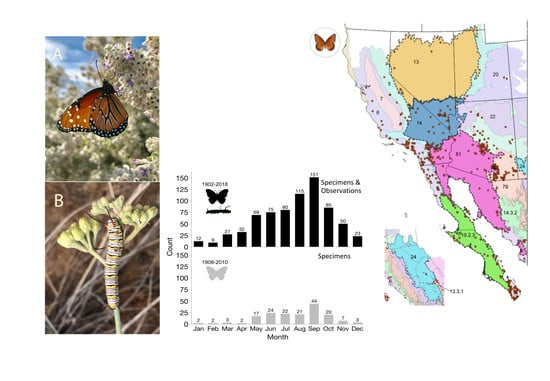
Ecology of the Western Queen Butterfly Danaus gilippus thersippus (Lepidoptera: Nymphalidae) in the Mojave and Sonoran Deserts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Distribution and Phenology
2.2. Host Plant Use and Phenology
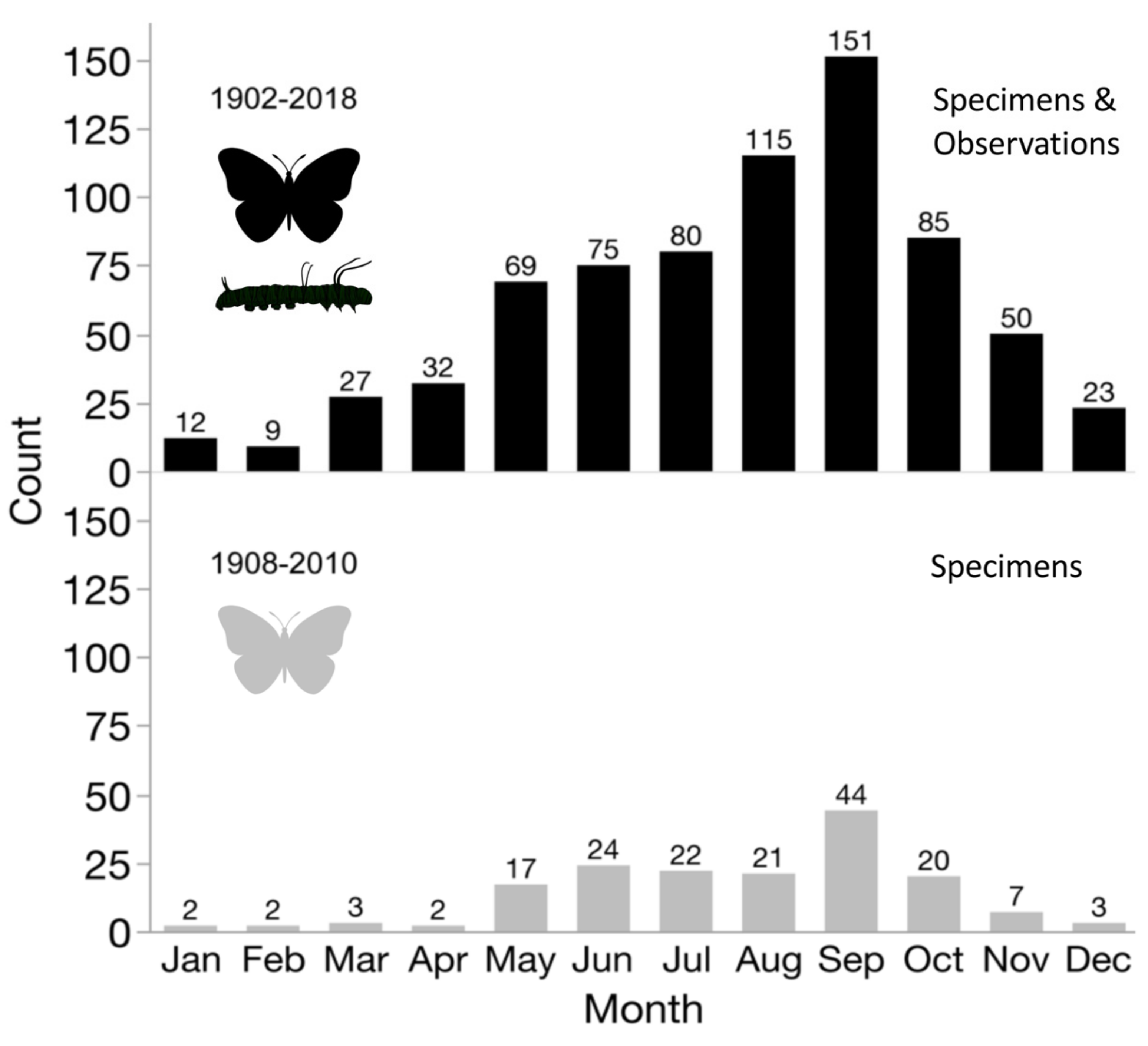
3. Results
3.1. Distribution and Phenology
3.2. Movement Patterns
3.3. Diet
3.4. Mating Behavior
3.5. Chemical Ecology
3.6. Predators, Parasites and Pathogens
4. Discussion
4.1. Distribution
4.2. Movement
4.3. Phenology
4.4. Diet
4.5. Chemical Ecology
4.6. Predators, Parasites and Pathogens
4.7. Suggestions for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Forister, M.L.; McCall, A.C.; Sanders, N.J.; Fordyce, J.A.; Thorne, J.H.; O’Brien, J.; Waetjen, D.P.; Shapiro, A.M. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 2088–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forister, M.L.; Cousens, B.; Harrison, J.G.; Anderson, K.; Thorne, J.H.; Waetjen, D.; Nice, C.C.; De Parsia, M.; Hladik, M.L.; Meese, R.; et al. Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol. Lett. 2016, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Walther, G.; Post, E.; Convey, P.; Menzel, A.; Parmensan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Gulberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Maclean, I.M.D. Recent evidence for the climate change threat to Lepidoptera and other insects. J. Insect Conserv. 2010, 15, 259–268. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [Green Version]
- Vale, C.G.; Brito, J.C. Desert-adapted species are vulnerable to climate change: Insights from the warmest region on Earth. Glob. Ecol. Conserv. 2015, 4, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Nabhan, G.; Buckley, S.; Dial, H. Pollinator Plants of the Desert Southwest: Native Milkweeds (Asclepias spp.); USDA-Natural Resources Conservation Service, Tucson Plant Materials Center: Tucson, AZ, USA, 2015; pp. 1–35. [Google Scholar]
- Brower, L.P.; Taylor, O.R.; Williams, E.H.; Slayback, D.A.; Zubieta, R.R.; Ramirez, M.I. Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Conserv. Diver. 2012, 5, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Pleasants, J.M.; Oberhauser, K.S. Milkweed loss in agricultural fields because of herbicide use: Effect on the monarch butterfly population. Insect Conserv. Divers. 2012, 6, 135–144. [Google Scholar] [CrossRef]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [Green Version]
- Ikerd, H.; Griswold, T. USDA-ARS Pollinating Insects Lab, Logan, Utah, USA. Insects collected on milkweeds in Arizona, California, Nevada, New Mexico and Utah. Personal communication, 2014. [Google Scholar]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 2017, 356, 1395–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2010, 108, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saul-Gershenz, L.; Millar, J.G.; McElfresh, J.S.; Williams, N.W. Deceptive signals and behaviors of a cleptoparasitic beetle show local adaptation to different host bee species. Proc. Natl. Acad. Sci. USA 2018, 115, 9756–9760. [Google Scholar] [CrossRef] [Green Version]
- California Native Plant Society (CNPS), Rare Plant Program. Inventory of Rare and Endangered Plants of California (online edition, v8-03 0.39). Available online: http://www.rareplants.cnps.org (accessed on 13 December 2019).
