RETRACTED: Cold Responses of the Mediterranean Fruit Fly Ceratitis capitata Wiedemann (Diptera: Tephritidae) in Blueberry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ceratitis Capitata Colony
2.2. Fruits and Quality Assessment
2.2.1. Colour Test
2.2.2. Size
2.2.3. Weight
2.2.4. Hardness
2.2.5. Relative Humidity
2.2.6. Degrees Brix, Acid, and Their Ratio (Sugar/Acid)
2.3. Natural Infestation
2.4. Life History Study
2.5. Cold Treatment Cabinet
2.6. Statistical Analysis
3. Results
3.1. Fruit Quality Assessment
3.2. Natural Infestation
3.3. Life History Study
3.4. Responses (Mortality) to Cold Treatment
3.4.1. Cold Treatment Bioassay
3.4.2. Modelling Analysis
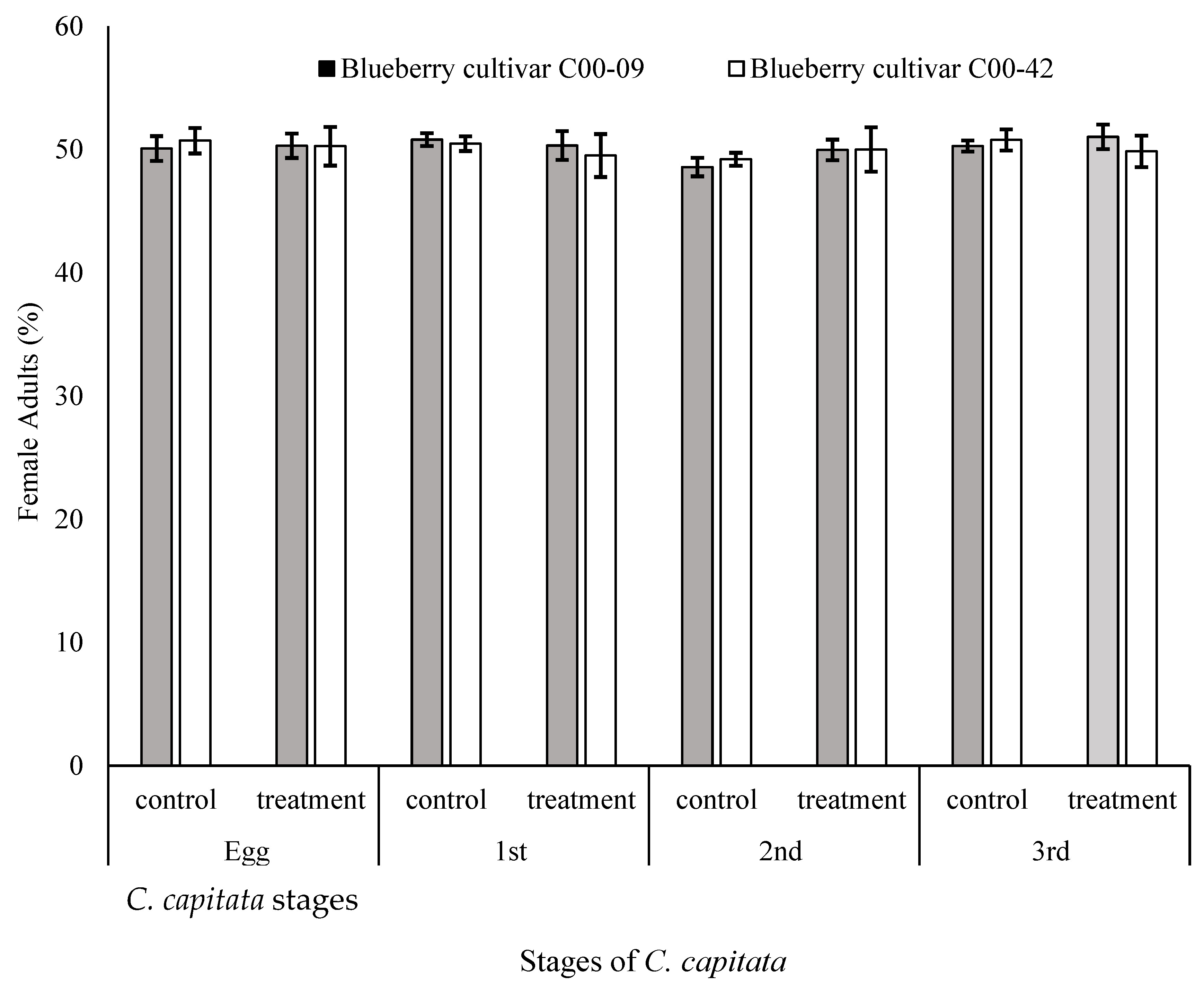
3.5. Sex Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sciarretta, A.; Tabilio, M.R.; Lampazzi, E.; Ceccaroli, C.; Colacci, M.; Trematerra, P. Analysis of the Mediterranean fruit fly [Ceratitis capitata (Wiedemann)] Spatio-temporal distribution in relation to sex and female mating status for precision IPM. PLoS ONE 2018, 13, e0195097. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.; Lopes, D.; Mexia, A.; Mumford, J. Seasonality of the Mediterranean Fruit Fly (Diptera: Tephritidae) on Terceira and Sao Jorge Islands, Azores, Portugal. J. Insect Sci. 2017, 17, iew097. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, A.; Gomulski, L.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica 2007, 131, 1. [Google Scholar] [CrossRef] [PubMed]
- Establishment of areas of low pest prevalence for fruit flies (Tephritidae). In International Standards for Phytosanitary Measures (ISPM. 30); Food and Agriculture Organization (FAO): Rome, Italy, 2017.
- De Meyer, M.; Robertson, M.P.; Mansell, M.W.; Ekesi, S.; Tsuruta, K.; Mwaiko, W.; Peterson, A.T. Ecological niche and potential geographic distribution of the invasive fruit fly Bactrocera invadens (Diptera, Tephritidae). Bull. Entomol. Res. 2010, 100, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Back, E.A.; Pemberton, C.E. Effect of cold-storage temperatures upon the Mediterranean fruit fly. J. Agric. Res. 1916, 5, 657–666. [Google Scholar]
