Multiscale Determinants Drive Parasitization of Drosophilidae by Hymenopteran Parasitoids in Agricultural Landscapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Biological Sampling and Explanatory Variables
2.3. Laboratory Work
2.4. Data Analyses
3. Results
3.1. Faunistic Surveys
3.2. Factors Affecting Parasitoids
3.2.1. Influence of Landscape Versus Local Variables on Community of Parasitoids and Drosophilids
3.2.2. Influence of Habitat and Microhabitat Types on Community and on Single Species of Parasitoids
3.2.3. Host Preference of Parasitoid Species in Multi-Species Baited Delta-Traps
4. Discussion
4.1. Species Composition in Zurich and Ticino
4.2. Influence of Landscape Versus Local Variables on Community of Parasitoids and Drosophilids
4.3. Influence of Habitat and Microhabitat Types on Community and on Single Species of Parasitoids
4.4. Host Preference of Parasitoid Species in Multi-Species Baited Delta-Traps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quicke, D.L. Parasitic Wasps; Chapman & Hall Ltd: New York, NY, USA, 1997; p. 485. [Google Scholar]
- Clarke, C.W.; Calatayud, P.A.; Sforza, R.F.; Ndemah, R.N.; Nyamukondiwa, C. Parasitoids’ Ecology and Evolution. Front. Ecol. Evol. 2019, 7, 485. [Google Scholar] [CrossRef] [Green Version]
- Thierry, M.; Hrček, J.; Lewis, O.T. Mechanisms structuring host–parasitoid networks in a global warming context: A review. Ecol. Entomol. 2019, 44, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Fleury, F.; Ris, N.; Allemand, R.; Fouillet, P.; Carton, Y.; Boulétreau, M. Ecological and genetic interactions in Drosophila-parasitoids communities: A case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in south-eastern France. In Drosophila Melanogaster, Drosophila Simulans: So Similar, so Different; Capy, P., Gibert, P., Boussy, I., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 11, pp. 181–194. [Google Scholar] [CrossRef]
- Gillespie, M.A.; Gurr, G.M.; Wratten, S.D. Beyond nectar provision: The other resource requirements of parasitoid biological control agents. Entomol. Exp. Appl. 2016, 159, 207–221. [Google Scholar] [CrossRef]
- Bianchi, F.J.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Veres, A.; Petit, S.; Conord, C.; Lavigne, C. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ. 2013, 166, 110–117. [Google Scholar] [CrossRef]
- Rusch, A.; Bommarco, R.; Ekbom, B. Conservation biological control in agricultural landscapes. Adv. Bot. Res. 2017, 81, 333–360. [Google Scholar] [CrossRef]
- Lavandero, B.; Wratten, S.; Shishehbor, P.; Worner, S. Enhancing the effectiveness of the parasitoid Diadegma semiclausum (Helen): Movement after use of nectar in the field. Biol. Control 2005, 34, 152–158. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Wratten, S.D. Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 2004, 85, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Tscharntke, T.; Karp, D.S.; Chaplin-Kramer, R.; Batáry, P.; DeClerck, F.; Gratton, C.; Hunt, L.; Ives, A.; Jonsson, M.; Larsen, A.; et al. When natural habitat fails to enhance biological pest control—Five hypotheses. Biol. Conserv. 2016, 204, 449–458. [Google Scholar] [CrossRef] [Green Version]