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Tepedino, V.J. The reproductive biology of rare rangeland plants and their vulnerability to insecticides. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G.L., Sampson, M.W., Technical Coordinators, Eds.; Technical Bulletin 180; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 2000; pp. III5.1–III.5-10. [Google Scholar]
- Pelton, E.M.; Schultz, C.B.; Jepsen, S.J.; Black, S.H.; Crone, E.E. Western monarch population plummets: Status, probable causes, and recommended conservation actions. Front. Ecol. Evol. 2019, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.A.; Petschenka, G.; Bingham, R.A.; Weber, M.G.; Rasmann, S. Toxic cardenolides: Chemical ecology and coevolution of specialized plant–herbivore interactions. New Phytol. 2012, 194, 28–45. [Google Scholar] [CrossRef]
- Dussourd, D.E.; Harvis, C.A.; Meinwald, J.; Eisner, T. Paternal allocation of sequestered plant pyrrolizidine alkaloid to eggs in the danaine butterfly, Danaus gilippus. Experientia 1989, 45, 896–898. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Armstrong, A.; Burney, A.; Greer, J.; Ryan, G.; Moore, K.; Diedhiou, I.; Grodsky, S.M.; Saul-Gershenz, L.; Davis, R.; et al. Techno-ecological synergies of solar energy produce beneficial outcomes across industrial-ecological boundaries to mitigate global environmental change. Nat. Sustain. 2019, 2, 560–568. [Google Scholar] [CrossRef]
- Grodsky, S.M.; Saul-Gershenz, L.; Moore-O’Leary, K.; Hernandez, R.R. Her Majesty’s desert throne: The ecology of queen butterfly oviposition on Mojave milkweed host plants. Insects 2020, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- Lepidopterists. Society Season Summary Website for Danaus gilippus thersippus Hosted by Florida Museum of Natural History at the University of Florida. pp. 314–316. Available online: https://www.lepsoc.org/content/season-summary (accessed on 11 May 2020).
- Forrest, J.R.K.; Thorp, R.W.; Kremen, C.; Williams, N.M. Contrasting patterns in species and functional-trait diversity of bees in an agricultural landscape. J. Appl. Ecol. 2015, 52, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Bartomeus, I.; Ascher, J.S.; Wagner, D.; Danforth, B.N.; Colla, S.; Kornbluth, S.; Winfree, R. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20654–20659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.gbif.org/ (accessed on 6 December 2019).
- Emmel, T.C.; Emmel, J.F. The Butterflies of Southern California; Science Series; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1973; Volume 26, pp. 1–148. [Google Scholar]
- Shields, O.; Emmel, J.F. A review of carrying pair behavior and mating times in butterflies. J. Res. Lepid. 1973, 12, 25–64. [Google Scholar]
- Howe, W.H. The Butterflies of North. America; Doubleday and Co.: New York, NY, USA, 1975; pp. 1–633. [Google Scholar]
- Pfeiler, E.; Ramírez, M.; Laclette, L.; Markow, T.A. Butterfly biodiversity in a threatened coastal desert ecosystem of northwestern Mexico, with a focus on the life history and ecology of potentially endangered species. J. Lepidopterists’ Soc. 2016, 70, 47–60. [Google Scholar] [CrossRef]
- Coolidge, K.R. The life history of Danaus berenice strigose Bates (Lepidoptera; Danaidae). Trans. Am. Entomol. Soc. 1926, 51, 27–33. [Google Scholar]
- Orsak, L.J. The Butterflies of Orange County, California; Miscellaneous Publication No. 3; Research Series; University of California, Irvine, Museum of Systematic Biology, University of California, Irvine, California, USA & Center for Pathobiology: Irvine, CA, USA, 1978; Volume 4, p. xii,349. [Google Scholar]
- GIBO, D.L. Notes on Danaus gilippus strigosus (Nymphalidae: Danainae) in Southern California. J. Lepidopterists’ Soc. 1993, 47, 160–161. [Google Scholar]
- Germano, D.J.; Rathbun, G.B.; Saslaw, L.R.; Cypher, B.R.; Cypher, E.A.; Vredenburgh, L.M. The San Joaquin Desert of California: Ecologically Misunderstood and Overlooked. Nat. Areas J. 2011, 31, 138–147. [Google Scholar] [CrossRef]
- EPA. Available online: https://www.epa.gov/eco-research/level-iii-and-iv-ecoregions-continental-united-states (accessed on 10 December 2019).
