo, P.; González, F.J.; Castillo, A. Size, shape and hue modulate attraction and landing responses of the braconid parasitoid Fopius arisanus to fruit odour-baited visual targets. Biocontrol 2012, 57, 405–414. [Google Scholar] [CrossRef]
- Wong, T.T.Y.; Ramadan, M.M. Mass rearing biology of larval parasitoids (Hymenoptera: Braconidae: Opiinae) of tephritid flies (Diptera: Tephritidae) in Hawaii. In Advances in Insect Rearing for Research and Pest Management; Westview Press: San Francisco, CA, USA, 1992; pp. 405–426. [Google Scholar]
- Manoukis, N.; Geib, S.; Seo, D.; McKenney, M.; Vargas, R.; Jang, E. An optimized protocol for rearing Fopius arisanus, a parasitoid of tephritid fruit flies. J. Vis. Exp. 2011, 53, e2901. [Google Scholar] [CrossRef] [Green Version]
- Dukas, R.; Duan, J.J. Potential fitness consequences of associative learning in a parasitoid wasp. Behav. Ecol. 2000, 11, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Giunti, G.; Canale, A.; Messing, R.H.; Donati, E.; Stefanini, C.; Michaud, J.P.; Benelli, G. Parasitoid learning: current knowledge and implications for biological control. Biol. Control 2015, l90, 208–219. [Google Scholar] [CrossRef]
- Spencer, J.P.; Fujita, B.H. A Procedural Manual for Mass Rearing Four Species of Tephritid Fruit Flies; United States Department of Agriculture, Agricultural Research Service: Honolulu, HI, USA, 1997. [Google Scholar]
- Gu, X.H.; Cai, P.M.; Yang, Y.C.; Yang, Q.Y.; Yao, M.Y.; Idrees, A.; Ji, Q.E.; Yang, J.Q.; Chen, J.H. The response of four braconid parasitoid species to methyl eugenol: Optimization of a biocontrol tactic to suppress Bactrocera dorsalis. Biol. Control 2018, 122, 101–108. [Google Scholar] [CrossRef]
- Revadi, S.; Vitagliano, S.; Rossi Stacconi, M.V.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.; Rota-Stabelli, O.; et al. Olfactory responses of Drosophila suzukii to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Raghu, S.; Halcoop, P.J. Bridging the morphological and biological species concepts: studies on the Bactrocera dorsalis (Hendel) complex (Diptera: Tephritidae: Dacinae) in South-east Asia. Biol. J. Linn. Soc. 2010, 93, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Pagadala Damodaram, K.J.; Kempraj, V.; Aurade, R.M.; Venkataramanappa, R.K.; Nandagopal, B.; Verghese, A.; Bruce, T. Oviposition site-selection by Bactrocera dorsalis is mediated through an innate recognition template tuned to γ-Octalactone. PLoS ONE 2014, 9, e85764. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Turlings, T.C.J.; Wäckers, F.L.; Vet, L.E.M.; Lewis, J.W.; Tumlinson, J.H. Learning of Host-Finding Cues by Hymenopterous Parasitoids. Insect Learning; Chapman & Hall: New York, NY, USA, 1993; pp. 51–78. [Google Scholar]
- Geervliet, J.B.F.; Verdel, M.S.W.; Snellen, H.; Schaub, J.; Dicke, M.; Vet, L.E.M. Coexistence and niche segregation by field populations of the parasitoids Cotesia glomerata and C. rubecula in the Netherlands: predicting field performance from laboratory data. Oecologia 2000, 124, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Gebreziher, H.G. The role of herbivore-induced plant volatiles (HIPVs) as indirect plant defense mechanism in a diverse plant and herbivore species: A review. Int. J. Agric. Environ. Food Sci. 2018, 2, 139–147. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Loughrin, J.H.; McCall, P.J.; Röse, U.S.; Lewis, W.J.; Tumlinson, J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. P. Natl. Acad. Sci. U.S.A. 1995, 92, 4169–4174. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, M.; Montoya, P.; Cruz-Lopez, L.; Rojas, J.C. Response of the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) to mango fruit volatiles. Environ. Entomol. 2005, 34, 576–583. [Google Scholar]