- Heather, N.W.; Hallman, G.J. Pest Management and Phytosanitary Trade Barriers; CABI: Wallingford, UK, 2008. [Google Scholar]
- Ghafir, S.A. Physiological and anatomical comparison between four different apple cultivars under cold-storage conditions. Acta Biol. Szeged. 2009, 53, 21–26. [Google Scholar]
- Richardson, H.H. Cold treatment of fruits. In Insects: The Yearbook of Agriculture; United States Government Publishing Office: Washington, DC, USA, 1952; pp. 404–406. [Google Scholar]
- Mangan, R.L.; Hallman, G.J. Temperature treatments for quarantine security: New approaches for fresh commodities. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; Westview Press: Boulder, CO, USA, 1998; pp. 201–234. [Google Scholar]
- Hallman, G.J.; Sharp, J.L. Radio frequency heat treatments. In Quarantine Treatments for Pests of Food Plants; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Gould, W.P.; Hennessey, M.K. Mortality of Anastrepha suspensa (Diptera: Tephritidae) in carambolas treated with cold water precooling and cold storage. Fla. Entomol. 1997, 80, 79–84. [Google Scholar] [CrossRef]
- Hallman, G.J.; Wang, L.; Uzel, G.D.; Cancio-Martinez, E.; Cáceres-Barrios, C.E.; Myers, S.W. and Vreysen, M.J.B. Comparison of Populations of Ceratitis capitata (Diptera: Tephritidae) from Three Continents for Susceptibility to Cold Phytosanitary Treatment and Implications for Generic Cold Treatments. J. Econ. Entomol. 2018, 112, 127–133. [Google Scholar] [CrossRef]
- Williamson, G.; Lyrene, P.M. Blueberry Varieties for Florida. Institute of Food and Agricultural Sciences. Hort. Sci. 2004, 967. [Google Scholar]
- Strik, B.C.; Yarborough, D. Blueberry production trends in North America, 1992 to 2003, and predictions for growth. Horttechnology 2005, 15, 391–398. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Brazelton, C. World Blueberry Acreage & Production. North American Blueberry Council. Available online: http://www.chilealimentos (accessed on 26 August 2013).