- Dingle, H.; Drake, V.A. What Is Migration? BioScience 2007, 57, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Ramenofsky, M.; Wingfield, J.C. Regulation of migration. BioScience 2007, 57, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.A. Mating of butterflies. J. Res. Lep. 1972, 11, 99–127. [Google Scholar]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Berlin, Germany, 2007; pp. 215–264. [Google Scholar]
- Forcone, A.; Galettott, L.; Bernardello, L. Floral Nectar Chemical Composition of Some Species from Patagonia. Biochem. Syst. Ecol. 1997, 25, 395–402. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. In Biochemical Aspects of Evolutionary Biology; Nitechki, M.H., Ed.; Univ. of Chicago Press: Chicago, IL, USA, 1982; pp. 131–171. [Google Scholar]
- Junior, S.V.; Celloto, V.R.; Vieira, L.G.E.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Floral nectar chemical composition of floral nectar in conventional and transgenic sweet orange, Citrus sinensis (L.) Osbeck, expressing an antibacterial peptide. Plant Syst. Evol. 2008, 275, 1–7. [Google Scholar] [CrossRef]
- Waller, G.D. Evaluating Responses of Honey Bees to Sugar Solutions Using an Artificial-Flower Feeder. Ann. Entomol. Soc. Am. 1972, 65, 857–862. [Google Scholar] [CrossRef]
- Adler, L. The ecological significance of toxic nectar. Oikos 2001, 91, 401–420. [Google Scholar] [CrossRef]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef]
- Negri, G.; Teixeira, E.W.; Alves, M.L.; Moreti, A.C.; Otsuk, I.P.; Borguini, R.G.; Salatino, A. Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. [Google Scholar] [CrossRef]
- Waser, N.M.; Price, M.V. Drought, pollen and nectar availability, and pollination success. Ecology 2016, 97, 1400–1409. [Google Scholar] [CrossRef] [Green Version]
- Baker, H.G.; Baker, I. Floral nectar sugar constituents in relation to pollinator type. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold Co., Inc.: New York, NY, USA, 1983; pp. 131–171. [Google Scholar]
- Singer, M.; Mace, K.; Bernays, E. Self-medication as adaptive plasticity: Increased ingestion of plant toxins by parasitized caterpillars. PLoS ONE 2009, 4, e4796. [Google Scholar] [CrossRef]
- Singer, M.S.; Carrière, Y.; Theuring, C.; Hartmann, T. Disentangling food quality from resistance against parasitoids: Diet choice by a generalist caterpillar. Am. Nat. 2004, 164, 423–429. [Google Scholar] [CrossRef]
- Manson, J.S.; Otterstatter, M.C.; Thomson, J.D. Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 2010, 162, 81–89. [Google Scholar] [CrossRef]
- Bingham, R.A.; Agrawal, A.A. Specificity and trade-offs in the induced plant defense of common milkweed Asclepias syriaca to two lepidopteran herbivores. J. Ecol. 2010, 98, 1014–1022. [Google Scholar] [CrossRef]
- Vannette, R.L.; Hunter, M.D. Plant defense theory re-examined: Nonlinear expectations based on the costs and benefits of resource mutualisms. J. Ecol. 2011, 99, 66–76. [Google Scholar] [CrossRef]
- Pereda-Miranda, R. Bioactive Natural Products from traditionally used Mexican Plants. In Phytochemistry of Medicinal Plants: Recent Advances in Phytochemistry; Arnason, J.T., Mata, R., Romeo, J.T., Eds.; Springer: Boston, MA, USA, 1995; Volume 29, pp. 83–112. [Google Scholar]
- Thorne, R.F.; Prigge, B.A.; Henrickson, J. A flora of the higher ranges and the Kelso Dunes of the eastern Mojave Desert in California. Aliso 1981, 10, 71–186. [Google Scholar] [CrossRef] [Green Version]
- Sakai, W.H. Notes on Danaus gilippus strigosus (Nymphalidae: Danainae) in Southern California. J. Lepidopterists’ Soc. 1993, 47, 160–161. [Google Scholar]
- Pliske, T.E. Attraction of Lepidoptera to Plants containing pyrrolizidine alkaloids. Environ. Ent. 1973, 4, 455–473. [Google Scholar] [CrossRef]
- Available online: https://www.fireflyforest.com/flowers/2770/asclepias-asperula-spider-milkweed/ (accessed on 8 September 2019).
- Sundell, E. Synopsis of gonolobus s. l. (Apocynaceae, Asclepiadoideae) in the United States and its territories, including lectotypification of Lachnostoma arizonicum. J. Ariz.-Nev. Acad. Sci. 1994, 27, 169–187. [Google Scholar]
- Sencio, K.; Rutowski, R.L. Daily Eclosion Patterns in nymphalid butterflies (Lepidoptera: Nymphalidae): Interspecific and intraspecific variation. J. Lepidopterists’ Soc. 2019, 73, 56–62. [Google Scholar]
- Rosatti, T.J.; Hoffmann, C.A. Asclepias asperula subsp. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=49306 (accessed on 8 September 2019).
- Rosatti, T.J.; Hoffmann, C.A. Asclepias curassavica in Jepson Flora Project [eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=14363 (accessed on 8 September 2019).
- Einem, G.E. Attraction of Male Queen Butterflies to Cardenolide- and Alkaloid-Containing Plants During Fall Migrations. News Lepidopterist’s Soc. 2004, 46, 94–97. [Google Scholar]
- Rosatti, T.J.; Hoffmann, C.A. Asclepias erosa in Jepson Flora Project [eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=1437114363 (accessed on 8 September 2019).
- Kearney, T.H.; Peebles, R.H. Arizona Flora, 2nd ed.; Univ. of California Press: Berkeley, CA, USA, 1960; p. 1085. [Google Scholar]
- Rosatti, T.J.; Hoffman, C.A. Asclepias fascicularis, in Jepson Flora Project (eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=14375 (accessed on 8 September 2019).
