Enzymology, Histological and Ultrastructural Effects of Ar-Turmerone on Culex pipiens pallens Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Ar-Turmerone
2.3. Treatment of Larvae, Pupae and Eggs by Ar-Turmerone Solution
2.4. Treatment of Fourth Instar Larvae by Ar-Turmerone Solution for Histological Study
2.5. Histological Observation
2.6. Transmission Electron Microscopy (TEM)
2.7. Enzymological Studies
2.7.1. Preparation of the Samples
2.7.2. Determination of AChE Activity
2.7.3. Determination of GST, CarE and P450 Activities
2.8. Data Analysis
3. Results
3.1. Pupicidal and Ovicidal Activities of Ar-Turmerone against C. pipiens Pallens
3.2. Larvicidal Activities of Ar-Turmerone against the First to Third Instar Larvae
3.3. Toxicity Behavior Observations of Larvae Treated by Ar-Turmerone
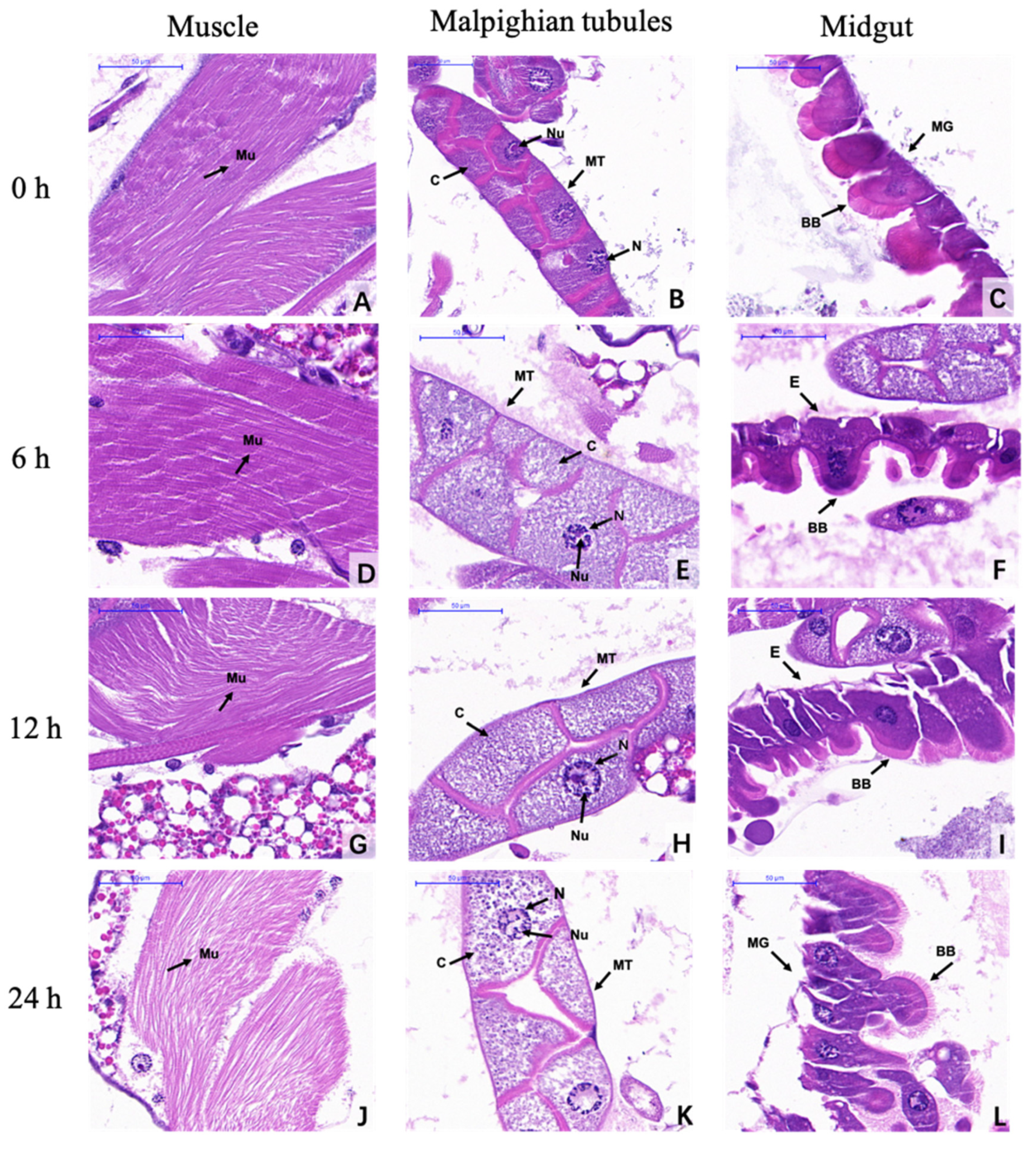
3.4. Histological Observations
3.5. Transmission Electron Microscopy (TEM) Observations
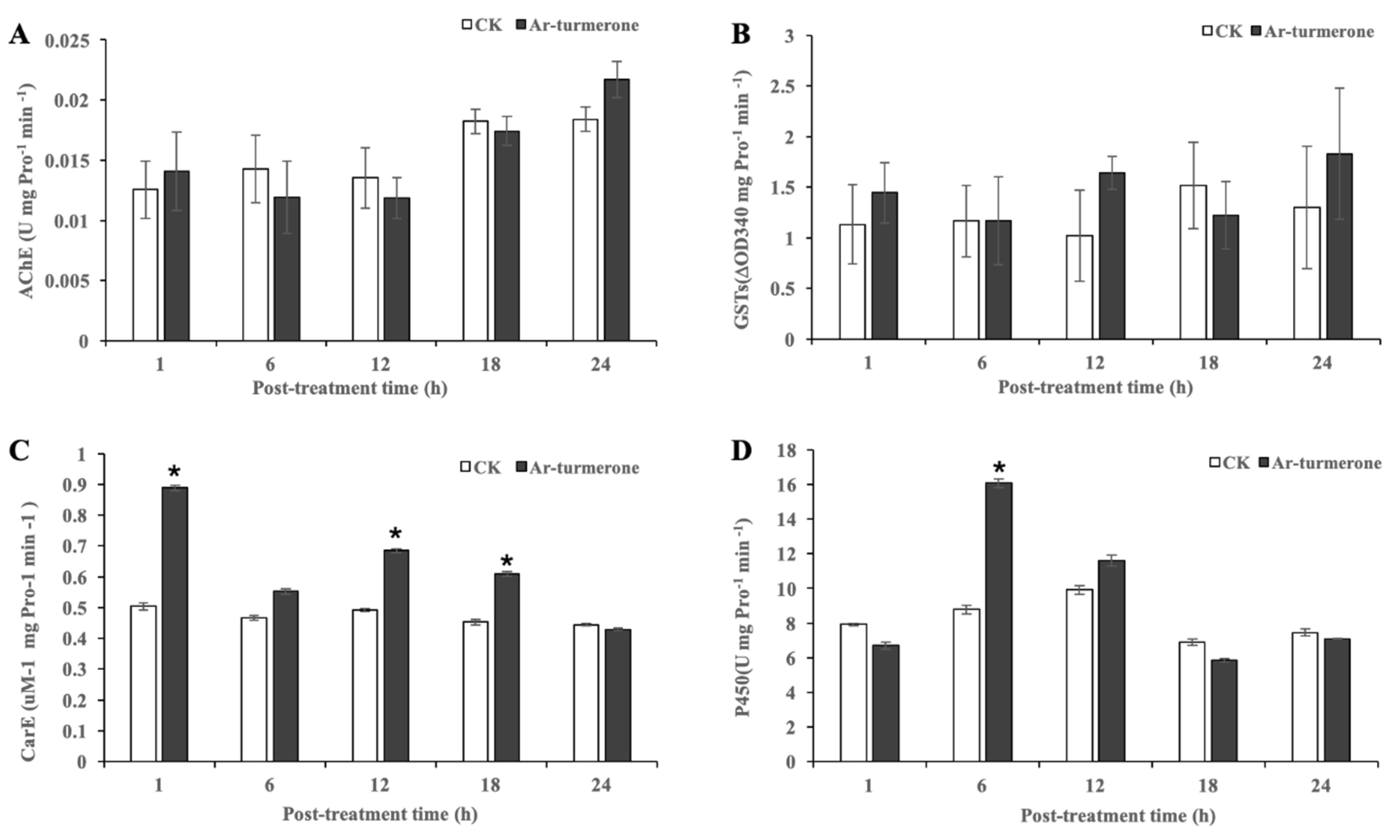
3.6. Activity of the Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medlock, J.M.; Hansford, K.M.; Schaffner, F. A Review of the Invasive Mosquitoes in Europe: Ecology, Public Health Risks, and Control Options. Vector Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, L.; Cheng, P.; Xu, J.; Huang, X.; Wang, H.; Song, X.; Liu, L.; Wang, H.; Kou, J. Trends in insecticide resistance in Culex pipiens pallens over 20 years in Shandong, China. Parasites Vectors 2019, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Champakaew, D.; Choochote, W.; Pongpaibul, Y.; Chaithong, U.; Jitpakdi, A.; Tuetun, B.; Pitasawat, B. Larvicidal efficacy and biological stability of a botanical natural product, zedoary oil-impregnated sand granules, against Aedes aegypti (Diptera, Culicidae). Parasitol. Res. 2007, 100, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Penilla, R.P.; Rodriguez, A.D.; Hemingway, J.; Torres, J.L.; Arredondo-Jimenez, J.I.; Rodriguez, M.H. Resistance management strategies in malaria vector mosquito control. Baseline data for a large-scale field trial against Anopheles albimanus in Mexico. Med. Vet. Entomol. 1998, 12, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Weill, M.; Lutfalla, G.; Mogensen, K.; Chandre, F.; Berthomieu, A.; Berticat, C.; Pasteur, N.; Philips, A.; Fort, P.; Raymond, M. Insecticide resistance in mosquito vectors. Nature 2003, 425, 366. [Google Scholar] [CrossRef]