- Sasso, R.; Gualtieri, L.; Russo, E.; Nugnes, F.; Gebiola, M.; Bernardo, U. The establishment of a rearing technique for the fruit fly parasitoid Baryscapus silvestrii increases knowledge of biological, ecological and behavioural traits. BioControl 2020, 65, 47–57. [Google Scholar] [CrossRef]
- Tanaka, N.; Steiner, L.F.; Ohinat, K.; Okamoto, R. Low-cost larval rearing medium for mass production of oriental and Mediterranean fruit flies. J. Econ. Entomol. 1969, 62, 967–968. [Google Scholar] [CrossRef]
- De Lima, C.P.F.; Jessup, A.J.; Mansfield, E.R.; Daniels, D. Cold treatment of table grapes infested with Mediterranean fruit fly Ceratitis capitata (Wiedemann) and Queensland fruit fly Bactrocera tryoni (Froggatt) Diptera: Tephritidae. N. Z. J. Crop Hortic. Sci. 2011, 39, 95–105. [Google Scholar] [CrossRef]
- Wright, G.; Lyrene, P. Blueberry plant named “C99-42. United States Plant Patent US PP20,695 P2, 2 February 2010. [Google Scholar]
- Wright, G.; Lyrene, P. Blueberry plant named “C00-09. United States Plant Patent US PP22,778 P3, 12 June 2012. [Google Scholar]
- National Association of Testing Authorities (NATA). General Accreditation Guidance: Liquid-in-Glass Thermometers-Selection and Use; Copyright National Association of Testing Authorities: Silverwater, Australia, 2019. [Google Scholar]
- Püntener, W. Manual for Field Trials in Plant Protection; Ciba-Geigy Limited: Basle, Switzerland, 1981. [Google Scholar]
- Sun, Y.P.; Shepaud, H. Methods of calculating and correcting the mortality of insects. J. Econ. Entomol. 1947, 40, 710–715. [Google Scholar] [CrossRef]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; WHO Press: London, UK, 2016. [Google Scholar]
- Krainacker, D.; Carey, J.R.; Vargas, R.I. Effect of larval host on life-history traits of the Mediterranean fruit fly, Ceratitis capitata. Oecologia 1987, 73, 583–590. [Google Scholar] [CrossRef]
- Grout, T.G.; Stephen, P.R.; Daneel, J.H.; Hattingh, V. Cold Treatment of Ceratitis capitata (Diptera: Tephritidae) in Oranges Using a Larval Endpoint. J. Econ. Entomol. 2011, 104, 1174–1179. [Google Scholar] [CrossRef]
- Jessup, A.J.; Sloggett, R.F.; Quinn, N.M. Quarantine disinfestation of blueberries against Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) by cold storage. J. Econ. Entomol. 1998, 91, 964–967. [Google Scholar] [CrossRef]
- De Lima, C.P.F.; Jessup, A.J.; Cruickshank, L.; Walsh, C.J.; Mansfield, E.R. Cold disinfestation of citrus (Citrus srm.) for Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). N. Z. J. Crop Hortic. Sci. 2007, 35, 39–50. [Google Scholar] [CrossRef]