- Asclepias latifolia. Available online: https://plants.usda.gov/core/profile?symbol=ASLA4 (accessed on 5 September 2019).
- Available online: https://www.butterfliesofamerica.com/L/t/Danaus_gilippus_thersippus_a.htm (accessed on 7 September 2019).
- Asclepias linaria. Available online: http://southwestdesertflora.com/WebsiteFolders/All_Species/Asclepiadaceae/Asclepias%20linaria,%20Pineneedle%20Milkweed.html (accessed on 5 September 2019).
- Asclepias linaria. Available online: https://bugguide.net/node/view/737092larva (accessed on 5 September 2019).
- Rosatti, T.J.; Hoffman, C.A. Asclepias nyctaginifolia in Jepson Flora Project (eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=14404 (accessed on 5 September 2019).
- Plagens, M.J. 1999–2010. Available online: http://www.arizonensis.org/sonoran/fieldguide/arthropoda/danaus.html (accessed on 5 September 2019).
- Rosatti, T.J.; Hoffman, C.A. Asclepias speciosa in Jepson Flora Project (eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=14422 (accessed on 8 September 2019).
- Saul-Gershenz, L. Personal communication, 2018.
- Plant List of Ivanpah Solar Site. Available online: https://www.a-state-of-change.com/IvanpahValley.html (accessed on 5 September 2019).
- Rosatti, T.J.; Hoffman, C.A. Asclepias subulata in Jepson Flora Project (eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=14425 (accessed on 8 September 2019).
- Rosatti, T.J.; Hoffman, C.A. Asclepias vestita in Jepson Flora Project (eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=14441 (accessed on 8 September 2019).
- Zavortink, T.J. Personal communication, 20 January 2020.
- Hartmann, T. Pyrrolizidine Alkaloids. In Herbivores: Their Interactions with Secondary Plant Metabolites, 2nd ed.; Rosenthal, G.A., Berenbaum, M.R., Eds.; Academic Press: San Diego, CA, USA, 1991; Volume I, pp. 96–102. [Google Scholar]
- Boppré, M.J. Lepidoptera and pyrrolizidine alkaloids, exemplification of complexity in chemical ecology. Chem. Ecol. 1990, 16, 165–184. [Google Scholar] [CrossRef]
- Keil, D.J. Ageratina herbacea, in Jepson Flora Project [eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=749 (accessed on 8 September 2019).
- Hartmann, T.; Witte, L. Chemistry, biology and chemoecology of the pyrrolizidine alkaloids. In Alkaloids: Chemical and Biological Perspectives; Pellitier, S.W., Ed.; Elsevier Science, Inc.: New York, NY, USA, 1995; Volume 9, pp. 155–234. [Google Scholar]
- Andre, J. Personal communication, 7 December 2019.
- Trock, D.K. Senecio flaccidus var. douglasii, in Jepson Flora Project [eds.) Jepson eFlora. 2012. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=8218 (accessed on 8 September 2019).
- Trock, D.K. Senecio flaccidus var. monoensis, in Jepson Flora Project (eds.) Jepson eFlora. 2012. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=8223 (accessed on 8 September 2019).
- Fu, P.P.; Yang, Y.C.; Xia, Q.; Chou, M.C.; Cui, Y.Y.; Lin, G. Pyrrolizidine alkaloids-tumorigenic components in Chinese herbal medicines and dietary supplements. J. Food Drug Anal. 2002, 10, 198–211. [Google Scholar]
- Kelly, R.B.; Ganders, F.R. Amsinckia tessellata var. tessellata, in Jepson Flora Project (eds.) Jepson eFlora. 2012. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=77020 (accessed on 8 September 2019).
- Kelly, R.B.; Wilken, D.H. Heliotropium curassavicum var. oculatum, in Jepson Flora Project (eds.) Jepson eFlora. 2012. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=59914 (accessed on 8 September 2019).
- CCH1. Nerium oleander locality, San Bernadino Co. Available online: http://ucjeps.berkeley.edu/cgi-bin/new_detail.pl?UCR166614 (accessed on 8 September 2019).
- Calflora. Matelea parviflora. Available online: https://www.calflora.org//cgi-bin/species_query.cgi?where-calrecnum=12666 (accessed on 8 September 2019).
- Butterflies and Moths of North America Website. Available online: https://www.butterfliesandmoths.org/species/Danaus-gilippus (accessed on 13 September 2019).
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Calflora: Consortium of California Herbaria. 2019. Berkeley, California: The Calflora Database [a Non-Profit Organization]. Available online: https://www.calflora.org/ (accessed on 8 September 2019).
- Flickr Image. Available online: https://www.flickr.com/photos/37247779@N07/45240260385/in/photolist-2bVJagp-rAfuTS-9zpBdy-riLG33-p1aGeR-24D5H3B-26Vf4FY-fus9oF-9zpBoy-5eV6LB-fqbyNB-7V3YqF-riUafM-bxEH5p-7KCvEf-MjqD4-Tszmt7-K5QJKy-XatMcw-bV8tGn-YxxoVX-4Jtn2o-VV3N4Q-29gEJrj-fpUxRM-26JWLxe-orw23n-fq8wfZ-22Won7E-25R6RCH-obpesa-28eXkTk-25DDfo1-orvBZa-279zq6G-bVNSsQ-MjqCR-aWuVNR-26Fdx3w-6WaUvd-bUz7Pg-fqqQX1-G36LT2-futPyT-H67nbV-2dBqnxH-25DBPSu-9vXwij-8oSAs2-H67ode (accessed on 31 October 2019).
- Keil, D.J. Cirsium mohavense, in Jepson Flora Project (eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=2198 (accessed on 8 September 2019).
- Cirsium mohavensis. Available online: https://bugguide.net/node/view/1261109 (accessed on 8 September 2019).
- Harley, R.M.; Rosatti, T.J. Condea emoryi in Jepson Flora Project (eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=98461 (accessed on 8 September 2019).
- Flora of North America. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=250067830 (accessed on 7 December 2019).