- Purcell, M.F.; Jackson, C.G.; Long, J.P.; Batchelor, M.A. Influence of guava ripening on parasitism of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), by Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) and other parasitoids. Biol. Control 1994, 4, 396–403. [Google Scholar] [CrossRef]
- Eitam, A.; Vargas, R. Host habitat preference of Fopius arisanus (Hymenoptera: Braconidae), a parasitoid of tephritid fruit flies. Ann. Entomol. Soc. Am. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Connor, E.C.; Rott, A.S.; Samietz, J.; Dorn, S. The role of the plant in attracting parasitoids: response to progressive mechanical wounding. Entomol. Exp.Appl. 2007, 125, 145–155. [Google Scholar] [CrossRef]
- Song, X.M.; Zhang, Y.; Qu, A.J. Orientation of Aphidius gifuensis Ashmead to tobacco and prey combination affected by insecticides. Tobacco Sci. Tech. 2011, 11, 76–78. [Google Scholar]
- Rafalimanana, H.; Kaiser, L.; Delpuech, J.M. Stimulating effects of the insecticide chlorpyrifos on host searching and infestation efficacy of a parasitoid wasp. Pest Manag. Sci. 2002, 58, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheshrao, K.; Thakre, S.; Saxena, N. Effect of soil application of chlorinated insecticides on amino acid composition of maize (Zea mays). Plant. Soil 1972, 37, 415–418. [Google Scholar]
- Roitberg, B.D.; Lalonde, R.G. Host marking enhances parasitism risk for a fruit-infesting fly Rhagoletis basiola. Oikos 1991, 61, 389–393. [Google Scholar] [CrossRef]
- Averill, A.L.; Prokopy, R.J. Host-marking pheromones. In Fruit Flies. Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1989; pp. 207–219. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, P.; Song, Y.; Huo, D.; Lin, J.; Zhang, H.; Zhang, Z.; Xiao, C.; Huang, F.; Ji, Q. Chemical Cues Induced from Fly-Oviposition Mediate the Host-Seeking Behaviour of Fopius arisanus (Hymenoptera: Braconidae), an Effective Egg Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae), within a Tritrophic Context. Insects 2020, 11, 231. https://doi.org/10.3390/insects11040231
Cai P, Song Y, Huo D, Lin J, Zhang H, Zhang Z, Xiao C, Huang F, Ji Q. Chemical Cues Induced from Fly-Oviposition Mediate the Host-Seeking Behaviour of Fopius arisanus (Hymenoptera: Braconidae), an Effective Egg Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae), within a Tritrophic Context. Insects. 2020; 11(4):231. https://doi.org/10.3390/insects11040231
Chicago/Turabian StyleCai, Pumo, Yunzhe Song, Da Huo, Jia Lin, Huameng Zhang, Zihao Zhang, Chunmei Xiao, Fengming Huang, and Qinge Ji. 2020. "Chemical Cues Induced from Fly-Oviposition Mediate the Host-Seeking Behaviour of Fopius arisanus (Hymenoptera: Braconidae), an Effective Egg Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae), within a Tritrophic Context" Insects 11, no. 4: 231. https://doi.org/10.3390/insects11040231
APA StyleCai, P., Song, Y., Huo, D., Lin, J., Zhang, H., Zhang, Z., Xiao, C., Huang, F., & Ji, Q. (2020). Chemical Cues Induced from Fly-Oviposition Mediate the Host-Seeking Behaviour of Fopius arisanus (Hymenoptera: Braconidae), an Effective Egg Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae), within a Tritrophic Context. Insects, 11(4), 231. https://doi.org/10.3390/insects11040231