- De Ros, G.; Anfora, G.; Grassi, A.; Ioriatti, C. The potential economic impact of Drosophila suzukii on small fruits production in Trentino (Italy). IOBC-WPRS Bull. 2013, 91, 317–321. [Google Scholar]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; De Toni, D.C.; Valente, V.L. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Kenis, M.; Tonina, L.; Eschen, R.; van der Sluis, B.; Sancassani, M.; Mori, N.; Haye, T.; Helsen, H. Non-crop plants used as hosts by Drosophila suzukii in Europe. J. Pest Sci. 2016, 89, 735–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyet, M.; Le Roux, V.; Gibert, P.; Meirland, A.; Prevost, G.; Eslin, P.; Chabrerie, O. The wide potential trophic niche of the Asiatic fruit fly Drosophila suzukii: The key of its invasion success in temperate Europe? PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, S.; Boycheva-Woltering, S.; Romeis, J.; Collatz, J. Trichopria drosophilae parasitizes Drosophila suzukii in seven common non-crop fruits. J. Pest Sci. 2020, 93, 627–638. [Google Scholar] [CrossRef]
- Tait, G.; Grassi, A.; Pfab, F.; Crava, C.M.; Dalton, D.T.; Magarey, R.; Ometto, L.; Vezzulli, S.; Rossi-Stacconi, M.V.; Gottardello, A.; et al. Large-scale spatial dynamics of Drosophila suzukii in Trentino, Italy. J. Pest Sci. 2018, 91, 1213–1224. [Google Scholar] [CrossRef]
- Bruck, D.J.; Bolda, M.; Tanigoshi, L.; Klick, J.; Kleiber, J.; DeFrancesco, J.; Gerdeman, B.; Spitler, H. Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag. Sci. 2011, 67, 1375–1385. [Google Scholar] [CrossRef]
- Pelton, E.; Gratton, C.; Isaacs, R.; Van Timmeren, S.; Blanton, A.; Guédot, C. Earlier activity of Drosophila suzukii in high woodland landscapes but relative abundance is unaffected. J. Pest Sci. 2016, 89, 725–733. [Google Scholar] [CrossRef]
- Cahenzli, F.; Bühlmann, I.; Daniel, C.; Fahrentrapp, J. The distance between forests and crops affects the abundance of Drosophila suzukii during fruit ripening, but not during harvest. Environ. Entomol. 2018, 47, 1274–1279. [Google Scholar] [CrossRef]
- Leach, H.; Moses, J.; Hanson, E.; Fanning, P.; Isaacs, R. Rapid harvest schedules and fruit removal as non-chemical approaches for managing spotted wing Drosophila. J. Pest Sci. 2018, 91, 219–226. [Google Scholar] [CrossRef]
- Haro-Barchin, E.; Scheper, J.; Ganuza, C.; De Groot, G.A.; Colombari, F.; van Kats, R.; Kleijn, D. Landscape-scale forest cover increases the abundance of Drosophila suzukii and parasitoid wasps. Basic Appl. Ecol. 2018, 31, 33–43. [Google Scholar] [CrossRef]
- Santoiemma, G.; Mori, N.; Tonina, L.; Marini, L. Semi-natural habitats boost Drosophila suzukii populations and crop damage in sweet cherry. Agric. Ecosyst. Environ. 2018, 257, 152–158. [Google Scholar] [CrossRef]
- Carton, Y.; Boulétreau, M.; van Alphen, J.J.M.; van Lenteren, J.C. The Drosophila parasitic wasps. In The Genetics and Biology of Drosophila; Ashburner, M., Carson, H.L., Thompson, J.N.J., Eds.; Academic Press: London, UK, 1986; Volume 3, pp. 347–394. [Google Scholar]
- Miller, B.; Anfora, G.; Buffington, M.; Daane, K.M.; Dalton, D.T.; Hoelmer, K.M.; Rossi Stacconi, M.V.; Grassi, A.; Ioriatti, C.; Loni, A.; et al. Seasonal occurrence of resident parasitoids associated with Drosophila suzukii in two small fruit production regions of Italy and the USA. Bull. Insectol. 2015, 68, 255–263. [Google Scholar]
- Mazzetto, F.; Marchetti, E.; Amiresmaeili, N.; Sacco, D.; Francati, S.; Jucker, C.; Dindo, M.L.; Lupi, D.; Tavella, L. Drosophila parasitoids in northern Italy and their potential to attack the exotic pest Drosophila suzukii. J. Pest Sci. 2016, 89, 837–850. [Google Scholar] [CrossRef]
- Gabarra, R.; Riudavets, J.; Rodríguez, G.A.; Pujade-Villar, J.; Arnó, J. Prospects for the biological control of Drosophila suzukii. BioControl 2015, 60, 331–339. [Google Scholar] [CrossRef]