- Sukumar, K.; Perich, M.J.; Boobar, L.R. Botanical derivatives in mosquito control: A review. J. Am. Mosq. Control. Assoc. 1991, 7, 210–237. [Google Scholar]
- Mathivanan, T.; Govindarajan, M.; Elumalai, K.; Krishnappa, K.; Ananthan, A. Mosquito larvicidal and phytochemical properties of Ervatamia coronaria Stapf. (Family: Apocynaceae). J. Vector Borne Dis. 2010, 47, 178–180. [Google Scholar]
- El-Wakeil, N.E. Botanical Pesticides and Their Mode of Action. Gesunde Pflanzen 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Gross, D.A. Botanical pesticides: Identification of a molecular target and mode of action studies. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Joshi, R.S.; Wagh, T.P.; Sharma, N.; Mulani, F.; Giri, A.P. Way toward “Dietary Pesticides”: Molecular Investigation of Insecticidal Action of Caffeic Acid against Helicoverpa armigera. J. Agric. Food Chem. 2014, 62, 10847–10854. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop. Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Mudili, V.; Anthuvan, A.J.; Siddaiah, C.; Sreepathi, M.H.; Vardhan, B.H. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT—Food Sci. Technol. 2016, 69, 522–528. [Google Scholar]
- Kamdem, S.L.S.; Belletti, N.; Tchoumbougnang, F.; Essiangang, J.J.; Montanari, C.; Tabanelli, G.; Lanciotti, R.; Gardini, F. Effect of mild heat treatments on the antimicrobial activity of essential oils of Curcuma longa, Xylopia aethiopica, Zanthoxylum xanthoxyloides and Zanthoxylum leprieurii against Salmonella enteritidis. J. Essent. Oil Res. 2014, 27, 52–60. [Google Scholar] [CrossRef]
- Ali, A.; Wang, Y.; Khan, I.A. Larvicidal and Biting Deterrent Activity of Essential Oils of Curcuma longa, Ar-turmerone, and Curcuminoids Against Aedes aegypti and Anopheles quadrimaculatus (Culicidae: Diptera). J. Med. Entomol. 2015, 52, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.N.; Chandra, A.; Nair, M.G. Novel Bioactivities of Curcuma longa Constituents. J. Nat. Prod. 1998, 61, 542–545. [Google Scholar] [CrossRef]
- Ramadasan, K.; VijayastelterB, L.; Kottarapat, J. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J. Pharmacol. 2011, 43, 526. [Google Scholar]