- Hallman, G.J.; Myers, S.W.; Jessup, A.J.; Islam, A. Comparison of in vitro Heat and Cold Tolerances of the New Invasive Species Bactrocera invadens (Diptera: Tephritidae) with Three Known Tephritids. J. Econ. Entomol. 2011, 104, 21–25. [Google Scholar] [CrossRef]
- Al-Behadili, F.J.M.; Bilgi, V.; Li, J.; Wang, P.; Taniguchi, M.; Agarwal, M.; Ren, Y.; Xu, W. Cold Response of the Mediterranean Fruit Fly (Ceratitis capitata) on a Lab Diet. Insects 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.; McBride, O. Effect of low temperatures on the Mediterranean fruit fly in infested fruit. J. Econ. Entomol. 1934, 27, 897–902. [Google Scholar] [CrossRef]
- Hill, A.R.; Rigney, C.J.; Sproul, A.N. Cold-Storage of Oranges as a Disinfestation Treatment against the Fruit-Flies Dacus Tryoni (Froggatt) and Ceratitis Capitata (Wiedemann) (Diptera, Tephritidae). J. Econ. Entomol. 1988, 81, 257–260. [Google Scholar] [CrossRef]
- Maurizio, B.; Ponton, F.; Taylor, P.W. Cool storage of Queensland fruit fly eggs for increased flexibility in rearing programs. Pest Manag. Sci. 2019, 75, 1056–1064. [Google Scholar]
- Jordan, H.; Tomberlin, J. Abiotic and biotic factors regulating inter-kingdom engagement between insects and microbe activity on vertebrate remains. Insects 2017, 8, 54. [Google Scholar] [CrossRef]
- Vanderzant, E.S. Physical aspects of artificial diets. Entomologia Experimentalis et Applicata. 1969, 12, 642–650. [Google Scholar] [CrossRef]
- Shibuya, K.; Onodera, S.; Hori, M. Toxic wavelength of blue light changes as insects grow. PLoS ONE 2018, 13, e0199266. [Google Scholar] [CrossRef]
| Fruit Quality | Test | Blueberry Cultivars | Mean ± SE | Unit | Significance |
|---|---|---|---|---|---|
| Biochemical | Sugar | C00-09 | 13.5 ± 0.14 | % | 0.3 |
| C99-42 | 11.3 ± 1.9 | % | |||
| Acid | C00-09 | 0.5 ± 0.03 | % | 0.1 | |
| C99-42 | 0.4 ± 0.04 | % | |||
| Sugar to acid | C00-09 | 26.1 ± 1.6 | % | 0.05 | |
| C99-42 | 34.1 ± 3.3 | % | |||
| Water content | C00-09 | 92.7 ± 0.7 | % | 0.0 | |
| C99-42 | 79.4 ± 1.4 | % | |||
| Physical | Weight | C00-09 | 3.2 ± 0.1 | gm | 0.0 |
| C99-42 | 2.0 ± 0.1 | gm | |||
| Diameter | C00-09 | 20.6 ± 0.3 | mm | 0.0 | |
| C99-42 | 17.9 ± 0.3 | mm | |||
| Colour R | C00-09 | 91.1 ± 21.5 | nm | 0.5 | |
| C99-42 | 104.5 ± 1.9 | nm | |||
| Colour G | C00-09 | 90.8 ± 4.5 | nm | 0.7 | |
| C99-42 | 92.7 ± 2.3 | nm | |||
| Colour B | C00-09 | 108.3 ± 4.2 | nm | 0.0 | |
| C99-42 | 92.2 ± 2.5 | nm | |||
| Hardness | C00-09 | 3.1 ± 0.1 | Kg/cm | 0.3 | |
| C99-42 | 6.2 ± 3.5 | Kg/cm |