- Knoll, V.; Ellenbroek, T.; Romeis, J.; Collatz, J. Seasonal and regional presence of hymenopteran parasitoids of Drosophila in Switzerland and their ability to parasitize the invasive Drosophila suzukii. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Kremmer, L.; Thaon, M.; Borowiec, N.; David, J.; Poirié, M.; Gatti, J.L.; Ris, N. Field monitoring of Drosophila suzukii and associated communities in south eastern France as a pre-requisite for classical biological control. Insects 2017, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Chabert, S.; Allemand, R.; Poyet, M.; Eslin, P.; Gibert, P. Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol. Control 2012, 63, 40–47. [Google Scholar] [CrossRef]
- Iacovone, A.; Ris, N.; Poirié, M.; Gatti, J.-L. Time-course analysis of Drosophila suzukii interaction with endoparasitoid wasps evidences a delayed encapsulation response compared to D. melanogaster. PLoS ONE 2018, 13, e0201573. [Google Scholar] [CrossRef] [Green Version]
- Rossi Stacconi, M.V.; Grassi, A.; Dalton, D.T.; Miller, B.; Ouantar, M.; Loni, A.; Ioriatti, C.; Walton, V.M.; Anfora, G. First field records of Pachycrepoideus vindemiae as a parasitoid of Drosophila suzukii in European and Oregon small fruit production areas. Entomologia 2013, 1, e3. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Panel, A.; Baser, N.; Ioriatti, C.; Pantezzi, T.; Anfora, G. Comparative life history traits of indigenous Italian parasitoids of Drosophila suzukii and their effectiveness at different temperatures. Biol. Control 2017, 112, 20–27. [Google Scholar] [CrossRef]
- Amiresmaeili, N.; Jucker, C.; Savoldelli, S.; Lupi, D. Understanding Trichopria drosophilae performance in laboratory conditions. Bull. Insectol. 2018, 71, 251–256. [Google Scholar]
- Wang, X.G.; Serrato, M.A.; Son, Y.; Walton, V.M.; Hogg, B.N.; Daane, K.M. Thermal performance of two indigenous pupal parasitoids attacking the invasive Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2018, 47, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgini, M.; Wang, X.G.; Wang, Y.; Chen, F.S.; Hougardy, E.; Zhang, H.M.; Chen, Z.Q.; Chen, H.Y.; Liu, C.X.; Cascone, P.; et al. Exploration for native parasitoids of Drosophila suzukii in China reveals a diversity of parasitoid species and narrow host range of the dominant parasitoid. J. Pest Sci. 2019, 92, 509–522. [Google Scholar] [CrossRef]
- Lue, C.H.; Borowy, D.; Buffington, M.L.; Leips, J. Geographic and seasonal variation in species diversity and community composition of frugivorous Drosophila (Diptera: Drosophilidae) and their Leptopilina (Hymenoptera: Figitidae) parasitoids. Environ. Entomol. 2018, 47, 1096–1106. [Google Scholar] [CrossRef]
- Spinedi, F.; Isotta, F. Il clima del Ticino. Dati Statistiche E Società 2004, 2, 5–39. [Google Scholar]
- Weber, M.; Sorg, L.; Flury, C. Landwirtschaft und Landschaft im Kanton Zürich. Handlungsbedarf für die Kantonale Politik; Technical Report; Amt für Landschaft und Natur: Zürich, Switzerland, 2014. [Google Scholar]
- DWD. Deutscher Wetterdiens. Available online: https://www.dwd.de/DE/leistungen/klimadatenwelt/europa/rs/schweiz/schweiz_node.html (accessed on 6 April 2020).
- McGarigal, K.; Cushman, S.A.; Neel, M.C.; Ene, E. FRAGSTATS: Spatial Pattern Analysis Program for Categorical Maps. In Documentation of the Computer Software Program; University of Massachusetts: Amherst, MA, USA, 2002; p. 182. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 29 May 2020).