- Liu, J.; Zhang, M.; Fu, W.; Hu, J.; Dai, G. Efficacy of bioactive compounds from Curcuma longa L. against mosquito larvae. J. Appl. Entomol. 2018, 142, 792–799. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, L.; Li, C.; Su, T.; Jin, J.; Guo, Y.; Ren, D.; Yang, Z.; Liu, Q.; Meng, F. A Survey of Insecticide Resistance in Aedes albopictus (Diptera: Culicidae) during a 2014 Dengue Fever Outbreak in Guangzhou, China. J. Econ. Entomol. 2016, 110, 239. [Google Scholar]
- Daniella, G.; Christelle, D.; Andric, G.; Cédric, R.; Thierry, G.; Frédéric, F.; Jean-Philippe, D.; Anubis, V.-R.; Florence, F. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect. Dis. Poverty 2017, 6, 38. [Google Scholar]
- Li, S.; Li, M.; Huang, Y.; Hua, R.; Lin, H.; He, Y.; Wei, L.; Liu, Z. Fumigant activity of Illicium verumfruit extracts and their effects on the acetylcholinesterase and glutathioneS-transferase activities in adultSitophilus zeamais. J. Pest Sci. 2013, 86, 677–683. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Shi, L.; Xu, Z.; He, L. Resistance selection and biochemical mechanism of resistance against cyflumetofen in Tetranychus cinnabarinus (Boisduval). Pestic. Biochem. Physiol. 2014, 111, 24–30. [Google Scholar] [CrossRef]
- Chellasamy, P.; Kadarkarai, M.; Kalimuthu, K.; Mahesh, K.P. Mosquito larvicidal, pupicidal, adulticidal, and repellent activity of Artemisia nilagirica (Family: Compositae) against Anopheles stephensi and Aedes aegypti. Parasitol. Res. 2012, 111, 2241–2251. [Google Scholar]
- Govindarajan, M.; Mathivanan, T.; Elumalai, K.; Krishnappa, K.; Anandan, A. Mosquito larvicidal, ovicidal, and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2011, 109, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, L.; Ma, Z.; Zhang, X. Microstructural and ultrastructural changes in the muscle cells of the oriental armyworm Mythimna separata Walker (Lepidoptera: Noctuidae) on treatment with wilforine. Pestic. Biochem. Physiol. 2017, 139, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lü, M.; Wu, W.; Liu, H. Effects of fraxinellone on the midgut ultrastructural changes of Mythimna separata Walker. Pestic. Biochem. Physiol. 2010, 98, 263–268. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Wu, X.; Yu, W.; Guo, S. Insecticidal Mechanism of Wintergreen Oil Against the Health Pest Paederus fuscipes (Coleoptera: Staphylinidae). J. Med. Entomol. 2018, 55, 155–162. [Google Scholar] [CrossRef]