| Blueberry | Treatment | Egg | 1st Instar | 2nd Instar | 3rd Instar | ||||
|---|---|---|---|---|---|---|---|---|---|
| (Days) | Pupariation (%) Mean (SE) | Emerged Adult (%) Mean (SE) | Pupariation (%) Mean (±SE) | Emerged Adult (%) Mean (±SE) | Pupariation (%) Mean (±SE) | Emerged adult (%) Mean (±SE) | Pupariation (%) Mean (±SE) | Emerged Adult (%) Mean (±SE) | |
| C00-09 | 0 | 100 (0) | 98 (0.2) | 100 (0) | 99 (0.1) | 100 (0) | 99 (0.1) | 100 (0) | 100 (0) |
| 1 | 52.6 (0.4) | 48.0 (0.4) | 59.2 (0.3) | 54.8 (0.3) | 74.8 (0.3) | 76.3 (0.2) | 82.7 (0.4) | 83.9 (0.4) | |
| 2 | 37.5 (0.5) | 32.8 (0.6) | 37.7 (0.3) | 30.8 (0.4) | 56.2 (0.6) | 58.0 (0.6) | 79.6 (0.3) | 763 (0.3) | |
| 3 | 37.5 (0.5) | 31.2 (0.4) | 29.6 (0.6) | 28.5 (0.6) | 56.2 (07) | 53.4 (0.4) | 75.9 (0.4) | 74.8 (0.3) | |
| 4 | 33.8 (0.8) | 26.7 (0.5) | 29.6 (0.5) | 24.8 (0.6) | 54.0 (0.5) | 48.4 (0.5) | 69.9 (0.3) | 68.7 (0.3) | |
| 5 | 27.0 (0.5) | 25.1 (0.3) | 26.6 (0.8) | 22.5 (0.3) | 51.8 (0.7) | 45.8 (0.5) | 62.4 (0.9) | 61.0 (0.8) | |
| 6 | 19.5 (0.4) | 17.5 (0.4) | 207.(0.6) | 19.5 (0.6) | 48.1 (0.6) | 42.7 (0.4) | 58.6 (0.4) | 50.3 (0.6) | |
| 7 | 12.0 (0.2) | 9.9 (0.2) | 19.2 (0.6) | 17.2 (0.5) | 34.5 (0.4) | 29.0 (0.4) | 45.1 (0.2) | 42.7 (0.3) | |
| 8 | 4.5 (0.2) | 3.8 (0.2) | 17.0 (0.5) | 13.5 (0.3) | 19.7 (0.4) | 17.5(0.5) | 37.5 (0.3) | 32.8 (0.4) | |
| 9 | 0 | 0 | 11.8 (0.4) | 4.5 (0.2) | 11.1 (0.2) | 6.1 (0.1) | 15.7 (0.3) | 15.2 (0.2) | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 9.7 (0.4) | 7.6 (0.2) | |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C99-42 | 0 | 100 (0) | 99 (0.1) | 100 (0) | 100 (0) | 100 (0) | 98 (0.2) | 100 (0) | 99 (0.1) |
| 1 | 49.6 (0.3) | 46.1 (0.3) | 58.6 (0.6) | 53.8 (0.7) | 70.4 (0.3) | 72.0 (0.2) | 81.4 (0.3) | 81.4 (0.3) | |
| 2 | 37.5 (0.2) | 33.0 (0.3) | 34.5 (0.7) | 30.7 (0.9) | 58.4 (0.3) | 56.(0.5) | 78.5 (0.6) | 77.7 (0.5) | |
| 3 | 33.8 (0.3) | 27.6 (0.2) | 27.0 (0.7) | 25.3 (0.7) | 53.8 (0.5) | 48.8 (0.6) | 74.8 (0.4) | 76.3 (0.3) | |
| 4 | 30.0 (0.5) | 30.0 (0.5) | 26.3 (0.7) | 23.0 (0.7) | 52.3 (0.4) | 48.0 (0.4) | 66.6 (0.3) | 66.6 (0.3) | |
| 5 | 22.5 (0.3) | 19.2 (0.2) | 23.3 (0.6) | 21.5 (0.6) | 50.0 (0.3) | 48.0 (0.2) | 62.9 (0.2) | 56.2 (0.2) | |
| 6 | 17.2 (0.5) | 12.3 (0.4) | 19.5 (0.8) | 15.3 (0.6) | 46.1 (0.2) | 38.4 (0.4) | 52.5 (0.4) | 45.1 (0.6) | |
| 7 | 9.7 (0.3) | 6.1 (0.1) | 18.7 (0.8) | 13.8 (0.6) | 31.5 (0.4) | 33.3 (0.2) | 40.7 (0.6) | 37.0 (0.5) | |
| 8 | 4.5 (0.2) | 2.3 (0.2) | 15.0 (0.3) | 12.3 (0.2) | 20.0 (0.3) | 14.4 (0.4) | 33.3 (0.2) | 26.6 (0.3) | |