- Lee, J.C.; Dreves, A.J.; Cave, A.M.; Kawai, S.; Isaacs, R.; Miller, J.C.; van Timmeren, S.; Bruck, D.J. Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann. Entomol. Soc. Am. 2015, 108, 117–129. [Google Scholar] [CrossRef]
- Perkins, R.C.L. Hymenoptera (supplement). In Fauna Hawaiiensis; Sharp, D., Ed.; Cambridge University Press: Cambridge, UK, 1910; pp. 600–686. [Google Scholar]
- Nixon, G.E.J. Diapriidae (Diapriinae) Hymenoptera, Proctotrupoidea. In Handbooks for the Identification of British Insects; Fitton, M.G., Ed.; Royal Entomological Society: London, UK, 1980; Volume 8, p. 55. [Google Scholar]
- Graham, M.W.R.D.V. The Pteromalidae of north-western Europe (Hymenoptera-Chalcidoidea). Bull. Br. Mus. Nat. Hist. Entomol. 1969, 16, 1–908. [Google Scholar]
- Forshage, M.; Nordlander, G. Identification key to European genera of Eucoilinae (Hymenoptera, Cynipoidea, Figitidae). Insect Syst. Evol. 2008, 39, 341–359. [Google Scholar] [CrossRef]
- Lue, C.H.; Driskell, A.C.; Leips, J.; Buffington, M.L. Review of the genus Leptopilina (Hymenoptera, Cynipoidea, Figitidae, Eucoilinae) from the Eastern United States, including three newly described species. J. Hymenopt. Res. 2016, 53, 35–76. [Google Scholar] [CrossRef]
- Nordlander, G. Revision of the genus Leptopilina Förster, 1869, with notes on the status of some other genera (Hymenoptera, Cynipoidea: Eucoitidae). Insect Syst. Evol. 1980, 11, 428–453. [Google Scholar] [CrossRef]
- Bächli, G.; Viljoen, F.; Escher, S.A.; Saura, A. The Drosophilidae (Diptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 2005; Volume 39, p. 362. [Google Scholar]
- Dray, S.; Blanchet, F.G.; Legendre, P. Packfor: Forward Selection with Permutation (Canoco p. 46). Version 0.0-8/r109. 2013. Available online: http://R-Forge.R-project.org/projects/sedar (accessed on 29 May 2020).
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Bray, J.R.; Curti, J.T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Anderson, M.; Ter Braak, C. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; ISBN 3-900051-07-0. Available online: https://www.r-project.org/ (accessed on 29 May 2020).
- Patot, S.; Martinez, J.; Allemand, R.; Gandon, S.; Varaldi, J.; Fleury, F. Prevalence of a virus inducing behavioural manipulation near species range border. Mol. Ecol. 2010, 19, 2995–3007. [Google Scholar] [CrossRef]