- Chen, Y.; Bertrand, C.; Dai, G.; Yuan, J. Biochemical mechanisms of acaricidal activity of 2,4-di-tert-butylphenol and ethyl oleate against the carmine spider mite Tetranychus cinnabarinus. J. Pest Sci. 2018, 91, 405–419. [Google Scholar] [CrossRef]
- Rahuman, A.A.; Gopalakrishnan, G.; Ghouse, B.S.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia 2000, 71, 553–555. [Google Scholar] [CrossRef]
- Haribalan, P.; Jin, J.M.; Ran, K.J.; Murugan, K.; Joon, A.Y. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasites Vectors 2015, 8, 237. [Google Scholar]
- Daniel, R.A.; Rajiv, G.M.; Gabriel, P.M.; Kedike, B.; Savarimuthu, I. Effect of niloticin, a protolimonoid isolated from Limonia acidissima L. (Rutaceae) on the immature stages of dengue vector Aedes aegypti L. (Diptera: Culicidae). Acta Tropica 2014, 139, 67–76. [Google Scholar]
- Wang, C.M.; Zheng, X.L. Toxicity of photoactivated insecticide K-01 to the larvae of Aedes albopictus. J. South. Med. Univ. 2006, 26, 431. [Google Scholar]
- Kala, S.; Singla, N.; Verma, P.; Naik, S.N.; Agarwal, A.; Patanjali, P.; Kumar, J. Nanoemulsion of cashew nut shell liquid bio-waste: Mosquito larvicidal activity and insights on possible mode of action. S. Afr. J. Bot. 2019, 127, 293–300. [Google Scholar] [CrossRef]
- Percy, J.; Fast, P.G. Bacillus thuringiensis crystal toxin: Ultrastructural studies of its effect on silkworm midgut cells. J. Invertebr. Pathol. 1983, 41, 86–98. [Google Scholar] [CrossRef]
- Endo, Y.; Nishiitsutsuji-Uwo, J. Mode of action of Bacillus thuringiensis δ-endotoxin: Histopathological changes in the silkworm midgut. J. Invertebr. Pathol. 1980, 36, 90–103. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Wu, W. Effects of Celangulin V on midgut cells and digestive enzyme activities of Mythimna separata (walker). Acta Entomol. Sin. 1998, 41, 258–262. [Google Scholar]
- Arshia, H.; Yee, L.S.; Lim, C.W.; Sofian, A.M.; Sanghiran, L.V.; Khalijah, A. Inhibition and Larvicidal Activity of Phenylpropanoids from Piper sarmentosum on Acetylcholinesterase against Mosquito Vectors and Their Binding Mode of Interaction. PLoS ONE 2016, 11, e0155265. [Google Scholar]