- Wojciechowski, M.F. Parkinsonia florida, in Jepson Flora Project [eds.) Jepson eFlora, Revision 1. 2013. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=36268 (accessed on 8 September 2019).
- University of California, Davis. BMEC Specimen of Danaus gilippus thersippus; Imperial Co.: Algodones Dunes, CA, USA.
- Hartmann, T.; Zimmer, M. Organ specific distribution and accumulation of pyrrolizidine alkaloids during the life history of two annual Senecio species. J. Plant Physiol. 1986, 122, 67–80. [Google Scholar] [CrossRef]
- Züst, T.; Petschenka, G.; Hastings, A.P.; Anurag, A.; Agrawal, A.A. Toxicity of Milkweed Leaves and Latex: Chromatographic Quantification Versus Biological Activity of Cardenolides in 16 Asclepias Species. J. Chem. Ecol. 2018, 45, 50–60. [Google Scholar]
- Brower, L.R.; van Zandt Brower, J.; Cranston, F.R. Courtship behavior of the queen butterfly, Danaus gilippus berenice (Cramer). Zool 1965, 50, 1–39. [Google Scholar]
- Burns, J.M. Duration of Copulation in Poanes hobomok (Lepidoptera: Hesperiidae) and Some Broader Speculations. Psyche 1970, 77, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Pliske, T.E.; Eisner, T. Sex pheromone of the queen butterfly: Biology. Science 1969, 164, 1170–1172. [Google Scholar] [CrossRef]
- Miller, L.D.; Clench, H.K. Some aspects of mating behavior in butterflies. Lepid. Soc. 1968, 22, 125–132. [Google Scholar]
- Malcolm, S.B. Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology 1995, 5, 101–117. [Google Scholar] [CrossRef]
- Meinwald, J.; Meinwald, Y.C.; Mazzocchi, P.H. Sex pheromone of the queen butterfly. Chemistry. Science 1969, 164, 1174–1175. [Google Scholar] [CrossRef]
- Dobler, S.; Petschenka, G.; Wagschal, V.; Flacht, L. Convergent adaptive evolution-how insects master the challenge of cardiac glycoside-containing host plants. Entomol. Exp. Appl. 2015, 157, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, S.B. Cardenolide-mediated interactions between plants and herbivores. In Herbivores: Their Interactions with Secondary Plant Metabolites, 2nd ed.; Rosenthal, G.A., Berenbaum, M.R., Eds.; The Chemical Participants; Academic Press: San Diego, CA, USA, 1991; Volume I, pp. 251–269. [Google Scholar]
- Rasmann, S.; Agrawal, A.A. Latitudinal patterns in plant defense: Evolution of cardenolides, their toxicity and induction following herbivory. Ecol. Lett. 2011, 14, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Evans, F.J.; Cowley, P.S. Cardenolides and spirostanols in Digitalis purpurea at various stages of development. Phytochemistry 1972, 11, 2971–2975. [Google Scholar] [CrossRef]
- Nelson, C.J.; Seiber, J.N.; Brower, L.P. Seasonal and intraplant variation of cardenolide content in the California milkweed Asclepias eriocarpa, and implications for plant defense. J. Chem. Ecol. 1981, 7, 981–1010. [Google Scholar] [CrossRef] [PubMed]
- Detzel, A.; Wink, M. Evidence for a cardenolide carrier in Oncopeltus fasciatus (Dallas) (Insecta: Hemiptera). Zeitschrift für Naturforschung 1995, 50, 127–134. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Brower, L.P.; Alonso, A. Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias humistrata. Ecol. Entomol. 2001, 26, 212–224. [Google Scholar] [CrossRef]
- Moranz, R.; Brower, L.P. Geographic and Temporal Variation of Cardenolide-Based Chemical Defenses of Queen Butterfly (Danaus gilippus) in Northern Florida. J. Chem. Ecol. 1998, 24, 905–932. [Google Scholar] [CrossRef]
- Stuhlfauth, T.; Klug, K.; Fock, H.P. The production of secondary metabolites by Digitalis lanata during CO2 enrichment and water-stress. Phytochemistry 1987, 26, 2735–2739. [Google Scholar] [CrossRef]
- Malcolm, S.B.; Zalucki, M.P. Milkweed latex and cardenolide induction may resolve the lethal plant defense paradox. Entomol. Exp. Appl. 1996, 80, 193–196. [Google Scholar] [CrossRef]
- Vannette, R.L.; Hunter, M.D. Genetic variation in expression of defense phenotype may mediate evolutionary adaptation of Asclepias syriaca to elevated CO2. Glob. Chang. Biol. 2011, 17, 1277–1288. [Google Scholar] [CrossRef]
- Jacobsohn, M.K.; Gert, M.; Jacobsohn, G.M. Production of a Fungistat and the role of fungi during germination of Digitalis purpurea L.cv. Gloxinia flora seeds. Ann. Bot. 1985, 56, 543–552. [Google Scholar] [CrossRef]
- Akhtar, N.; Malik, A.; Ali, S.N.; Kazmi, S.U. Proceragenin, an antibacterial cardenolide from Calotropis procera. Phytochemistry 1992, 31, 2821–2824. [Google Scholar] [CrossRef]
- Lefèvre, T.; Oliver, L.; Hunter, M.D.; de Roode, J.C. Evidence for trans-generational medication in nature. Ecol. Lett. 2010, 13, 1485–1493. [Google Scholar] [CrossRef]