- De Jong, Y.; Verbeek, M.; Michelsen, V.; de Place Bjørn, P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—All European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collatz, J.; Agroscope, Zurich, Switzerland. Personal communication, 2019.
- Shorrocks, B. An ecological classification of European Drosophila species. Oecologia 1977, 26, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Cooke, B.; Roland, J. Spatial analysis of large-scale patterns of forest tent caterpillar outbreaks. Ecoscience 2000, 7, 410–422. [Google Scholar] [CrossRef]
- Tscharntke, T.; Brandl, R. Plant-insect interactions in fragmented landscapes. Annu. Rev. Entomol. 2004, 49, 405–430. [Google Scholar] [CrossRef]
- Anderson, R.M.; Dallar, N.M.; Pirtel, N.L.; Connors, C.J.; Mickley, J.; Bagchi, R.; Singer, M.S. Bottom-Up and top-down effects of forest fragmentation differ between dietary generalist and specialist caterpillars. Front. Ecol. Evol. 2019, 7, 452. [Google Scholar] [CrossRef] [Green Version]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martínez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef] [Green Version]
- Boccaccio, L.; Petacchi, R. Landscape effects on the complex of Bactrocera oleae parasitoids and implications for conservation biological control. BioControl 2009, 54, 607–616. [Google Scholar] [CrossRef]
- Peters, R.S. New habitat and host records and notes on the life history of Pachycrepoideus vindemmiae (Rondani, 1875). Mitt. Hamb. Zool. Mus. Inst. 2009, 106, 39–49. [Google Scholar]
- Van Alphen, J.J.M.; Janssen, A.R.M. Host selection by Asobara tabida Nees (Braconidae; Alysiinae) a larval parasitoid of fruit inhabiting Drosophila species, 2: Host species selection. Neth. J. Zool. 1982, 32, 194–214. [Google Scholar] [CrossRef]
- Janssen, A. Optimal host selection by Drosophila parasitoids in the field. Funct. Ecol. 1989, 469–479. [Google Scholar] [CrossRef]
- Janssen, A.; Driessen, G.; De Haan, M.; Roodbol, N. The impact of parasitoids on natural populations of temperate woodland Drosophila. Neth. J. Zool. 1988, 38, 61–73. [Google Scholar] [CrossRef]