- Jadhav, K.B.; Rajini, P.S. Neurophysiological alterations in Caenorhabditis elegans exposed to dichlorvos, an organophosphorus insecticide. Pestic. Biochem. Physiol. 2009, 94, 79–85. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Holden, L.; Sturgess, N.; Weiner, M.; Sheets, L.; Sargent, D.; Soderlund, D.M.; Choi, J.; Symington, S.; Clark, J.M.; et al. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology 2009, 30 (Suppl. 1), S17–S31. [Google Scholar] [CrossRef]
- Evans, P.D. Studies on the mode of action of octopamine, 5-hydroxytryptamine and proctolin on a myogenic rhythm in the locust. J. Exp. Biol. 1984, 110, 231–251. [Google Scholar]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular Mechanisms of Metabolic Resistance to Synthetic and Natural Xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Yang, M.L.; Zhang, J.Z.; Zhu, K.Y.; Xuan, T.; Liu, X.J.; Guo, Y.P.; Ma, E.B. Mechanisms of organophosphate resistance in a field population of oriental migratory locust, Locusta migratoria manilensis (Meyen). Arch. Insect Biochem. Physiol. 2009, 71, 3–15. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Song, D.N.; Wu, H.H.; Yang, H.M.; Zhang, J.Z.; Li, L.J.; Ma, E.B.; Guo, Y.P. Effect of Dietary Cadmium on the Activity of Glutathione S-Transferase and Carboxylesterase in Different Developmental Stages of the Oxya chinensis (Orthoptera: Acridoidea). Environ. Entomol. 2014, 43, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Nutchaya, K.; Wanchai, P.; Opender, K.; Vasakorn, B. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014, 87, 721–729. [Google Scholar]
- Rachokarn, S.; Piyasaengthong, N.; Bullangpoti, V. Impact of botanical extracts derived from leaf extracts Melia azedarach L. (Meliaceae) and Amaranthus viridis L. (Amaranthaceae) on populations of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) and detoxification enzyme activities. Commun. Agric. Appl. Biol. Sci. 2008, 73, 451. [Google Scholar] [PubMed]
- Rattanapan, A. Effects of rotenone from derris crude extract on esterase enzyme mechanism in the beet armyworm, Spodoptera exiqua (Hubner). Commun. Agric. Appl. Biol. Sci. 2009, 74, 437–444. [Google Scholar] [PubMed]