- Bertol, J.W.; Maia de Pádua, R.; Kreis, W.; Monte Barardi, C.R.; Braga, F.C.; Oliveira Simões, C.M. Antiherpes activity of glucoevatromonoside, a cardenolide isolated from a Brazilian cultivar of Digitalis lanata. Antivir. Res. 2011, 92, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.J. A model for cardenolide and cardenolide glycoside storage by the butterfly. In Biology and Conservation of the Monarch Butterfly; Malcolm, S.B., Zalucki, M.P., Eds.; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1993; pp. 83–90. [Google Scholar]
- Rasmann, S.; Johnson, M.D.; Agrawal, A.A. Induced responses to herbivory and jasmonate in three milkweed species. J. Chem. Ecol. 2009, 35, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D.; Wagner, M.G.; Toennes, S.W.; Boppré, M. Selective sequestration of cardenolide isomers by two species of Danaus butterflies (Lepidoptera: Nymphalidae: Danainae). Chemoecology 2012, 22, 269–272. [Google Scholar] [CrossRef]
- Oyeyele, S.O.; Zalucki, M.P. Cardiac glycosides and oviposition by Danaus plexippus on Asclepias fruticosa in south-east Queensland (Australia), with notes on the effect of plant nitrogen content. Ecol. Entomol. 1990, 15, 177–186. [Google Scholar] [CrossRef]
- Holzinger, F.; Wink, M. Mediation of cardiac glycoside insensitivity in the monarch butterfly (Danaus plexippus): Role of an amino acid substitution in the ouabain binding site of Na+, K+-ATPase. J. Chem. Ecol. 1996, 22, 1921–1937. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Lajeunesse, M.J.; Fishbein, M. Evolution of latex and its constituent defensive chemistry in milkweeds (Asclepias): A phylogenetic test of plant defense escalation. Entomol. Exp. Appl. 2008, 128, 126–138. [Google Scholar] [CrossRef]
- Dussourd, D.E.; Eisner, T. Vein-cutting behavior: Insect counter- ploy to the latex defense of plants. Science 1987, 237, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, P.H., Jr. A Host-Parasite Catalog of North American Tachinidae (Diptera); United States Department of Agriculture, Miscellaneous Publication: Washington, DC, USA, 1978; Volume 1319, p. ii-860. [Google Scholar]
- Prudic, K.L.; Olson, C. A new parasitoid of Danaus gilippus thersippus (Nymphalidae: Danainae) in South-Eastern Arizona. J. Lepidopterists’ Soc. 2005, 59, 118–119. [Google Scholar]
- d ‘Araújo, E.; Silva, A.G.; Gonçlaves, R.; Galvão, A.J.L.; Gonçlaves, M.; do Nascimento, S.; de Simoni, L. Quarto catalogo dos insectos que vivem nas plantas do Brasil. Rio de Janeiro 1968, 1, xxvii+62. [Google Scholar]
- McLaughlin, R.E.; Myers, J. Ophryocystis elektroscirrha sp. n., a Neogregarine pathogen of the monarch butterfly Danaus plexippus (L.) and the Florida queen butterfly D. gilippus berenice Cramer. J. Protozool. 1970, 17, 300–305. [Google Scholar] [CrossRef]
- Fink, L.S.; Brower, L.P.; Waide, R.B.; Spitzer, P.R. Overwintering Monarch Butterflies as Food for Insectivorous Birds in Mexico. Biotropica 1983, 15, 151–153. [Google Scholar] [CrossRef]
- Grodsky, S.M.; Saul-Gershenz, L.; Moore-O’Leary, K.A.; Whitney, J.P.; Hernandez, R.R. Hare don’t care! Consumption of a rare desert milkweed containing phytochemicals by the black-tailed jackrabbit. J. Arid Environ. 2018, 174, 103991. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Hoffacker, M.K.; Murphy-Mariscal, M.L.; Wu, G.C.; Allen, M.F. Solar energy development and land cover change. Proc. Natl. Acad. Sci. USA 2015, 112, 13579–13584. [Google Scholar] [CrossRef] [Green Version]
- Leong, K.L.H.; Yoshimura, M.A.; Kaya, H.K. Occurrence of a neogregarine protozoan, Ophryocystis elektroscirrha McLaughlin and Myers, in populations of monarch and queen butterflies. Pan-Pac. Entomol. 1997, 73, 49–51. [Google Scholar]
- Barriga, P.A.; Sternberg, E.D.; Lefèvre, T.; de Roode, J.C.; Altizer, S. Occurrence and host specificity of a neogregarine protozoan in four milkweed butterfly hosts (Danaus spp.). J. Invertebr. Pathol. 2016, 140, 75–82. [Google Scholar] [CrossRef] [Green Version]


| Location | Specimen Based Records | Observation Based Records | Total Data Records |
|---|---|---|---|
| US | 182 | 556 | 738 |
| Baja Ca | 53 | 13 | 66 |
| Baja Ca Sur | 96 | 15 | 111 |
| Sonora | 12 | 9 | 21 |
| Sinaloa | 5 | 67 | 72 |
| Western Mex. Subtotal | 166 | 104 | 270 |
| Total | 348 | 660 | 1008 |
 Host plant used by queen butterflies |  Found in desert |  Larval host plant |  Nectar plant |  Desert distribution in Arizona (AZ), California (CA), Nevada (NV), Utah (UT) and Mexico (Mex) |
|---|---|---|---|---|
| Apocynaceae | ||||
| Asclepias albicans S. Watson | Y [58,59] | Y [29,59,60] | Y [58] | AZ: La Paz, Maricopa, Pinal, Yuma [29,61] CA: Imperial, Riverside, San Bernardino, San Diego; Mex: Baja, CA |