- Woltz, J.M.; Lee, J.C. Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol. Control 2017, 110, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Boycheva Woltering, S.; Romeis, J.; Collatz, J. Influence of the rearing host on biological parameters of Trichopria drosophilae, a potential biological control agent of Drosophila suzukii. Insects 2019, 10, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi Stacconi, M.V.; Grassi, A.; Ioriatti, C.; Anfora, G. Augmentative releases of Trichopria drosophilae for the suppression of early season Drosophila suzukii populations. BioControl 2019, 64, 9–19. [Google Scholar] [CrossRef]
| Canton 1 | Locality | Site Code | Landscape Type 2 | Lat. [N]/Lon. [E] 3 | Elevation [m a.s.l.] |
|---|---|---|---|---|---|
| TI | Vezia | 1-Vezi | Homo | 46°01′16″/8°55′55″ | 334.5 |
| TI | Giornico | 2-Gior | Homo | 46°23′48″/8°52′36″ | 377.3 |
| TI | Contone | 3-Cont | Hete | 46°08′54″/8°55′47″ | 211.4 |
| TI | Arbedo | 4-Arbe | Hete | 46°13′11″/9°03′13″ | 264.0 |
| TI | Davesco | 5-Dave | Hete | 46°01′48″/8°58′30″ | 377.9 |
| TI | Corteglia | 6-Cort | Homo | 45°51′51″/8°59′36″ | 426.4 |
| TI | Mezzana | 7-Mezz | Hete | 45°51′08″/8°59′58″ | 327.1 |
| TI | Stabio | 8-Stab | Homo | 45°51′13″/8°55′36″ | 409.4 |
| TI | Gordola | 9-Gord | Homo | 46°10′53″/8°52′12″ | 216.1 |
| TI | Sementina | 10-Seme | Homo | 46°10′52″/8°58′27″ | 374.9 |
| TI | Malvaglia | 11-Malv | Homo | 46°24′34″/8°59′01″ | 429.9 |
| TI | Novazzano | 12-Nova | Hete | 45°50′41″/8°57′57″ | 378.0 |
| TI | Sessa | 13-Sess | Homo | 46°00′30″/8°49′46″ | 527.3 |
| TI | Monteggio | 14-Mont | Hete | 45°59′44″/8°48′51″ | 408.8 |
| TI | Biasca | 15-Bias | Hete | 46°20′47″/8°58′10″ | 283.5 |
| TI | Giubiasco | 16-Giub | Hete | 46°09′49″/8°58′48″ | 211.4 |
| ZH | Reckenholz | 17-Reck | - | 47°25′45″/8°31′51″ | 442 |
| ZH | Waidhof | 18-Waid | - | 47°25′23″/8/31′36″ | 457 |
| ZH | Seebach | 19-Seeb | - | 47°25′29″/8°31′60″ | 436 |
| ZH | Rieder | 20-Ried | - | 47°25′50″/8°31′40″ | 447 |
| ZH | Rümlang | 21-Ruml | - | 47°26′10″/8°32′15″ | 463 |
| ZH | Bahn | 22-Bahn | - | 47°25′08″/8°30′59″ | 456 |
| ZH | Glaubten | 23-Glau | - | 47°24′35″/8°31′18″ | 495 |
| ZH | Buchegg | 24-Buch | - | 47°24′09″/8°31′49″ | 478 |
| Spatial Scale | Response Variables | Explanatory Variables | Analyses 1 |
|---|---|---|---|
| Landscape (500 m radius) | Parasitoid and drosophilid communities | % crop cover % woody cover Crop patch complexity Woody patch complexity | Community level |