| 9 | 0 | 0 | 9.7 (0.3) | 4.6 (0.5) | 10 (0.2) | 4.8 (0.2) | 13.3 (0.4) | 11.8 (0.4) | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 8.1 (0.3) | 4.4 (0.3) | |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Development | Blueberry | Mortality | Pupae Recovery as End Point | Adults Recovery as and Point | ||||
|---|---|---|---|---|---|---|---|---|
| Stage | Cultivars | (LT)% | Treatment (Days) | 95% Confidence Limits | Treatment (Days) | 95% Confidence Limits | ||
| Lower | Upper | Lower | Upper | |||||
| Eggs | C00-09 | 90 | 6.7 | 6.0 | 7.7 | 6.3 | 5.8 | 6.9 |
| 95 | 8.1 | 7.2 | 9.4 | 7.8 | 7.2 | 8.6 | ||
| 99 | 10.8 | 9.5 | 12.9 | 10.7 | 9.7 | 11.9 | ||
| C99-42 | 90 | 6.4 | 5.9 | 7.0 | 5.7 | 5.3 | 6.2 | |
| 95 | 7.8 | 7.2 | 8.6 | 7.1 | 6.5 | 7.8 | ||
| 99 | 10.5 | 9.6 | 11.7 | 9.7 | 8.8 | 10.9 | ||
| 1st | C00-09 | 90 | 8.0 | 6.9 | 9.6 | 7.1 | 6.2 | 8.5 |
| 95 | 9.8 | 8.5 | 12.1 | 9.0 | 7.8 | 10.9 | ||
| 99 | 13.3 | 11.3 | 17.0 | 12.4 | 10.6 | 15.6 | ||
| C99-42 | 90 | 7.6 | 6.6 | 9.2 | 6.9 | 6.0 | 8.8 | |
| 95 | 9.5 | 8.2 | 11.7 | 8.6 | 7.6 | 10.3 | ||
| 99 | 13.0 | 11.0 | 16.6 | 12.0 | 10.3 | 14.7 | ||
| 2nd | C00-09 | 90 | 9.1 | 8.0 | 11.1 | 8.7 | 7.7 | 10.1 |
| 95 | 10.7 | 9.2 | 13.2 | 10.1 | 8.9 | 11.9 | ||
| 99 | 13.6 | 11.5 | 17.2 | 12.7 | 11.1 | 15.3 | ||
| C99-42 | 90 | 9.2 | 8.0 | 10.9 | 8.7 | 7.6 | 10.3 | |
| 95 | 10.7 | 9.3 | 12.9 | 10.1 | 8.8 | 12.2 | ||
| 99 | 13.4 | 11.5 | 16.7 | 12.8 | 10.9 | 15.9 | ||
| 3rd | C00-09 | 90 | 10.3 | 9.3 | 11.8 | 10.0 | 9.1 | 11.2 |
| 95 | 11.6 | 10.4 | 13.5 | 11.3 | 10.3 | 12.8 | ||
| 99 | 14.1 | 12.5 | 16.9 | 13.7 | 12.3 | 15.8 | ||
| C99-42 | 90 | 10.0 | 9.1 | 11.4 | 9.5 | 8.8 | 10.5 | |
| 95 | 11.3 | 10.2 | 13.0 | 10.7 | 9.8 | 11.9 | ||
| 99 | 13.8 | 12.3 | 16.1 | 13.1 | 11.9 | 14.7 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Behadili, F.J.M.; Agarwal, M.; Xu, W.; Ren, Y. RETRACTED: Cold Responses of the Mediterranean Fruit Fly Ceratitis capitata Wiedemann (Diptera: Tephritidae) in Blueberry. Insects 2020, 11, 276. https://doi.org/10.3390/insects11050276
Al-Behadili FJM, Agarwal M, Xu W, Ren Y. RETRACTED: Cold Responses of the Mediterranean Fruit Fly Ceratitis capitata Wiedemann (Diptera: Tephritidae) in Blueberry. Insects. 2020; 11(5):276. https://doi.org/10.3390/insects11050276
Chicago/Turabian StyleAl-Behadili, Farhan J.M., Manjree Agarwal, Wei Xu, and Yonglin Ren. 2020. "RETRACTED: Cold Responses of the Mediterranean Fruit Fly Ceratitis capitata Wiedemann (Diptera: Tephritidae) in Blueberry" Insects 11, no. 5: 276. https://doi.org/10.3390/insects11050276