| Asclepias angustifolia (Schweigg) | Y [62] | AZ: Cochise, Pima, Santa Cruz; Mex [58,61] | ||
| Asclepias asperula (Decne.) Woodson | Y [58,60] | AZ: all counties except Yuma [29,61]; CA: Riverside, San Bernardino; NV [63]; Mex | ||
| Asclepias curassavica | Y [29,40] | AZ: Pima; CA: Los Angeles, Orange, San Diego [29,64] | ||
| Asclepias cutleri Woodson | Y | AZ: Apache, Coconino, Navajo; UT [58,61] | ||
| Asclepias erosaTorr. | Y [61] | Y [29,58] | AZ: Coconino, La Paz, Mohave, Yuma [61,65]; CA: Imperial, Inyo, Riverside, San Bernardino, San Diego [66]; NV; Mex: Baja CA, Sonora | |
| Asclepias fascicularis Decne. | Y | Y [34,40,58] | AZ: Pima [65,67]; CA: Inyo, Los Angeles, Riverside, San Bernardino, San Diego [68]; Mex: Baja CA | |
| Asclepias involucrata Engelmann ex Torrey /macrosperma | Y [61] | AZ: Apache, Cochise, Coconino, Graham, Mohave, Navajo, Pima, Santa Cruz, Yavapai [58,61]; Mex | ||
| Asclepias latifolia (Torr.) Raf. | Y [6] | AZ: Apache, Cochise, Coconino Greenlee, Mohave, Navajo, Yavapai [58,61]; CA: Inyo [69,70], San Bernardino; UT [58] | ||
| Asclepias linaria Cavanilles | Y | Y [71,72] | Y | AZ: Cochise, Gila, Graham, Greenlee, Maricopa, Pima, Pinal, Santa Cruz [72], Yavapai [58,61,63,65,67,71]; Mex |
| Asclepias nytaginifolia A. Gray | Y [58,61] | Y [24] | AZ: Apache, Cochise, Coconino, Gila, Graham, La Paz, Maricopa, Mohave, Navajo, Pima, Santa Cruz, Yavapai, Yuma [29,61,73,74]; CA: San Bernardino; NV [73]; Mex: Sonora, Baja CA | |
| Asclepias speciosa Torrey | Y | Y [74] | AZ: Apache, Coconino, Gila, Greenlee, Navajo [61,63]; CA: Inyo [70,75], NV: Clark, Nye | |
| Asclepias subulata Decne. | Y [58,61] | Y [59,76,77] | Y [76] | AZ; Gila, Pinal, Maricopa, Mojave, Yuma [58,61] CA: Imperial, Riverside, San Bernardino, San Diego; CA [78]; NV; Mex: Baja CA, Sonora |
| Asclepias vestita Hook & Arn. | Y | CA: Inyo, Los Angeles [70], San Bernardino [79] | ||
| Funastrum cynanchoides (Decne.) Schltr. | Y [21,76] | Y [80] | Y | AZ: Cochise, Coconino, Graham, Pima, Pinal, Santa Cruz, Yavapai [61]; CA: Imperial, Orange, Riverside, San Bernardino, San Diego; Mex |
| Funastrum cynanchoides ssp hartwegii (Vail) R. Holm | Y | Y [80] | CA: Imperial, Riverside, San Bernardino, San Diego; UT; Mex | |
| Funastrum hirtellum(A. Gray) Schltr. | Y [61] | Y [29] | AZ: Coconino, La Paz, Mohave [61,67]; CA: Inyo, Imperial, Riverside, San Bernardino, San Diego; NV | |
| Funastrum utahense (Engelm.) Liede & Meve | Y [61,77] | Y | AZ: La Paz, Mohave [67]; CA: Imperial, Riverside, San Bernardino, San Diego; NV |
 Nectar plant used by queen butterfly containing PAs |  Found in desert |  Larval host plant |  Nectar plant |  Distribution in Arizona (AZ), California (CA), Nevada (NV), and Utah (UT) and Mexico (Mex) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Asteraceae | |||||||||
| Ageratina [81,82] (Eupatorium) herbacea (S. Gray) R. M. King & H. Rob. PAs | Y | Y other spp [65] | AZ: Pima; CA: San Bernardino [83] | ||||||
| Senecio† [70,81,83,84] faccidus Less. Var. douglasii (D.C.) B. L. Turner & T. Barkley PAs | Y | Y [85], Figure S1 | CA: Inyo, Orange, Riverside, San Bernardino, San Diego [86]; Mex: Baja Ca | ||||||
| Senecio † [70,81] faccidus var. monoensis (Greene) B. L. Turner & T. M. Barkley PAs | Y [77] | Y [86], Figure S1 | AZ, CA: Inyo, Mono; Riverside, San Bernardino, San Diego [87]; Mex: Baja Ca | ||||||
| Chromolaena odorata (L.) R. M. King & H. Rob. [58,82,88] PAs | Y [65] | Mex [61] | |||||||
| Boraginaceae | |||||||||
| Amsinkia† [70,81,85,89] tessellata A. Gray PAs | Y [80] | † | CA: Imperial, Inyo, Riverside, San Bernardino [77], San Diego | ||||||
| Amsinkia† [70,81,85] tessellata A. Gray var. tessellata PAs | Y | † | AZ; CA: Imperial, Inyo, Riverside, San Bernardino [77], San Diego [45]; Mex: Baja Ca | ||||||
| Cryptantha [81] angustifolia (Torr.) Greene PAs | Y | † | AZ: La Paz, Maricopa, Mohave, Pima, Yuma [63]; CA; Imperial, Inyo, Riverside, San Bern.; NV: Clark, Nye | ||||||
| Cryptantha [81] nevadensis A. Nelson & P.B. Kenn. PAs | Y | † | AZ: Coconino, La Paz, Mohave, Maricopa, Pima, Yuma [63]; CA: Kern, Inyo, Imperial, Los Angeles, Riverside, San Bernardino, San Diego, Santa Barbara; NV: Clark, Nye, UT: Washington | ||||||
| Cryptantha [81] utahensis (A. Gray) Greene PAs | Y | † | AZ: Maricopa, Mohave [63]; CA: Inyo, Imperial, Riverside, San Bernardino; NV: Clark, Nye; UT: Washington | ||||||
| Heliotropium [70,81,85] curassavicum L. var. oculatum (A. Heller) I.M. Johnst. ex Tidestr. PAs | Y | Y [9] | CA: Imperial, Inyo, Orange, Riverside, San Bernardino, San Diego [90]; NV; Mex [33] | ||||||
| Helitropium spp. | Y | Y † | CA: Riverside, San Bernardino, Inyo [90] | ||||||