| Local (100 m radius) | Parasitoid and drosophilid communities | % vineyards % berries Presence wild-berries Presence wild trees Drosophilid species | Community level |
| Habitat (<50 m radius) | Parasitoid communities and single species of parasitoids | Habitat type (crop, woody, ecotone) period | Community and Single species level |
| Microhabitat (<0.2 m radius) | Parasitoid communities and single species of parasitoids | Microhabitat type (ground, canopy) period | Community and Single species level |
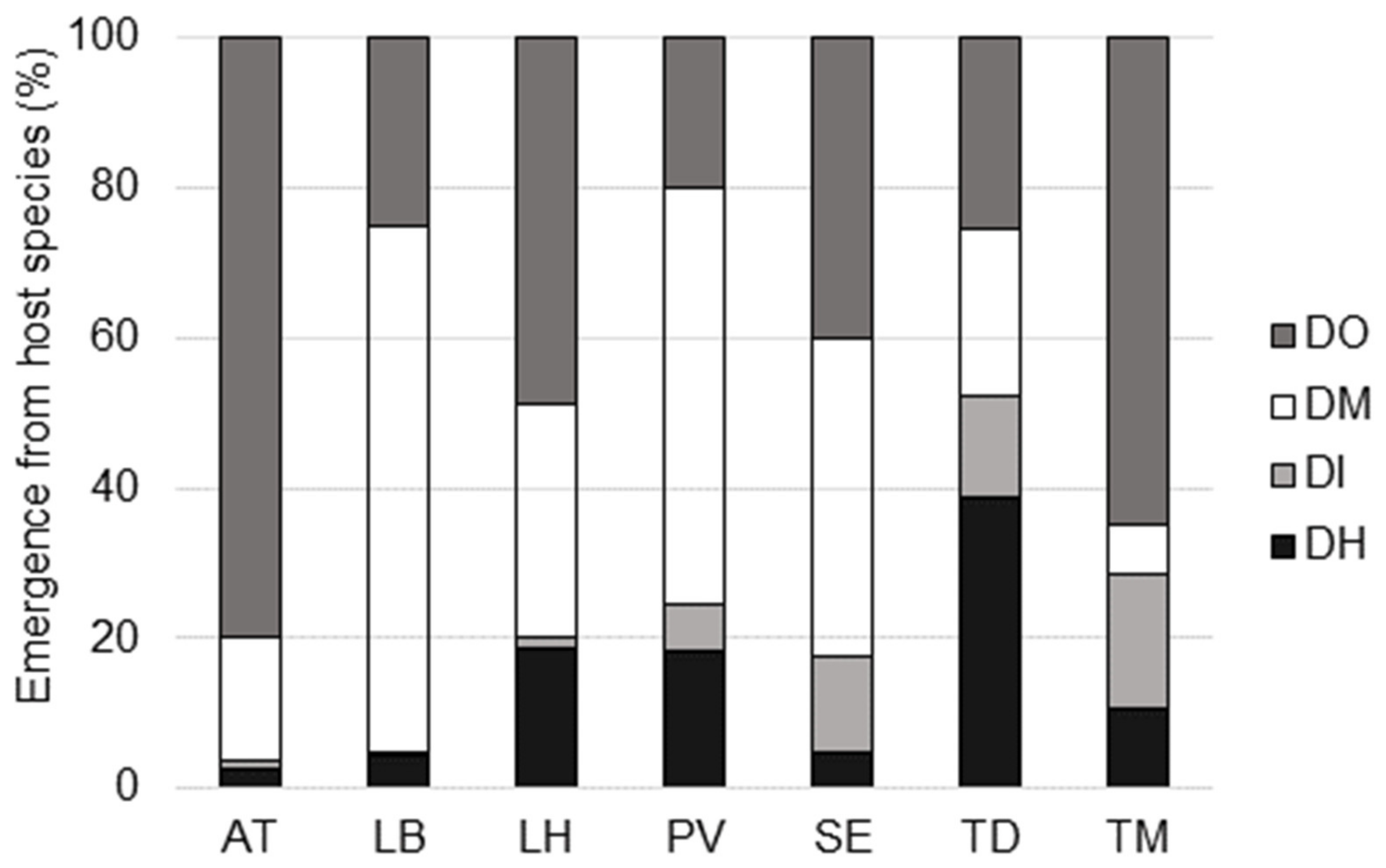
| Within Delta-trap (Host preference) | Single species of parasitoids | Host species (DH, DI, DM, DO) | Single species level |
| Species | Host Stage Parasitized | Ticino | Zurich | ||||
|---|---|---|---|---|---|---|---|
| # Traps 1 | # Ind. 2 | # Sites 3 | # Traps | # Ind. | # Sites | ||
| Braconidae | |||||||
| Asobara tabida | larva | 8 | 34 | 4 | 11 | 119 | 7 |
| Figitidae | |||||||
| Leptopilina boulardi | larva | 17 | 649 | 8 | - | - | - |
| Leptopilina heterotoma | larva | 16 | 426 | 11 | 83 | 5316 | 8 |
| Diapriidae | |||||||
| Trichopria drosophilae | pupa | 44 | 926 | 13 | 1 | 24 | 1 |
| Trichopria modesta | pupa | - | - | - | 21 | 204 | 8 |
| Pteromalidae | |||||||
| Pachycrepoideus vindemmiae | pupa | 27 | 646 | 13 | 16 | 289 | 8 |
| Spalangia erythromera | pupa | 3 | 13 | 3 | 5 | 27 | 4 |
| Vrestovia brevior | pupa | 1 | 4 | 1 | - | - | - |
| Environmental Variables | Parasitoids | Drosophilids | ||
|---|---|---|---|---|
| R2adj a | p Value b | R2adj a | p Value b | |
| Landscape level | ||||
| crop_500 | - | ns | - | ns |
| woody_500 | - | ns | - | ns |
| PD_crop_500 | - | ns | - | ns |
| PD_woody_500 | - | ns | - | ns |
| Total | 23% | ns | 34% | . |
| Local level (potential plant hosts of drosophilids) | ||||
| vineyard_100 | - | ns | - | ns |
| berries_100 | - | ns | - | ns |
| berries_wild | - | ns | - | ns |
| tree_wild | - | ns | - | ns |
| Total | 20% | ns | 27% | ns |
| Local level (potential drosophilid hosts of parasitoids) | ||||
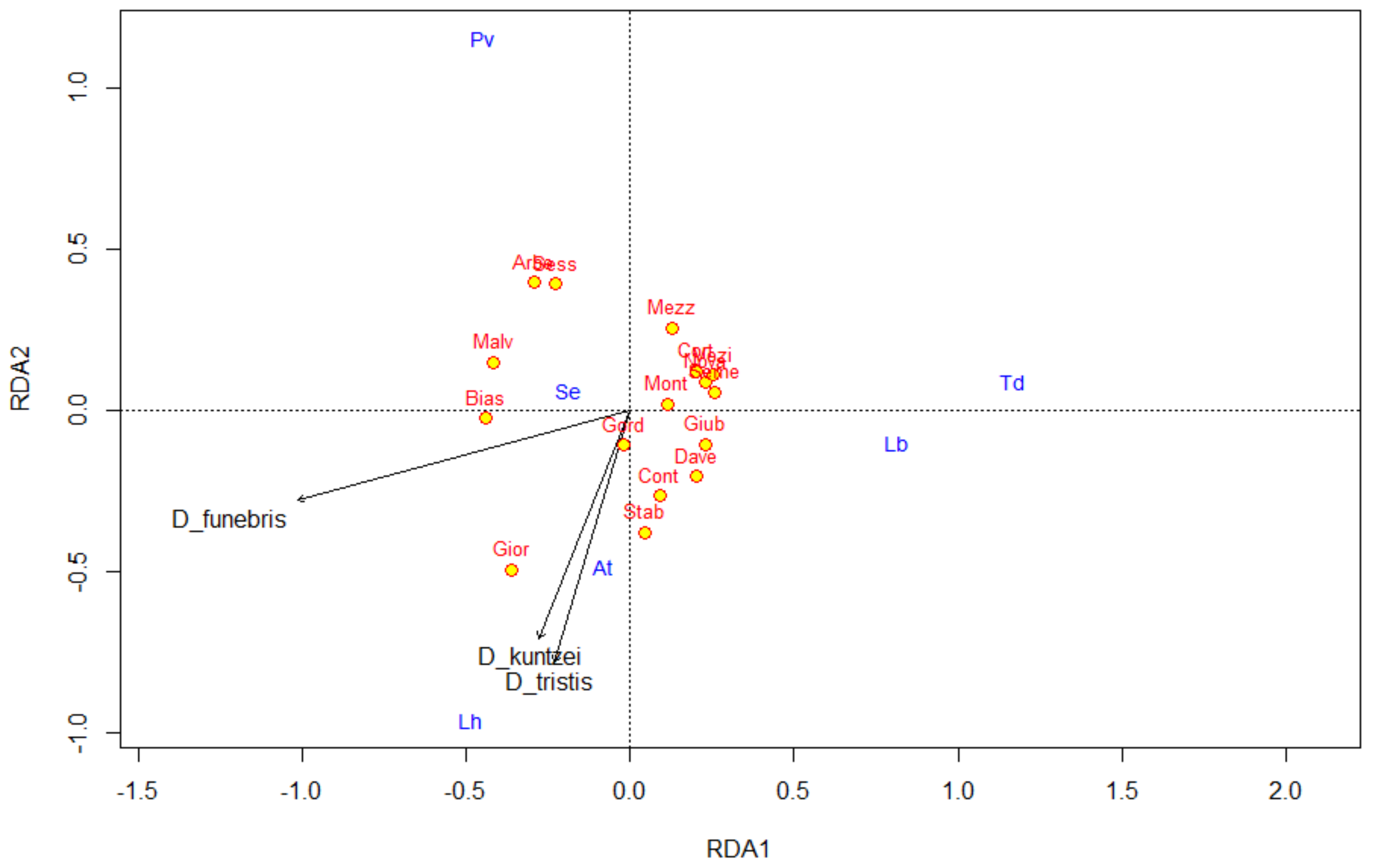
| D_funebris | 27% | ** | - | - |
| D_tristis | 13% | * | - | - |
| D_kuntzei | 12% | * | - | - |
| Total | 52% | *** | - | - |
| Source of Variation | Df | SS | MS | Pseudo F | p (perm) a | EV (%) |