- Sukhirun, N.; Pluempanupat, W.; Bullangpoti, V.; Koul, O. Bioefficacy of Alpinia galanga (Zingiberaceae) rhizome extracts, (E)-p-acetoxycinnamyl alcohol, and (E)-p-coumaryl alcohol ethyl ether against Bactrocera dorsalis (Diptera: Tephritidae) and the impact on detoxification enzyme activities. J. Econ. Entomol. 2011, 104, 1534–1540. [Google Scholar] [CrossRef] [PubMed]

| Compound | Life Stages | Concentration (ppm) | Mortality ± SE (%) | LC50 (95%CL) a (ppm) |
|---|---|---|---|---|
| ar-turmerone | Larvae b | - | - | 138.86 (127.04–150.93) |
| Pupae | 10,000 | 13.96 ± 2.93 | 13,172.03 (12,575.49–13,773.53) | |
| 12,000 | 25.97 ± 3.27 | |||
| 14,000 | 60.61 ± 11.61 | |||
| 16,000 | 86.04 ± 2.93 | |||
| 18,000 | 94.41 ± 2.83 | |||
| Eggs | 10,000 | 33.27 ± 4.18 | 11,040.19 (101,07.68–11,748.17) | |
| 12,000 | 65.14 ± 2.64 | |||
| 14,000 | 82.41 ± 4.04 | |||
| 16,000 | 79.26 ± 0.74 | |||
| 18,000 | 87.92 ± 3.07 | |||
| 1% Temephos | Larvae | 50 | 100 | - |
| Pupae | 20,000 | 90 | - | |
| Eggs | 40,000 | 0 | - | |
| 1% DMSO | Larvae | - | 0 | - |
| Pupae | - | 0 | - | |
| Eggs | - | 0 | - |
| Compound | Ages | LC50 (95%CL) a (ppm) | χ2 | p-Value |
|---|---|---|---|---|
| ar-turmerone | 1th | 27.168 (10.175–51.84) | 4.55 | 0.72 |
| 2th | 59.655 (51.460–66.716) | 13.02 | 0.45 | |
| 3th | 85.713 (75.476–92.796) | 5.76 | 0.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Fernandez, D.; Gao, Y.; Silvie, P.; Gao, Y.; Dai, G. Enzymology, Histological and Ultrastructural Effects of Ar-Turmerone on Culex pipiens pallens Larvae. Insects 2020, 11, 336. https://doi.org/10.3390/insects11060336
Liu J, Fernandez D, Gao Y, Silvie P, Gao Y, Dai G. Enzymology, Histological and Ultrastructural Effects of Ar-Turmerone on Culex pipiens pallens Larvae. Insects. 2020; 11(6):336. https://doi.org/10.3390/insects11060336
Chicago/Turabian StyleLiu, Jia, Diana Fernandez, Yanjin Gao, Pierre Silvie, Yongdong Gao, and Guanghui Dai. 2020. "Enzymology, Histological and Ultrastructural Effects of Ar-Turmerone on Culex pipiens pallens Larvae" Insects 11, no. 6: 336. https://doi.org/10.3390/insects11060336
APA StyleLiu, J., Fernandez, D., Gao, Y., Silvie, P., Gao, Y., & Dai, G. (2020). Enzymology, Histological and Ultrastructural Effects of Ar-Turmerone on Culex pipiens pallens Larvae. Insects, 11(6), 336. https://doi.org/10.3390/insects11060336