| Tournefortia [81] floribunda PAs (related to Heliotropium) | Y | Mex: Mexicali [24] | |||||||
| Apocynaceae | |||||||||
| Nerium oleander * L. PAs Naturalized, * not native | Y | Y [29,58] | CA: Los Angeles, Orange, Riverside, San Bernardino [91], San Diego [91] | ||||||
| Matelea parvifolia (Torr.) Woodson [70] PAs (Rare) | Y | Y [70] | CA: Riverside, San Bernardino, San Diego [92] | ||||||
| Euphorbiaceae | |||||||||
| Croton† californicus [93] Müll. Arg. Possibly glutarimide alkaloids & sesquiterpene guaiane-type alkaloids with antibiotic properties [94] | Y | Y [70] † | CA: Inyo, Kern, Los Angeles, Orange, Riverside, San Bernardino, San Diego [95] | ||||||
| Lamiaceae | |||||||||
| Condea emoryi (Torr.) Harley & J.F. B. Pastore Triterpenoids, butelinic acid which have anti-tumor, anti-inflammatory, antimalarial properties [60] | Y [18] | Y [18,38] | AZ [67]; CA: Imperial, Riverside, San Bernardino, San Diego [95]; Mex: Ensenada, Mexicali | ||||||
 Nectar host plant used by queen butterfly |  Found in desert |  Larval host plant |  Nectar plant | 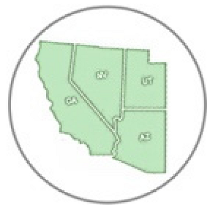 Distribution in Arizona (AZ), California (CA), Nevada (NV), Utah (UT) and Mexico (Mex) |
|---|---|---|---|---|
| Asteraceae | ||||
| Acamptoppaus sphaeorcephalus (Harv. & A. Gray) A. Gray | Y | Y [57] | AZ: Coconino, Gila, Graham, La Paz, Maricopa, Mohave, Pima, Pinal, Yavapai [67]; CA: Imperial, Inyo, Kern, Los Angeles, Riverside, San Bernardino, San Diego [95]; NV; Clark, Lincoln; UT: Washington, Kane, San Juan | |
| Baileya multiradiata [96] Harv. & A. Gray ex Torr. | Y | Y [96] | AZ: Pima; CA: Inyo, Los Angeles, Orange, Riverside, San Bernardino, San Diego [95] | |
| Cirsium mohavensis (Greene) Petr. | Y [97] | Y [70,98] | AZ 41; CA: Inyo [70,97,98] | |
| Heliomeris longifolia [Figure 3] (Robbins. & Greenm.) Cockerell | Y [57] | Y (Figure 3) | AZ: Cochise, Pima [57], Santa Cruz; NV, UT, Mex: central | |
| Palafoxia arida B. L. Turner & M. I. Morris | Y [32] | Y [32] | CA: Imperial Inyo, Riverside, San Bernardino, San Diego; Mex: Sonora [32] | |
| Ericameria (Chrysothamnus) nauseosa (Pursh) G. L. Nesom & G. I. Baird var mohavensis | Y | Y | CA: Inyo, Los Angeles, Orange, Riverside, San Bernardino [37], [99] | |
| Helianthus annuus L. | Y [100] | Y (Figure 3) | CA: Imperial, Inyo, Los Angeles, Orange, Riverside, San Diego [99] | |
| Verbesina encelioides * (Cav.) Benth, & Hook f. ex A. Gray * Not native | Y | Y | CA: Orange, Los Angeles, Riverside, San Bernardino, San Diego | |
| Senecio flaccidus Less. monoensis or douglasii † PAs | Y † [42,100] | Y † [27] | CA: Inyo, Kern, Los Angeles, Riverside, San Bernardino, San Diego [86,87] | |
| Xanthisma spinulosum (Pursh) D. R. Morgan & R. L. Hartm. | Y [100] | Y [57] | AZ [100]; CA: Riverside, San Bernardino [45], NV; UT; Mex | |
| Lamiaceae | ||||
| Condea emoryi (Torr.) Harley & J.F. B. Pastore | Y [77] | Y [18] | AZ; CA: Imperial, Riverside, San Bernardino, San Diego [100]; Mex: Ensenada, Mexicali [99] | |
| Fabaceae | ||||
| Parkinsonia florida (A. Gray) S. Watson | Y | Y | AZ [25]; CA: Imperial [101,102], Riverside, San Bernardino, San Diego [39]; Mex: northwest | |
| Verbenaceae | ||||
| Phyla lanceolata (Michx.) Greene | Y | Y [24] | CA: Inyo, Los Angeles, Orange, San Bernardino, San Diego | |
 Queen movement |  Found in desert |  Larval host plant |  Nectar plant | 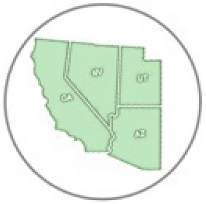 Distribution in Arizona (AZ), California (CA), Nevada (NV), Utah (UT) and Mex |
| Danaus gilippus thersippus Long distance movement documented by tagging [S2] 1. AZ to CA: 598.85 km 2. AZ to CA: 671.42 km 3. AZ to CA: 1404.31 km | Y | See above | AZ: Cochise, Coconino, Maricopa, Mohave, Pima, Santa Cruz (SI) [67]; NV: Clark, Nye; CA: Imperial, Orange, Riverside, San Diego, San Bernardino; UT: Washington [S-2]; Mex: (SI) Baja Ca |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saul-Gershenz, L.; Grodsky, S.M.; Hernandez, R.R. Ecology of the Western Queen Butterfly Danaus gilippus thersippus (Lepidoptera: Nymphalidae) in the Mojave and Sonoran Deserts. Insects 2020, 11, 315. https://doi.org/10.3390/insects11050315
Saul-Gershenz L, Grodsky SM, Hernandez RR. Ecology of the Western Queen Butterfly Danaus gilippus thersippus (Lepidoptera: Nymphalidae) in the Mojave and Sonoran Deserts. Insects. 2020; 11(5):315. https://doi.org/10.3390/insects11050315
Chicago/Turabian StyleSaul-Gershenz, Leslie, Steven M. Grodsky, and Rebecca R. Hernandez. 2020. "Ecology of the Western Queen Butterfly Danaus gilippus thersippus (Lepidoptera: Nymphalidae) in the Mojave and Sonoran Deserts" Insects 11, no. 5: 315. https://doi.org/10.3390/insects11050315