|---|---|---|---|---|---|---|
| PERMANOVA | ||||||
| Period | 4 | 7.74 | 1.93 | 6.38 | 0.001 | 11.1 |
| Habitat | 2 | 3.02 | 1.51 | 4.99 | 0.003 | 4.3 |
| Microhabitat | 1 | 3.56 | 3.56 | 11.73 | 0.001 | 5.1 |
| Period × Habitat | 6 | 2.91 | 4.50 | 1.60 | ns | 4.2 |
| Period × Microhabitat | 4 | 1.39 | 0.35 | 1.15 | ns | 2.0 |
| Habitat × Microhabitat | 2 | 1.70 | 0.85 | 2.80 | 0.002 | 2.4 |
| Period × Habitat × Microhabitat | 6 | 2.24 | 0.37 | 1.23 | ns | 3.2 |
| Residuals | 156 | 47.28 | 0.30 | - | - | 67.7 |
| Total | 181 | 69.83 | - | - | - | 100 |
| PERMDISP | ||||||
| Period | 4 | - | - | 3.74 | 0.006 | - |
| Total | 177 | - | - | - | - | - |
| May–Jun | - | - | - | - | 0.03 | - |
| May–Aug | - | - | - | - | 0.06 | - |
| May–Sep | - | - | - | - | 0.001 | - |
| May–Oct | - | - | - | - | 0.07 | - |
| Jun–Aug | - | - | - | - | ns | - |
| Jun–Sep | - | - | - | - | ns | - |
| Jun–Oct | - | - | - | - | ns | - |
| Aug–Sep | - | - | - | - | ns | - |
| Aug–Oct | - | - | - | - | ns | - |
| Sep–Oct | - | - | - | - | ns | - |
| Habitat | 2 | - | - | 3.96 | 0.02 | - |
| Total | 179 | - | - | - | - | - |
| ecotone-crop | - | - | - | - | ns | - |
| woody-crop | - | - | - | - | 0.02 | - |
| woody-ecotone | - | - | - | - | ns | - |
| Microhabitat | 1 | - | - | 0.25 | ns | - |
| Total | 180 | - | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivellone, V.; Meier, M.; Cara, C.; Pollini Paltrinieri, L.; Gugerli, F.; Moretti, M.; Wolf, S.; Collatz, J. Multiscale Determinants Drive Parasitization of Drosophilidae by Hymenopteran Parasitoids in Agricultural Landscapes. Insects 2020, 11, 334. https://doi.org/10.3390/insects11060334
Trivellone V, Meier M, Cara C, Pollini Paltrinieri L, Gugerli F, Moretti M, Wolf S, Collatz J. Multiscale Determinants Drive Parasitization of Drosophilidae by Hymenopteran Parasitoids in Agricultural Landscapes. Insects. 2020; 11(6):334. https://doi.org/10.3390/insects11060334
Chicago/Turabian StyleTrivellone, Valeria, Michela Meier, Corrado Cara, Lucia Pollini Paltrinieri, Felix Gugerli, Marco Moretti, Sarah Wolf, and Jana Collatz. 2020. "Multiscale Determinants Drive Parasitization of Drosophilidae by Hymenopteran Parasitoids in Agricultural Landscapes" Insects 11, no. 6: 334. https://doi.org/10.3390/insects11060334





